Abstract
Background
Intercellular adhesion molecule-1 (ICAM-1) modulates cell–cell adhesion and is a receptor for cognate ligands on leukocytes. Upregulation of ICAM-1 has been demonstrated in malignant transformation of adenomas and is associated with poor prognosis for many malignancies. ICAM-1 is upregulated on the invasive front of pancreatic metastases and melanomas. These data suggest that the upregulated ICAM-1 expression promotes malignant progression. We hypothesize that the downregulation of ICAM-1 will mitigate tumor progression.
Methods
Mouse colon adenocarcinoma cells (MC38) were evaluated for the expression of ICAM-1 using Western immunoblot analysis. Short hairpin RNA (shRNA) transduction was used to downregulate ICAM-1. Tumor invasion determined via a modified Boyden chamber was used as a surrogate of tumor progression examining MC38 cells, MC38 ICAM-1 knockdowns, and MC38 transduced with vehicle control. The cells were cultured in full media for 24 h and serum-starved for 24 h. A total of 5 × 104 cells were plated and allowed to migrate for 24 h using full media with 10% fetal bovine serum as a chemoattractant. Inserts were fixed and stained with crystal violet. Blinded investigators counted the cells using a stereomicroscope. Statistical analysis was performed by analysis of variance with Fischer protected least significant difference and a P value of <0.05 was considered statistically significant.
Results
ICAM-1 was constitutively expressed on MC38 cells. Transduction with anti–ICAM-1 shRNA vector downregulated ICAM-1 protein expression by 30% according to the Western blot analysis (P < 0.03) and decreased ICAM-1 messenger RNA expression by 70% according to the reverse transcription–polymerase chain reaction. shRNA knockdown cells had a significant reduction in invasion >45% (P < 0.03). There were no significant differences between the invasion rates of MC38 and MC38 vehicle controls.
Conclusions
Downregulation of ICAM-1 mitigates MC38 invasion. These data suggest that targeted downregulation of tumor ICAM-1 is a potential therapeutic target.
Keywords: Intercellular adhesion-molecule 1, Macrophage, Neutrophil, Alpha-actinin, Extracellular matrix
1. Introduction
Intercellular adhesion molecule-1 (ICAM-1), a member of the immunoglobulin superfamily, is a cell surface glycoprotein expressed by multiple cells, but prominently on endothelia [1]. Previous studies have shown that ICAM-1 plays an important role in neutrophil adhesion, migration, and facilitation of respiratory burst [2]. Of particular importance is the interaction between the neutrophil membrane protein, β2-integrin, and ICAM-1 on cell membrane surfaces [2]. More recently, it has been observed that ICAM-1 is highly expressed on the leading edge of many types of neoplastic cells, such as gastric carcinoma, melanoma, and pancreatic cancer [3,4]. In the case of gastric and pancreatic carcinomas, increased circulating levels and increased surface expression of ICAM-1 on tumor cells are associated with a poor prognosis [3,4].
It has previously been shown that binding ICAM-1 leads to structural changes in the cytoskeleton of endothelia that separate tricellular junctions [5]. Furthermore, the tumor microenvironment contains ample binding sites for multiple adhesion molecules to augment cancer progression [6].
Based on these data, we hypothesize that tumor-associated ICAM-1 mediates tumor progression in the absence of immune cells. To test this hypothesis, ICAM-1 was downregulated using short hairpin RNA (shRNA) and immune cell–independent tumor invasion was examined in vitro.
2. Materials and methods
2.1. Cell culture and reagents
The murine colon adenocarcinoma cell line MC38 was obtained from the Division of Cancer Treatment and Diagnosis Tumor Repository, National Cancer Institute, Frederick, MD, in association with the National Institutes of Health. All culture media were purchased from Life Technologies Corporation, Grand Island, NY. Cells were grown and maintained at 37°C in 5% carbon dioxide and RMPI-1640 containing 10% fetal bovine serum and 1% penicillin–streptomycin. During serum starvation, the cells were incubated in Roswell Park Memorial Institute-1640 containing 0.5% fetal bovine serum and 1% penicillin–streptomycin.
2.2. Western blot analysis
MC38 cells were plated in six-well culture plates with 200,000 cells/well in full media for 24 h and then serum-starved for 12 h before lysis. Cells were lysed with mammalian protein extraction reagent lysis buffer (Thermo Fisher Scientific, Rockford, IL) and mixed in a 3:1 ratio with a Laemmli buffer/BME solution. Cell lysate protein density was equalized using mass spectrometry before beginning the immunoblotting. Proteins were resolved using 4%–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Bio-Rad Laboratories, Hercules, CA). The proteins were transferred to nitrocellulose membranes, blocked, and probed with goat anti–ICAM-1 monoclonal antibody (mAb) as well as with rabbit anti–GAPDH mAb (Santa Cruz Biotechnology, Dallas, TX). GAPDH was used to assess overall protein density of the cell lysates. Blocking solution contained 5% nonfat milk in 1× phosphate-buffered saline (PBS) with 0.1% Tween-20. The ICAM-1 antibodies were diluted in 5% nonfat milk and 1× PBS with 0.1% Tween-20. After incubation with horse-radish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology), signal was detected using West Dura chemiluminescent substrate (Thermo Fisher Scientific).
2.3. Short hairpin RNA knockout of ICAM-1
MC38 cells were plated into six-well culture plates in full media for 24 h before viral infection (day 1). On day 2, the media were removed and replaced with full media containing polybrene (8 μg/mL). Lentiviral particles containing the shRNA with the ICAM-1 knockdown plasmids were added to half of the wells, whereas other wells received the lentiviral particles containing plasmids with nontargeting control, which will serve as a vehicle control (all lentiviral particles were acquired from Functional Genomics Facility, University of Colorado at Boulder, Boulder, CO). The lentiviral particle used to knockdown ICAM-1 was the single clone (TRCN0000065923) that has been validated by Sigma-Aldrich (St. Louis, MO), whereas the vehicle control was a nontargeting control (SHC002). Cells were incubated overnight, then the media containing polybrene and lentiviral particles were replaced with full media. Starting on day 4, full media containing puromycin (5 μg/mL) were used. The puromycin in this concentration kills untransduced MC38 cells, allowing selection of stable MC38 viral transductants. ICAM-1 protein density was assessed in these two cell groups alongside MC38 control cells according to the Western blot analysis protocol explained previously.
2.4. Real-time polymerase chain reaction
Untransduced MC38 cells, MC38 cells transduced with vehicle control, and MC38 cells transduced with ICAM-1 knockdown were grown in six-well plates at a density of 200K and 300K cells/well with full media, overnight. Messenger RNA (mRNA) was collected using Qiagen (Valencia, CA) mini RNA prep kit. All cells were analyzed for mRNA expression of ICAM-1 using quantitative real-time reverse transcriptase–polymerase chain reaction as previously described (Abcam, Cambridge, MA). mRNA for ICAM-1 was normalized to mRNA for GAPDH in the same samples (Abcam) and untransduced MC38 cells were designated a relative value of 1.
2.5. Cell invasion assay
The invasion chamber apparatus we used was BD BioCoat Matrigel Matrix (BD Biosciences, San Jose, CA). The three types of cells were plated for culture in six-well plates, according to the cell culture protocol described previously: an MC38 control cell group, a lentiviral MC38 vehicle control group, and the MC38 cell group with shRNA ICAM-1 knockdown. The cells were cultured in full media for 24 h and then serum-starved for 24 h in preparation for introduction into a 24-well Boyden-style chamber assay. A total of 5 × 104 cells were added to six Matrigel-precoated transwell inserts. The inserts were added to six wells of a 24-well cell culture plate.
The cells were allowed to migrate for 24 h from serum-starved media in the top chamber to full media with 10% fetal bovine serum as a chemoattractant in the bottom chamber. Once the migration period was complete, the inserts were rinsed and the cells were removed from the top of the membrane by scrubbing gently with a moist cotton swab. Membranes were then fixed with methanol and stained with 0.5% crystal violet. The membranes were then removed from the inserts with a scalpel and fixed to slides.
Light microscopy was used to evaluate the invasion of cells. A blinded investigator observed all cell slides and each membrane was sampled in three fields at random. Migrated cells were then counted and recorded for each field and the data were compiled.
2.6. Statistical analysis
Analysis of variance tests were performed when comparing the data groups. Statview (SAS Institute, Inc, Cary, NC) was used for all statistical analysis. Statistical significance was considered at P < 0.05.
3. Results
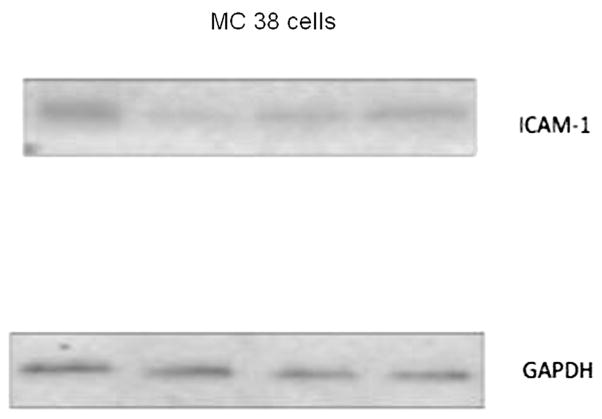
3.1. ICAM-1 is constitutively expressed on MC38 cells
Western immunoblot analysis of MC38 cell lysates revealed constitutive expression of ICAM-1 (Fig. 1). The ICAM-1 bands were detected on the nitrocellulose membrane at approximately 102 kDa in untransduced MC 38 cells. The GAPDH bands, used as a marker of overall protein density, were detected at approximately 39 kDa.
Fig. 1.
Western blot of untransduced MC 38 cell lysate shows the presence of ICAM-1 protein.
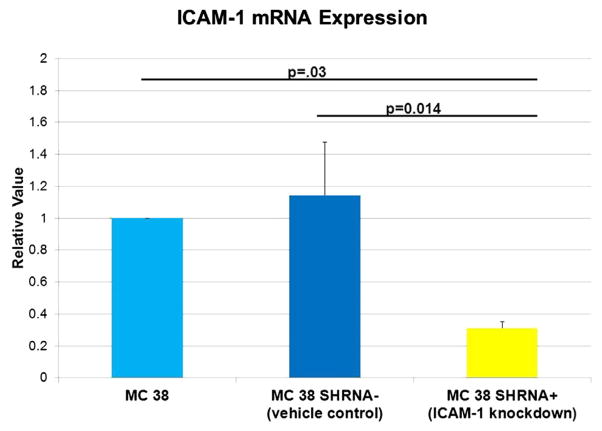
3.2. RT-PCR comparison of ICAM-1 mRNA in all cell lines
Untransduced MC 38 cells had a relative value of ICAM-1 mRNA expression of 1 and the MC 38 cells transduced with the vehicle control were found to have a relative value of ICAM-1 mRNA expression of 1.1; there is no difference in mRNA expression of ICAM-1 between these groups. MC 38 cells transduced with anti–ICAM-1 vector had a relative value of ICAM-1 mRNA expression of 0.31, which is approximately 70% less mRNA expression when compared with untransduced MC38 cells and MC38 cells transduced with the vehicle control (P = 0.03 and P = 0.014) (Fig. 2).
Fig. 2.
Reverse transcription–polymerase chain reaction revealed decreased ICAM-1 mRNA expression in MC 38 cells transduced with anti–ICAM-1 shRNA vector. There is no difference in ICAM-1 mRNA expression between untransduced MC 38 cells and MC 38 cells transduced with vehicle control. There was decreased ICAM-1 mRNA expression in the MC 38 cells transduced with anti–ICAM-1 shRNA vector. P = 0.03 when compared with untransduced MC 38 cells and P = 0.014 when compared with MC 38 cells transduced with vehicle control. N = 5 runs in triplicate. (Color version of figure is available online.)
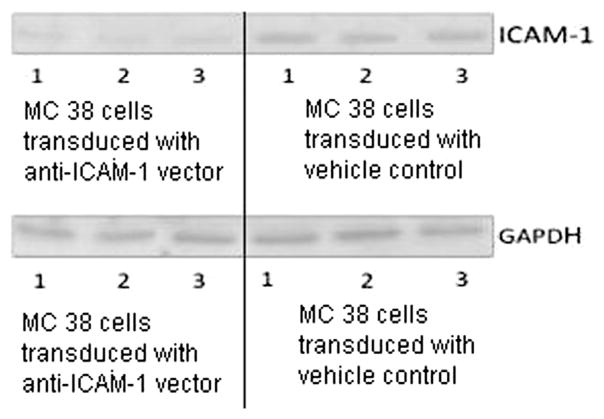
3.3. Western blot demonstrated ICAM-1 downregulation after transduction
As there was no difference between mRNA levels in the untransduced MC38 cells and the MC38 vehicle control, we now compared only the vehicle control with the MC38 cells transduced with the anti–ICAM-1.
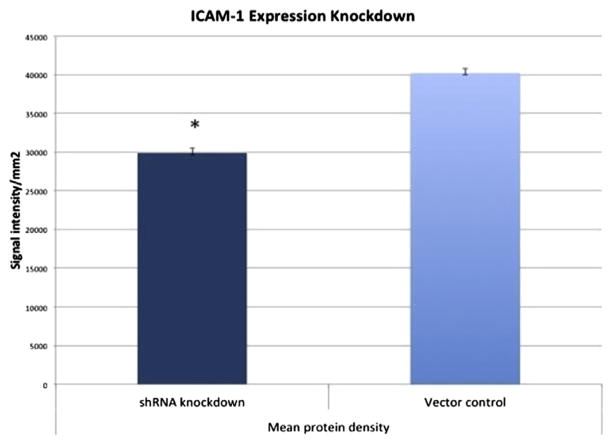
The lentiviral transduction with an anti–ICAM-1 shRNA vector molecule downregulated the ICAM-1 expression of MC38 cells. ICAM-1 bands were again detected at approximately 102 kDa (Fig. 3); however, densitometry analysis of Western immunoblot membranes revealed that ICAM-1 expression had been reduced by 30% (P < 0.03) when compared with control cells (Fig. 4). GAPDH was detected at approximately 39 kDa, and its density was not different between the test groups.
Fig. 3.
Western blot comparing ICAM-1 expression of MC 38 cells transduced with vehicle control and anti–ICAM-1 shRNA vector.
Fig. 4.
Densitometry analysis of Western blot comparing MC 38 cells transduced with vehicle control and anti–ICAM-1 shRNA vector. *P < 0.03 compared with the control group. The x-axis separates the test cell group from the vector control and the y-axis represents the signal intensity of the observed ICAM-1 bands using densitometry software. (Color version of figure is available online.)
3.4. ICAM-1 downregulation corresponds with decreased cell invasion
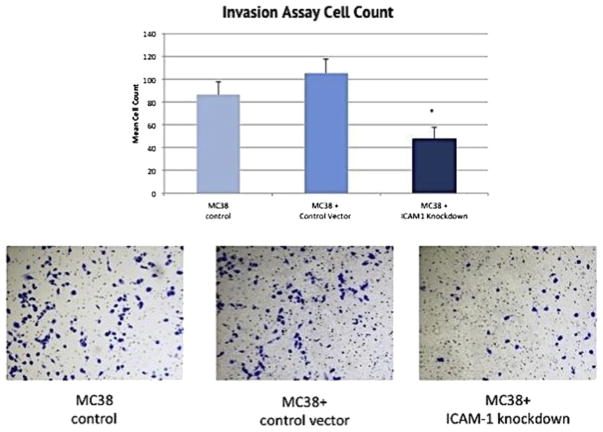
The reduction of ICAM-1 expression among the MC38 cells with a confirmed shRNA knockdown corresponded with a significant reduction of cell invasion in the Matrigel chambers. Cell invasion was decreased by more than 45% (P < 0.03) was observed when compared with the control cells using an average cell count of measured fields (Fig. 5). There was no difference in the invasion rate between the untransduced MC38 control cell group and the MC38 cells transduced with the vehicle control molecule (Fig. 5).
Fig. 5.
Analysis of rate of MC38 cell invasion through the membrane of the invasion chamber. *P <0.03 compared with the control group. Images following the graph show microscopic images of MC38 cells within the matrix of the chamber membrane and stained with crystal violet. The x-axis of the graph separates the three cell groups and the y-axis measures the mean count of invaded cells. (Color version of figure is available online.)
4. Discussion
ICAM-1 is a cell surface glycoprotein of the immunoglobulin superfamily that, although has been studied extensively, is still revealing an important function in pathophysiological processes. Recently, it has been suggested that ICAM-1 potentiates metastatic progression of pancreatic adenocarcinoma [3,4].
Furthermore, work examining the role of ICAM-1 in tumor progression using transgenic ICAM-1 null murine models has revealed that a lack of intrinsic ICAM-1 does not prevent hepatic metastases of pancreatic cancer. However, in this model, use of anti–ICAM-1 mAb mitigates tumor progression, thus implicating tumor-expressed ICAM-1 as having a putative role in tumor metastasis [3]. Despite these recent findings, it had not yet been elucidated whether tumor-associated ICAM-1 plays a significant role in tumor cell invasive potential that is independent of immune cell association. It is well understood that the immune response can be important in limiting the growth of neoplasms [7]. However, more recent interest has been directed at immunomodulation within the tumor microenvironment that can promote tumor growth and metastasis [8]. Interestingly, it has been shown that the release of neutrophil elastase is mediated by cross-linking of β2-integrin moieties on neutrophils, suggesting that ICAM-1 may play a key role in facilitating tumor progression [8.9]. Despite the potential facilitating role for ICAM-1 via ligation of leukocytes, it is clear that ICAM-1 has immune cell–independent function [5]. Furthermore, the tumor microenvironment hosts abundant binding sites for numerous adhesion molecules to augment cancer progression [6]. It is well known that ICAM-1 has an intimate association with cytoskeletal proteins such as alpha-actinin that can facilitate cellular movement, and this may be sufficient to promote tumor invasion in the absence of immune cells [2,10].
In this series of experiments, we attempted to gain a better understanding of the immune cell–independent significance of colon adenocarcinoma expression of ICAM-1. These results implicate ICAM-1 as a mediator of immune cell–independent tumor invasion. The intimate binding of ICAM-1 to cytoskeleton proteins may grant tumor cells the ability to contort cell structure and move within an extracellular matrix [8,10].
Although these data suggest that the targeted down-regulation of tumor-expressed ICAM-1 is a potential therapeutic target to limit tumor progression, the challenges of using systemic selective ICAM-1 block may be similar to those of efalizumab. This drug, which was voluntarily withdrawn from the market in 2009, acts by binding to the CD11a subunit of lymphocyte function-associated antigen 1. Its action provided enough immunosuppressive effect to precipitate side effects, such as bacterial sepsis, viral meningitis, and PML [11,12]. Previous work by Vaporciyan et al. [13] has demonstrated that alternate delivery methods for anti–ICAM-1 therapy produce alternate effects. It is possible that regionalized therapy or metronomic dosing [14] may be of benefit in limiting tumor progression.
Footnotes
This work was presented at the eighth Academic Surgical Congress, New Orleans, LA.
References
- 1.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245. [PubMed] [Google Scholar]
- 2.Barnett CC, Moore EE, Mierau GW, et al. ICAM-1-CD18 interaction mediates neutrophil cytotoxicity through protease release. Am J Physiol. 1998;274:C1634. doi: 10.1152/ajpcell.1998.274.6.C1634. [DOI] [PubMed] [Google Scholar]
- 3.Roland C, Dineen SP, Toombs JE, et al. Tumor-derived intercellular adhesion molecule-1 mediates tumor-associated leukocyte infiltration in orthotopic pancreatic xenografts. Exp Biol Med. 2010;235:263. doi: 10.1258/ebm.2009.009215. [DOI] [PubMed] [Google Scholar]
- 4.Roland C, Harken AH, Sarr MG, Barnett CC. ICAM-1 expression determines malignant potential of cancer. Surgery. 2007;141:705. doi: 10.1016/j.surg.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Gopalan PK, Burns AR, Simon SI, Sparks S, McIntire LV, Smith CW. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J Leukoc Biol. 2000;68:47. [PubMed] [Google Scholar]
- 6.Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal. 2009 Dec;3:255. doi: 10.1007/s12079-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disis M. Immune regulation of cancer. J Clin Oncol. 2010;28:4531. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson D, Meng X, Fullerton DA, et al. Activation state of stromal inflammatory cells in murine metastatic pancreatic adenocarcinoma. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1067. doi: 10.1152/ajpregu.00320.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett CC, Moore EE, Moore FA, Biffl WL, Partrick DA. Soluble intercellular adhesion molecule-1 provokes polymorphonuclear leukocyte elastase release by CD18. Surgery. 1996;120:395. doi: 10.1016/s0039-6060(96)80315-1. [DOI] [PubMed] [Google Scholar]
- 10.Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992;118:1223. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwab N, Ulzheimer JC, Fox RJ, et al. Fatal PML associated with efalizumab therapy: insights into integrin αLβ2 in JC virus control. Neurology. 2012;78:458. doi: 10.1212/WNL.0b013e3182478d4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauvreau GM, Becker AB, Boulet LP, et al. The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2003;112:331. doi: 10.1067/mai.2003.1689. [DOI] [PubMed] [Google Scholar]
- 13.Mulligan MS, Vaporciyan AA, Warner RL, et al. Compartmentalized roles for leukocytic adhesion molecules in lung inflammatory injury. J Immunol. 1995;154:1350. [PubMed] [Google Scholar]
- 14.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]