Abstract
Plasmodium falciparum infection during pregnancy causes maternal anemia and low birth weight (LBW), but the effect of frequency and timing of infection on the severity of these adverse effects is unknown. We conducted a cohort study recruiting 2462 pregnant women in Malawi. Microscopy was used to diagnose malaria at enrollment, follow-up and delivery. Birth weight and maternal hemoglobin were measured at delivery. The association between timing and frequency of infection and LBW and maternal anemia was analyzed using a binomial regression model. Compared with uninfected women, (i) the risk of LBW increased with the number of malaria episodes [one episode: prevalence ratio (PR) 1.62 (95% CI 1.07–2.46); two episodes: PR 2.41 (95% CI 1.39–4.18)]; (ii) the risk for maternal anemia increased with the number of malaria episodes [one episode: PR 1.15 (95% CI 0.86–1.54); two episodes: PR 1.82 (95% CI 1.28–2.62)]; and (iii) the risk of LBW was higher with infection in the second (PR 1.71; 95% CI 1.06–2.74) than third trimester or at delivery (PR 1.55; 95% CI 0.88–2.75). The timing and frequency of P. falciparum infection during pregnancy affected the risk of LBW but only frequency of infection had an effect on the risk of maternal anemia. Identification of gestational periods when malaria causes most adverse outcomes will facilitate effective targeting of interventions.
Keywords: Malaria, Plasmodium falciparum, Pregnancy, Timing, Frequency, Malawi
1. Introduction
Each year more than 50 million pregnant women in areas endemic for Plasmodium falciparum infection are at risk of its complications [1], which are dependent on transmission rates and levels of acquired immunity. In low transmission areas, complications include anemia, severe malaria [2,3], and problems of pregnancy such as spontaneous abortions, still births and low birth weight (LBW). In high transmission areas, women of reproductive age have acquired partial immunity to malaria [4]. Consequently, they are not at risk for severe disease, but are at an increased risk for developing anemia and delivering babies with LBW. The actual mechanisms by which P. falciparum infection during pregnancy causes LBW and anemia still remain unclear. Multiple factors have been cited as contributing to the pathophysiology of LBW [5–10] and maternal anemia [11–13], but the role of each of these factors has not been elucidated clearly.
To our knowledge, there have been very few studies that have investigated the effect of frequency of P. falciparum infection during pregnancy on the risk of LBW and maternal anemia [14]. Some studies that have examined the relationship between timing of malaria infection during pregnancy and the risk for complications have used placental histology to draw inferences regarding the evolution of infection during pregnancy [4,7,15–21]. The assessment of malaria at a single time point may result in misclassification of the exposure to malaria during pregnancy. Other studies have limited recruitment to primigravidae [22]. Plasmodium falciparum infection in multigravidae is not infrequent, and also poses a significant risk for severe anemia and LBW [18].
We therefore conducted a prospective study to examine women for the presence of parasitemia at several time points during pregnancy and at delivery to investigate the impact of timing and frequency of P. falciparum malaria on the risk of LBW and maternal anemia. This information could aid policy makers to identify the crucial time point when treatment and intermittent presumptive therapy (IPTp) would be most effective in preventing the complications of infection of malaria during pregnancy.
2. Methods
2.1. Study site
The study was conducted at Mpemba and Madziabango health centers in Blantyre District, which has a population of approximately 950 000 [23] and is located in the southern region of Malawi. Malaria transmission is perennial peaking during the rainy season (November–March). Plasmodium falciparum causes over 90% of all malaria infections. The two health centers have maternity beds for delivery of low risk pregnancies, a malaria microscopy laboratory with a trained medical technologist, and four full-time and nine part-time nurses. There are 18 traditional birth attendants (TBAs) who attend births in the community. Women with high risk pregnancies are referred to a tertiary hospital located approximately 10 km away for management.
2.2. Study population and recruitment
All pregnant women attending routine antenatal care at the two health facilities were invited to participate in the study. A total of 2462 women were recruited between April 2002 and July 2003. Signed or witnessed verbal consent was sought for participation in the study before enrollment. A standardized questionnaire was used to obtain information on demographics, past obstetric history, malaria symptoms, and factors associated with malaria prevention measures such as use of insecticide-treated bed nets (ITNs) and antimalarial drugs in pregnancy. Study participants were given study numbers to retain until delivery for easy identification.
At enrollment, women received routine antenatal assessment, which included height, weight, temperature and blood pressure measurements, and abdominal examination. A sample of venous blood was collected for estimation of hemoglobin concentration and preparation of thick blood films. The women were given a dose of sulfadoxine-pyrimethamine (SP) as IPTp under direct observation by the antenatal clinic personnel. Antenatal records were checked to ensure that SP was not given at intervals of less than 1 month.
2.3. Follow-up
The follow-up visits were scheduled according to standard antenatal routine care. Depending on the gestation age at enrollment, women were seen at approximately the following gestational ages: 12, 26, 32 and 36–38 weeks, and at delivery. At these visits, women received routine antenatal care and a standardized interview was conducted to obtain information on malarial illnesses and use of antimalarial drugs between visits. A peripheral blood sample was obtained for preparation of thick films, and the women were given a second dose of SP according to the Malawi national policy schedule. The women were encouraged to deliver at the health centers.
2.4. Delivery
Blood samples were obtained soon after delivery for measurement of maternal hemoglobin concentration and examination of peripheral, placental and cord thick films. Newborns delivered at the health centers were weighed within 24 h of delivery using a digital scale. A system was set up such that community nurses and TBAs were able to identify study participants who delivered at home. The TBAs were trained to prepare peripheral, placental and cord thick films from these women, and to forward them to the clinics through community health workers. Newborns delivered in the community were weighed using color-coded scales that indicated green if the newborn weighed 2500 g or more and red if the newborn weighed less than 2500 g.
2.5. Laboratory methods
Malaria diagnosis was performed using microscopy of Giemsa-stained thick blood films. Parasite densities were estimated using an assumed leukocyte count of 6000 leukocytes/μl of blood. A thick film was considered negative if no parasites were detected after examining 100 microscopic fields each containing approximately 20 white blood cells. All thick films were read by two skilled microscopists and any discrepancies (positive vs. negative or more than 25% difference in parasite density) were resolved by repeating the readings. Hemoglobin concentration was determined using a HemoCue machine (HemoCue Incorporated, Angelholm, Sweden).
2.6. Outcome definitions
Newborns were classified as having normal birth weight (≥2500 g) or LBW (<2500 g), regardless of gestational age. Anemia was defined as maternal hemoglobin concentration <11 g/dl [24]. Anemia was further classified into mild anemia (9–10.9 g/dl), moderate anemia (7–8.9 g/dl) and severe anemia (<7 g/dl).
2.7. Statistical analysis
Data analysis was performed using STATA version 9.0 software (Stata Corp., College Station, TX, USA). Comparison of baseline demographic characteristics was performed using the χ2 test for categorical data, t-test for normally distributed and Mann-Whitney test for non-normally distributed continuous variables. The frequency of malaria infection was classified according to the number of episodes detected during the study clinic visits during pregnancy. The timing of malaria infection was defined as: (1) parasitemia in the second trimester (gestation age 13–26 weeks), and (2) parasitemia in the third trimester or at delivery (gestation age >26 weeks to term). Only nine women presented for first trimester antenatal care, and these women were therefore excluded from the analysis.
Multivariate analyses were performed using a binomial regression model to estimate the association between timing and frequency of P. falciparum infection during pregnancy and LBW or maternal anemia. The analysis was stratified by the total number of visits during follow-up. Effect measure modification was assessed by including an interaction term and using the log-likelihood test at an α-level of 0.10. Confounding was assessed using the change in estimate criteria. Variables were considered confounders if there was an absolute change in estimate of 0.10. A backward elimination strategy was used to identify the best model. Covariates that were considered as potential confounders included maternal age, body mass index (BMI), gravidity and use of ITNs. All P-values are two-sided, and CIs were calculated at the 95% level. Statistical significance was set at P ≤ 0.05.
3. Results
3.1. Study population
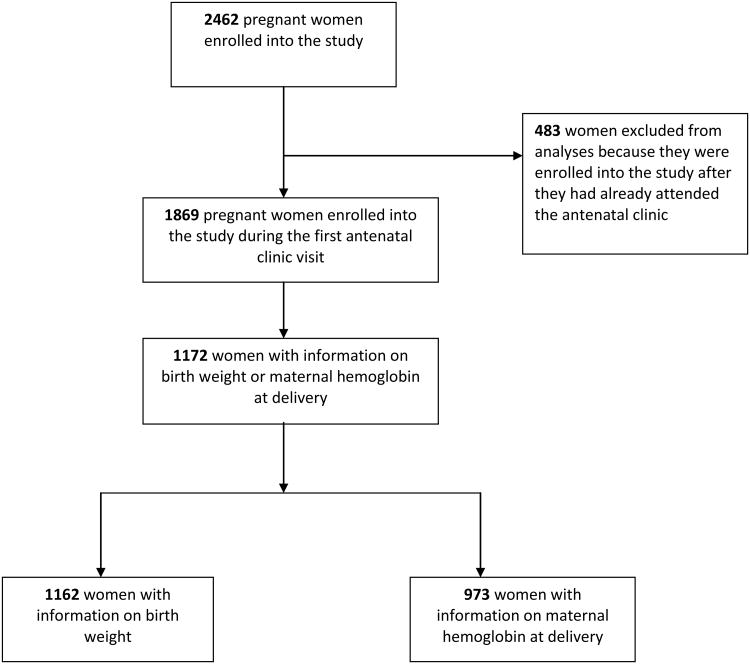
Of the 2462 women enrolled into the study, birth weight was recorded for 1548 newborns and information on hemoglobin concentration at delivery was available for 1253 women. Data was missing for women who had moved or delivered outside the study area, or failed to return for follow-up and could not be found by the study team. Women who are included in this analysis and those lost to follow-up are compared in Supplementary Table 1. Only women who were enrolled into the study at their first antenatal visit were included in this analysis; 1172 women with information on birth weight (n = 1162) and/or hemoglobin concentration at delivery (n = 973) (Figure 1). The characteristics of these women are shown in Table 1. The mean maternal age was 23.8 (SD 5.6) years, the mean gestation age at enrollment was 23.9 (SD 3.7) weeks, 15.3% of the women reported owning ITNs and the total number of visits per woman were: two [38.4%, (n = 451)], three [52.1% (n = 610)] and four [9.5% (n = 111)].
Figure 1.
The flow of study participants from enrollment to delivery.
Table 1. Baseline characteristics of the women enrolled into the study.
| Baseline characteristic | All women (n = 1172) | Malaria positivea (n = 372) | Malaria negativeb (n = 800) | P-value |
|---|---|---|---|---|
| Age (years) | 23.8 ± 5.6 | 22.3 ± 5.2 | 24.6 ± 5.7 | <0.01 |
| Gravidity | ||||
| Primigravidae | 306 (26.3) | 172 (46.7) | 134 (16.8) | <0.01 |
| Multigravidae | 858 (73.7) | 196 (53.3) | 662 (83.2) | |
| Bed net usage | 176 (15.3) | 46 (12.5) | 130 (16.7) | NS |
| Gestation age (weeks) | 23.9 ± 3.7 | 23.2 ± 3.8 | 24.2 ± 3.7 | <0.01 |
| Body mass index (kg/m2) | 21.6 ± 2.4 | 21.3 ± 2.4 | 21.7 ± 2.4 | 0.02 |
| Hemoglobin (g/dl) | 10.2 ± 1.4 | 9.9 ± 1.4 | 10.3 ± 1.3 | <0.01 |
| Maternal anemiac | 790 (67.4) | 266 (71.9) | 524 (65.8) | 0.04 |
| Routine SP treatment ≥2 doses | 720 (61.4) | 263 (70.7) | 457 (57.1) | <0.01 |
| Total number of visits | ||||
| 2 | 451 (38.4) | 109 (29.3) | 342 (42.8) | <0.01 |
| 3 | 610 (52.1) | 209 (56.2) | 401 (50.1) | |
| 4 | 111 (9.5) | 54 (14.5) | 57 (7.1) |
Data are mean ± SD or number (%); SP: sulfadoxine-pyrimethamine; NS: not significant.
Women who had at least one episode of P. falciparum infection detected during pregnancy or at delivery
Women who had no P. falciparum infection detected during pregnancy or at delivery
Anemia was defined as a hemoglobin concentration <11 g/dl.
3.2. Prevalence of malaria
The overall prevalence of parasitemia at enrollment was 20.1% (n = 235) and significantly higher in primigravidae than multigravidae [41.2% vs. 12.2%; prevalence ratio (PR) 3.36; 95% CI 2.69–4.21]. The cumulative prevalence of parasitemia throughout pregnancy was also higher in primigravidae than multigravidae (56.2% vs. 22.8%; PR 2.46; 95% CI 2.10–2.88). More women had parasitemia during the antenatal visits than at delivery irrespective of gravidity [19.1% (n = 222) vs. 7.2% (n = 84); P < 0.001], and 5.3% (n = 62) of the women had parasitemia both in the antenatal period and at delivery (Supplementary Table 2).
3.3. Risk factors for low birth weight
The mean birth weight was 3018.2 g (SD 482.3 g), and the prevalence of LBW was 9.3% (n = 108). Gravidity, maternal age, use of ITNs and the presence of maternal anemia at delivery were significantly associated with LBW in bivariate analyses. Women had an increased risk of delivering a LBW baby if they were primigravidae (16.5% vs. 6.8%; PR 2.42; 95% CI 1.70–3.45), were less than 20 years old (14.8% vs. 7.5%; PR 1.99; 95% CI 1.38–2.86), had anemia at delivery (12.8% vs. 7.9%; PR 1.61; 95% CI 1.06–2.46) and if they were not using ITNs (10.1% vs. 5.2%; PR 1.96; 95% 1.01–3.80). After adjustment for other baseline characteristics, only gravidity was still significantly associated with the risk of LBW; the risk was higher among primigravidae than multigravidae (PR 2.78; 95% CI 1.60–4.79).
3.4. Risk factors for maternal anemia
The prevalence of mild, moderate and severe anemia at enrollment was 52.8% (n = 616), 13.6% (n = 159) and 1.3% (n = 15), respectively. The prevalence of mild, moderate and severe anemia at delivery was 19.4% (n = 189), 3.1% (n = 30) and 0.1% (n = 1) respectively. Although not statistically significant, in bivariate analyses, the risk of anemia at delivery was higher in primigravidae than multigravidae (25.9% vs. 21.3%; PR 1.17; 95% CI 0.91–1.49), women less than 20 years old (25.5% vs. 21.8%; PR 1.20; 95% CI 0.94–1.52), women who had malaria during pregnancy (26.0% vs. 21.0%; PR 1.20; 95% CI 0.98–1.47) and in women who had their first antenatal clinic visit in the third trimester vs. the second trimester of pregnancy (30.9% vs. 22.1%; PR 1.40; 95% CI 0.93–2.12). The risk of maternal anemia at delivery was lower in women who received two doses (18.2% vs. 28.2%; PR 0.64; 95% CI 0.50–0.82) or three doses (25.8% vs. 28.2%; PR 0.92; 95% CI 0.63–1.34) compared with one dose of SP as IPTp. None of the variables were statistically significantly associated with anemia at delivery after adjustment for other baseline characteristics.
3.5. The effect of frequency of Plasmodium falciparum infection on low birth weight and maternal anemia
Compared with women who had no malaria, the prevalence of LBW increased with the number of episodes detected during pregnancy [one episode (13.0% vs. 7.0%; PR 1.62; 95% CI 1.07–2.46) and two episodes (21.4% vs. 7.0%; PR 2.41; 95% CI 1.39–4.18)], adjusting for maternal age, gravidity, BMI, total number of visits and use of ITNs. This effect was seen both in primigravidae and multigravidae.
The risk of maternal anemia at delivery also increased with the number of episodes of infection detected during pregnancy [one episode (23.4% vs. 21.2%; PR 1.15; 95% CI 0.86–1.54) and two episodes (36.4% vs. 21.2%; PR 1.82; 95% CI 1.28–2.62)], after adjusting for maternal age, gravidity, BMI, total number of visits and the use of ITNs. Additionally, the risk of maternal anemia at delivery was significantly higher in women who had two or more episodes of malaria detected compared with women who had one detected episode of malaria (PR 1.58; 95% CI 1.08–2.33) (Table 2).
Table 2. The effect of frequency of Plasmodium falciparum infection during pregnancy on low birth weight and maternal anemia.
| N (%) | Crude prevalence ratio (95% CI) | Adjusted prevalence ratiob (95% CI) | |
|---|---|---|---|
| Low birth weight | |||
| No malaria | 52 (7.0) | 1.00 | 1.00 |
| One episode detecteda | 36 (13.0) | 1.86 (1.25–2.79) | 1.62 (1.07–2.46) |
| Two or more episodes detecteda | 15 (21.4) | 3.07 (1.83–5.17) | 2.41 (1.39–4.18) |
| Two or more vs. one episode detected* | 1.65 (0.96–2.84) | 1.49 (0.87–2.54) | |
| Maternal anemia | |||
| No malaria | 132 (21.2) | 1.00 | 1.00 |
| One episode detecteda | 53 (23.4) | 1.10 (0.83–1.46) | 1.15 (0.86–1.54) |
| Two or more episodes detecteda | 24 (36.4) | 1.72 (1.21–2.44) | 1.82 (1.28–2.62) |
| Two or more vs. one episode detectedc | 1.56 (1.05–2.32) | 1.58 (1.08–2.33) |
N: number of children with low birth weight or women with anemia (hemoglobin <11 g/dl).
Comparing women with either one or two or more episodes of malaria with women who had no malaria (referent group) during pregnancy.
Adjusted for mother's age, gravidity, number of visits, body mass index and insecticide-treated bed net use.
Comparing women who had two vs. one (referent group) episode of detected malaria.
3.6. The effect of timing of malaria infection on low birth weight and maternal anemia
The risk of LBW also varied with the timing of the infection during pregnancy. Compared with women who had no infection during pregnancy, the risk of LBW was higher in women who had infection detected in the second trimester only (PR 1.71; 95% CI 1.06–2.74) than in women who had infection detected during the third trimester or at delivery (PR 1.55; 95% CI 0.88–2.75).
There was no significant difference in the risk of maternal anemia at delivery in women who had parasitemia detected in the second trimester only (PR 1.19; 95% CI 0.83–1.71) or third trimester only (PR 1.12; 95% CI 0.75–1.66) compared with women who no parasitemia after adjusting for other baseline characteristics (Table 3).
Table 3. The effect of timing of Plasmodium falciparum infection during pregnancy on low birth weight and maternal anemia.
| Time of malaria infection | N (%) | Crude prevalence ratio (95% CI) | Adjusted prevalence ratioa (95% CI) |
|---|---|---|---|
| Low birth weight | |||
| No malaria | 52 (7.0) | 1.00 | 1.00 |
| Second trimester onlyb | 24 (15.6) | 2.24 (1.06–2.74) | 1.71 (1.06–2.74) |
| Third trimester or delivery only c | 13 (10.6) | 1.52 (0.85–2.70) | 1.55 (0.88–2.75) |
| Third trimester or delivery vs. second trimesterd | 0.68 (0.36–1.28) | 0.91 (0.49–1.69) | |
| Maternal anemia | |||
| No malaria | 132 (21.2) | 1.00 | 1.00 |
| Second trimester onlyb | 29 (24.2) | 1.14 (0.80–1.62) | 1.19 (0.83–1.71) |
| Third trimester or delivery only c | 25 (22.2) | 1.04 (0.71–1.54) | 1.12 (0.75–1.66) |
| Third trimester or delivery vs. second trimesterd | 0.91 (0.57–1.48) | 0.93 (0.58–1.51) |
Adjusted for maternal age, gravidity, insecticide-treated bed net use, body mass index and number of visits.
Comparing women who had malaria in the second trimester (13–26 weeks gestation age) to women who had no malaria during pregnancy.
Comparing women who had malaria during the third trimester (>26 weeks to term) to women who had no malaria during pregnancy.
Comparing women who had malaria in the third trimester or at delivery to women who had malaria in the second trimester (referent group).
4. Discussion
In a large sample of pregnant women in Malawi, we found that the risk of LBW and maternal anemia increased with the number of episodes of infection detected during pregnancy, irrespective of gravidity. Timing of infection also affected the risk of LBW. Second trimester infection increased the risk of LBW more than infection detected in third trimester or at delivery.
Plasmodium falciparum infection during pregnancy is characterized by the sequestration of infected erythrocytes in the maternal placental vascular space [25]. Studies have shown that this sequestration causes an infiltration of inflammatory cells and elevation of cytokine levels in the infected placenta [9,10,26]. It has been suggested that this inflammatory response reduces the materno-fetal exchange of nutrients across the placenta, leading to growth restriction in the fetus. This might also be exacerbated by depletion of nutrients by the growing parasites or by white cells infiltrating the blood spaces of the placenta, and by mechanical blockage due to thickening of the basement membrane [7–10]. We found that the risk of LBW increased with the number of episodes experienced during pregnancy. This suggests that the damage caused to the placenta by the sequestration of malaria parasites is cumulative.
Timing of infection during pregnancy affected the risk of LBW, which was higher in women with infection early in pregnancy than infection detected late in pregnancy. Maximum fetal growth occurs between 20 and 28 week gestation, this would explain why infection in the second trimester increased the risk of LBW more than infection in the third trimester. Other studies have also suggested that timing of infection determines the risk of adverse outcomes. A study conducted in Malawi found that infection early in pregnancy was associated with intrauterine growth retardation while infection late in pregnancy was associated with preterm delivery [22]. Another study conducted in Burkina Faso found that the risk of LBW was higher after 6 months gestation [14]. Other studies have found that women with infections early in pregnancy did not show histological changes seen in infected placentae at delivery [8,27], indicating that there is resolution of histopathological changes after clearance of parasites in the placenta.
We did not find a significant difference in the risk of maternal anemia at delivery between women who had no parasitemia and those with parasitemia detected in the second or third trimester of pregnancy. However, the risk of maternal anemia also increased with the number of episodes of infection detected during pregnancy both in primigravidae and multigravidae. Compared with women who had no infection during pregnancy, the mean hemoglobin concentration was 0.3 g/dl lower in women who had one episode, and 0.6 g/dl lower in women with two or more episodes of malaria infection detected during pregnancy. The increased risk of developing maternal anemia with repeated infections may be particularly relevant in countries like Malawi where there are high rates of co-morbidities including HIV, hookworm infection and nutritional deficiencies [28].
Determining the precise period during pregnancy when malaria infection causes most harm has important public health implications. Most malaria-endemic areas have limited resources and most women do not receive the recommended two doses of SP during pregnancy due to limited access to health facilities, lack of drugs and poor seeking behavior [29,30]. Targeting interventions to the period when malaria causes the most damage can ensure optimal pregnancy outcomes in resource constrained settings.
There were several limitations in our study. We did not obtain information on HIV infection, which is associated with an increased risk of infection with malaria and its complications, and this could potentially have biased our results. Because only nine women presented during their first trimester of pregnancy in our study, consistent with other observations from women in rural areas of Malawi [31], we were unable to investigate the impact of malaria infection during the first trimester of pregnancy. Despite extensive attempts at follow-up including using TBAs to collect samples from home deliveries, loss to follow-up was common and occurred in part because of the highly mobile nature of this peri-urban study population. Women who were lost to follow-up were younger, primigravidae and were less likely to own ITNs. They were therefore more at risk of having malaria and would have potentially increased the effect seen in our cohort.
Future research studies could improve the identification of malaria at delivery by including the examination of placental histology. Recruiting women in the first trimester of pregnancy could aid our understanding of the impact of malaria during embryogenesis. Fetal growth monitoring using more sensitive methods such as obstetric ultrasound would provide more information on when malaria causes LBW during pregnancy.
In conclusion, the frequency of malaria infection during pregnancy has an effect on the risk of LBW and maternal anemia. The risk of maternal anemia and LBW increased with the number of episodes of malaria experienced during pregnancy. Additionally, the timing of infection also had an effect on the risk of LBW, increasing when infection occurred early in pregnancy during the second trimester. There is a need to conduct more studies to identify the crucial time period during pregnancy when malaria infection causes the greatest impact in order to target interventions to improve the effectiveness of malaria prevention strategies in pregnancy.
Supplementary Material
Acknowledgments
We thank the entire staff of the Mpemba and Madziabango health centers, Mrs Ebby Chaluluka, and all the midwives for co-operation and support in this research project. We are especially grateful to the mothers who volunteered to participate in this study.
Funding: This study was funded by CDC grant CDC/ASPH/ASTDR S1935-21/21. SJR was supported by a Wellcome Trust Senior Research Fellowship. LK was funded by a grant from the Fogarty International Center, 2D43TW006568. The CDC had no role in the design and conduct of the study, data collection, analysis and interpretation of the data, in the preparation, review, or approval of the manuscript.
Footnotes
Authors' contributions: IM, MC, SJR and SRM designed the study protocol; IM, MC, SJR, SRM and LK carried out the clinical assessment and data collection; LK, SJR and SRM carried out the data analysis and interpretation; LK, SJR and SRM drafted and revised the manuscript. All authors contributed to and read and approved the final manuscript. LK, SJR and SRM are guarantors of the paper.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the College of Medicine Research and Ethics Committee in Blantyre, Malawi and the Institutional Review Board of the University of North Carolina, Chapel Hill, NC, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Geneva: World Health Organization; 2005. [accessed 30 December 2008]. World Malaria Report, 2005. WHO/HTM/MAL/2005.1102. http://rbm.who.int/wmr2005/ [Google Scholar]
- 2.Nosten F, Rogerson SJ, Beeson JG, McGready R, Mutabingwa TK, Brabin B. Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol. 2004;20:425–432. doi: 10.1016/j.pt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Luxemburger C, McGready R, Kham A, Morison L, Cho T, Chongsuphajaisiddhi T, et al. Effects of malaria during pregnancy on infant mortality in an area of low malaria transmission. Am J Epidemiol. 2001;154:459–465. doi: 10.1093/aje/154.5.459. [DOI] [PubMed] [Google Scholar]
- 4.McGregor IA. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg. 1984;33:517–525. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 5.Brabin BJ, Romagosa C, Abdelgalil S, Menéndez C, Verhoeff FH, McGready R, et al. The sick placenta-the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Verhoeff FH, Brabin BJ, van Buuren S, Chimsuku L, Kazembe P, Wit JM, et al. An analysis of intra-uterine growth retardation in rural Malawi. Eur J Clin Nutr. 2001;55:682–689. doi: 10.1038/sj.ejcn.1601200. [DOI] [PubMed] [Google Scholar]
- 7.Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55(1 Suppl):33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- 8.Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 9.Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–2530. [PubMed] [Google Scholar]
- 10.Moormann AM, Sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, et al. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis. 1999;180:1987–1993. doi: 10.1086/315135. [DOI] [PubMed] [Google Scholar]
- 11.Omodeo-Sale F, Motti A, Basilico N, Parapini S, Olliaro P, Taramelli D. Accelerated senescence of human erythrocytes cultured with Plasmodium falciparum. Blood. 2003;102:705–711. doi: 10.1182/blood-2002-08-2437. [DOI] [PubMed] [Google Scholar]
- 12.Greve B, Lehman LG, Lell B, Luckner D, Schmidt-Ott R, Kremsner PG. High oxygen radical production is associated with fast parasite clearance in children with Plasmodium falciparum malaria. J Infect Dis. 1999;179:1584–1586. doi: 10.1086/314780. [DOI] [PubMed] [Google Scholar]
- 13.Verhoeff FH, Brabin BJ, Chimsuku L, Kazembe P, Broadhead RL. An analysis of the determinants of anaemia in pregnant women in rural Malawi—a basis for action. Ann Trop Med Parasitol. 1999;93:119–133. doi: 10.1080/00034989958609. [DOI] [PubMed] [Google Scholar]
- 14.Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. Am J Trop Med Hyg. 2007;76:849–54. [PubMed] [Google Scholar]
- 15.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 16.Matteelli A, Donato F, Shein A, Muchi JA, Leopardi O, Astori L, et al. Malaria and anaemia in pregnant women in urban Zanzibar, Tanzania. Ann Trop Med Parasitol. 1994;88:475–483. doi: 10.1080/00034983.1994.11812894. [DOI] [PubMed] [Google Scholar]
- 17.Shulman CE, Graham WJ, Jilo H, Lowe BS, New L, Obiero J, et al. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg. 1996;90:535–539. doi: 10.1016/s0035-9203(96)90312-0. [DOI] [PubMed] [Google Scholar]
- 18.Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, et al. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health. 2001;6:770–778. doi: 10.1046/j.1365-3156.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 19.Matteelli A, Caligaris S, Castelli F, Carosi G. The placenta and malaria. Ann Trop Med Parasitol. 1997;91:803–810. doi: 10.1080/00034989760563. [DOI] [PubMed] [Google Scholar]
- 20.Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 21.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41:1370–1374. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan AD, Nyirenda T, Cullinan T, Taylor T, Harlow SD, James SA, et al. Malaria infection during pregnancy: intrauterine growth retardation and preterm delivery in Malawi. J Infect Dis. 1999;179:1580–1583. doi: 10.1086/314752. [DOI] [PubMed] [Google Scholar]
- 23.National Statistical Office. Malawi population and housing census Summary of final results, volume 1. Zomba, Malawi: National Statistical Office; 1998. [Google Scholar]
- 24.WHO. Prevention and management of severe anaemia in pregnancy. Geneva: World Health Organization; 1993. [Google Scholar]
- 25.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol. 1982;109:330–342. [PMC free article] [PubMed] [Google Scholar]
- 26.Abrams ET, Brown H, Chensue SW, Turner GD, Tadesse E, Lema VM, et al. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J Immunol. 2003;170:2759–2764. doi: 10.4049/jimmunol.170.5.2759. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan AD, Nyirenda T, Cullinan T, Taylor T, Lau A, Meshnick SR. Placental haemozoin and malaria in pregnancy. Placenta. 2000;21:417–421. doi: 10.1053/plac.1999.0479. [DOI] [PubMed] [Google Scholar]
- 28.Ekvall H. Malaria and anemia. Curr Opin Hematol. 2003;10:108–114. doi: 10.1097/00062752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Ashwood-Smith H, Coombs Y, Kaimira N, Bokosi M, Lungu K. Availability and use of sulfadoxine-pyrimethamine (SP) in pregnancy in Blantyre District: a safe motherhood and Blantyre integrated malaria initiative (BIMI) joint survey. Malawi Med J. 2002;14:8–11. [PMC free article] [PubMed] [Google Scholar]
- 30.Rogerson SJ, van den Broek NR, Chaluluka E, Qongwane C, Mhango CG, Molyneux ME. Malaria and anemia in antenatal women in Blantyre, Malawi: a twelve-month survey. Am J Trop Med Hyg. 2000;62:335–340. doi: 10.4269/ajtmh.2000.62.335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.