Abstract
Objective
Examine the effects of obesity and metabolic syndrome on outcome in bipolar disorder.
Method
The Comparative Effectiveness of a Second Generation Antipsychotic Mood Stabilizer and a Classic Mood Stabilizer for Bipolar Disorder (Bipolar CHOICE) study randomized 482 participants with bipolar disorder in a six-month trial comparing lithium- and quetiapine-based treatment. Baseline variables were compared between groups with and without obesity, with and without abdominal obesity, and with and without metabolic syndrome, respectively. The effects of baseline obesity, abdominal obesity, and metabolic syndrome on outcomes were examined using mixed effects linear regression models.
Results
At baseline, 44.4% of participants had obesity, 48.0% had abdominal obesity, and 27.3% had metabolic syndrome; neither obesity, nor abdominal obesity, nor metabolic syndrome were associated with increased global severity, mood symptoms, or suicidality, or with poorer functioning or life satisfaction. Treatment groups did not differ on prevalence of obesity, abdominal obesity, or metabolic syndrome. By contrast, among the entire cohort, obesity was associated with less global improvement and less improvement in total mood and depressive symptoms, suicidality, functioning, and life satisfaction after six months of treatment. Abdominal obesity was associated with similar findings. Metabolic syndrome had no effect on outcome.
Conclusion
Obesity and abdominal obesity, but not metabolic syndrome, were associated with less improvement after six months of lithium- or quetiapine-based treatment.
Keywords: Bipolar Disorder, Obesity, Metabolic Syndrome, Outcome
Introduction
Bipolar disorder is associated with increased rates of obesity, including abdominal obesity (1–3), as well as metabolic syndrome (4, 5), of which abdominal obesity is the most common component (6). Indeed, obesity may predict a bipolar diagnosis among patients with major depressive episodes (7), and obesity-related medical conditions (e.g., circulatory disorders) contribute to the premature mortality of people with bipolar disorder (8). Moreover, many, though not all (9–11), studies suggest obesity in bipolar disorder is associated with greater psychiatric illness burden (1–3, 12–20). However, most of this work has been cross-sectional, and results of prospective studies exploring the effect of obesity on illness outcome in bipolar disorder are mixed.
In the Maintenance Therapies for Bipolar Disorder protocol (12), obese patients receiving lithium-based maintenance treatment had shorter euthymic periods and more frequent depressive relapses compared to non-obese patients. In the Treatment of Early Age Mania (TEAM) study, obesity was associated with poorer response to risperidone relative to lithium (21). By contrast, in an open-label investigation of ziprasidone, obese patients, compared with non-obese patients, had a lower discontinuation rate and more weight loss (22). Finally, in a longitudinal analysis of individuals with bipolar disorder who completed waves 1 and 2 of the National Epidemiologic Survey on Alcohol and Related Conditions (which were 3 years apart), obese individuals in wave 1 were more likely to have developed major depressive episodes in wave 2, but this finding was explained by baseline demographic variables (11). The two prospective studies that evaluated metabolic syndrome found it had no effects on treatment response (23, 24).
Aims of the study
The aims of the present study were to prospectively assess the effects of two measures of adiposity and of metabolic syndrome on treatment outcome in a large, heterogeneous sample of individuals with bipolar disorder in a comparative effectiveness study. We hypothesized that increased adiposity and presence of metabolic syndrome at baseline would predict poorer six-month outcome.
Material and methods
Procedure
The Comparative Effectiveness of a Second Generation Antipsychotic Mood Stabilizer and a Classic Mood Stabilizer for Bipolar Disorder (Bipolar CHOICE) study (25–27) was a six-month, multi-site, randomized pragmatic trial comparing a classic mood stabilizer, lithium, to an antipsychotic commonly used to treat bipolar disorder, quetiapine. Study rationale, design, methods, and results have been reported previously (25–27). Study physicians were able to prescribe additional medications as needed as long as it was consistent with guidelines used to treat bipolar disorder and personalized to the clinical needs of the patient (adjunctive personalized therapy [APT]) (28). The present study examined the effect of baseline anthropometric measures and presence of metabolic syndrome on outcome measures of global illness severity, mood symptoms, suicidality, functioning, and quality of life after six months of prospective treatment.
Participants
The Bipolar CHOICE study enrolled 482 individuals across 11 sites aged 18–68 years between 2011 and 2012 (26, 27). Participants were required to have a DSM-IV TR diagnosis of bipolar I or II disorder (determined using an electronic version of the Extended Mini-International Neuropsychiatric Interview [MINI]) (29) and to be at least mildly symptomatic (defined as a Clinical Global Impression Severity Scale for Bipolar Disorder [CGI-BP Severity] ≥3) (30).The MINI was also used to assess comorbid psychiatric pathology (e.g., anxiety disorders and binge eating). Exclusion criteria were limited to maximize generalizabilty, but included history of nonresponse or intolerable side effects with lithium or quetiapine, and substance dependence within the past 30 days. Participants were randomized to lithium plus APT or quetiapine plus APT. The Institutional Review Boards of the 11 study sites approved the study protocol. All participants provided written informed consent before completion of any study procedures.
Global illness severity was assessed with the CGI-BP Severity (30), and mood symptom severity and suicidality were assessed with the Bipolar Inventory of Symptoms Scale (BISS) (31, 32). Functional impairment was measured with the LIFE-Range of Impaired Functioning Tool (LIFE-RIFT) (33). Life satisfaction was assessed with the self-report Quality-of-Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) (34).
Weight, height, waist circumference, vital signs, and fasting lipid and glucose levels were obtained at baseline. Adiposity definitions were based on those recommended by the National Institute of Health, the National Heart, Lung, and Blood Institute, and The Obesity Society (35, 36). Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2 and abdominal obesity was defined a waist circumference ≥ 102 cm for men or ≥88 cm for women. Presence of metabolic syndrome was assessed using National Cholesterol Education Program (NCEP) criteria (37). Baseline psychotropic medications were recorded; those defined as causing weight gain were amitriptyline, aripiprazole, clomipramine, clozapine, doxepin, imipramine, lithium, mirtazapine, olanzapine, quetiapine, risperidone, and valproate (27).
Statistical Analyses
We calculated summary statistics of baseline demographic and clinical variables including adiposity measures and metabolic syndrome. We used chi-square tests to confirm that prevalence of obesity, abdominal obesity, and metabolic syndrome were not different between the two randomized treatment groups; similarly, we used a t-test to determine whether mean BMI differed between treatment groups. To examine baseline demographic and clinical differences between patients with and without obesity, patients with and without abdominal obesity, and patients with and without metabolic syndrome, we used t-tests for continuous variables and chi-square tests for categorical variables.
To examine whether baseline obesity predicted worse outcomes over the study, we employed mixed effects linear regression models with random intercepts and slopes and fixed effects for baseline obesity status, time, study site, and the obesity-by-time interaction. First, we tested whether the effect of baseline obesity on outcome was different between randomized treatment groups. If the difference was non-significant, we pooled the two treatment groups and examined the overall effect of obesity on outcome. For those outcomes measured at each study visit, we used the natural logarithm of time (log-time), since this fit the data much better. Using these models, we calculated the difference in 6-month change of each outcome between obesity groups. Similar mixed effects models were fit to examine the effects of baseline abdominal obesity, baseline metabolic syndrome, and baseline BMI on the various outcomes.
Because of the potential for residual confounding, statistically significant results and clinically relevant variables were subjected to additional analyses that compared treatment outcomes in obese versus non-obese patients. We applied stabilized inverse probability weighting (sIPW) to the baseline cohort to estimate the conditional probability of being obese (38). In other words, each observation was weighted by the inverse of the probability of being obese conditional on potential confounding factors. Stabilized weights were then calculated by using the marginal probability of being obese as the numerator, and the conditional probability as the denominator. We considered age, race, gender, current anxiety disorder, marital status, history of binge eating, and currently receiving any weight-gain medications as potential confounders. Summary statistics for weights and probabilities were calculated and adequate model fit was confirmed with the Hosmer and Lemeshow test.
Analyses were conducted using SAS 9.2 statistical software (SAS Institute, Inc., 1994) and figures were created using R 3.0.1 (www.r-project.org). A two-tailed significance threshold of P < 0.05 was used with no correction for multiple comparisons.
Results
At baseline, the mean (SD) BMI was 29.8 (7.5); 44.4% of participants had obesity, 48.0% had abdominal obesity, and 27.3% had metabolic syndrome. Selected demographic and clinical features of the total sample are reported in Table 1. Obese patients and non-obese patients did not differ regarding baseline measures of global illness severity, mood symptoms, suicidality, functioning, or quality of life (Table 1). Obesity, however, was associated with female gender, older age, race, marital status, and history of binge eating (all P’s < .05) (Table 1). Similar findings were seen in patients with and without abdominal obesity. There were no differences in prevalence of obesity, abdominal obesity, and metabolic syndrome, or in mean BMI between the two treatment groups (all P’s > .05, data not shown). Overall, patients receiving lithium-based treatment and those receiving quetiapine-based treatment displayed similar improvement on all outcome variables (26).
Table 1.
Selected baseline demographic and clinical variables among CHOICE participants by obesity status
| Variable | Overall(N=482) | Yes Obesity(N=212) | No Obesity(N=270) | Pa |
|---|---|---|---|---|
|
| ||||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age (mean ± SD years) | 39 ± 12 | 41 ± 11 | 37 ± 13 | <0.001 |
| CGI-BP Severity | 4.5 ± 0.9 | 4.4 ± 0.8 | 4.6 ± 0.9 | 0.061 |
| BISS total | 56.1 ± 18.8 | 55.8 ± 19.7 | 56.4 ± 18.2 | 0.736 |
| BISS depression | 37.6 ± 14.1 | 37.4 ± 14.1 | 37.7 ± 14.1 | 0.792 |
| LIFE-RIFT total | 14.2 ± 3.4 | 14.3 ± 3.3 | 14.2 ± 3.4 | 0.856 |
| Q-LES-Q overall | 41.6 ± 24.5 | 42.6 ± 24.5 | 40.8 ± 24.6 | 0.429 |
|
| ||||
| % | % | % | ||
|
| ||||
| Female gender | 58.8 | 64.6 | 54.1 | 0.021 |
| Race | 0.025 | |||
| White | 72.2 | 71.7 | 72.6 | |
| Black | 19.9 | 23.6 | 17.0 | |
| Other | 7.9 | 4.7 | 10.4 | |
| Married/Living as Married | 31.1 | 37.7 | 25.9 | 0.005 |
| Any Anxiety Disorder | 58.2 | 61.8 | 54.9 | 0.132 |
| Binge Eating | 25.6 | 30.6 | 21.6 | 0.026 |
| Weight Gain Medicationb | 21.8 | 24.1 | 20.0 | 0.284 |
P is based on either t-test (continuous) or chi-square test (categorical) between obese and non-obese groups.
Weight-gain medication = amitriptyline, aripiprazole, clomipramine, clozapine, doxepin, imipramine, lithium, mirtazepine, olanzapine, quetiapine, risperidone, and valproate.
Key: BISS = Bipolar Inventory of Symptoms Scale; CGI-BP Severity = Clinical Global Impression Severity Scale for Bipolar Disorder; CI = confidence interval; LIFE-RIFT = Life-Range of Impaired Functioning Tool; Q-LES-Q = Quality-of-Life Enjoyment and Satisfaction Questionnaire
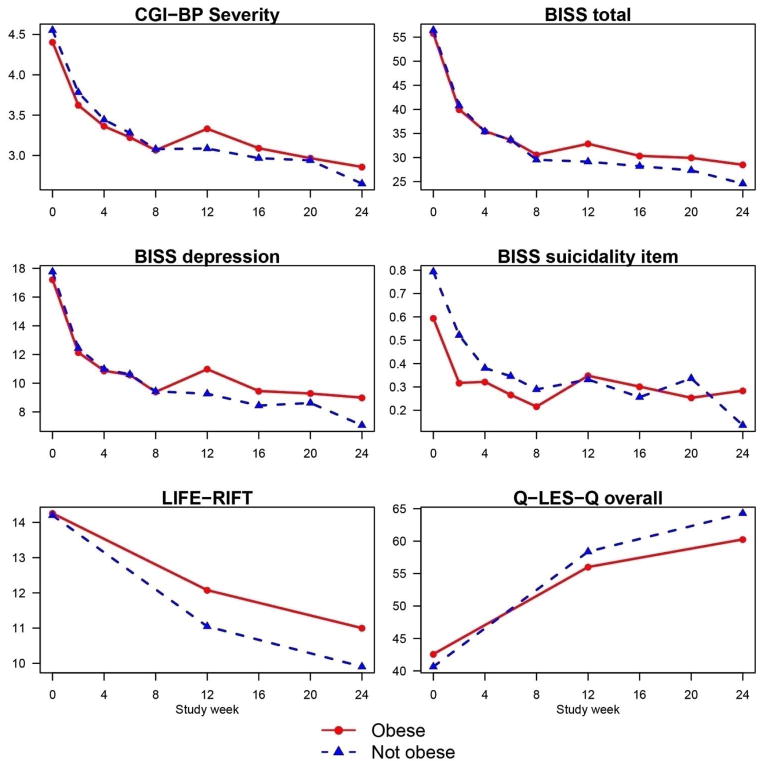
The effects of baseline adiposity on outcome were not different between randomized treatment groups (all P’s > 0.05, data not shown), but adiposity measures moderated outcome (Tables 2–4). Compared to non-obese patients, obese patients showed significantly less improvement in global illness severity, depressive and total mood symptomatology, suicidality, functioning, and overall life satisfaction regardless of treatment assignment (Table 2). Moreover, the difference in improvement between obese and non-obese patients was most apparent in the last three months of the trial (Figure 1). Similarly, compared to participants without abdominal obesity, participants with abdominal obesity improved less on measures of overall severity, depression, suicidality, functioning, and life satisfaction (Table 3). In addition, increased baseline BMI predicted significantly less improvement in global illness severity, suicidality, and overall life satisfaction (Table 4). By contrast, metabolic syndrome had no effect on outcome (data not shown).
Table 2.
Effect of baseline obesity status on change in clinical outcomes at six months
| Variable | Baseline | Estimated change from baseline | Estimated difference in 6-month change | Estimated difference in 6-month change(by sIPW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Overall | No obesity | Yes obesity | Overall | No obesity | Yes obesity | Yes – No | Yes – No | |||
|
| ||||||||||
| Mean ± SD (N) | Mean ± SD (N) | Mean ± SD (N) | Mean [95%CI] | Mean [95%CI] | Mean [95%CI] | Mean [95%CI] | P | Mean [95%CI] | P | |
| CGI-BP Severity | 4.5 ± 0.9 (478) | 4.6 ± 0.9 (266) | 4.4 ± 0.8 (212) | −1.61 [−1.73, −1.50] | −1.70 [−1.85, −1.55] | −1.36 [−1.53, −1.18] | 0.34 [0.12, 0.57] | 0.003 | 0.35 [0.08, 0.61] | 0.010 |
| BISS total | 56.1 ± 18.8 (478) | 56.4 ± 18.1 (266) | 55.8 ± 19.7 (212) | −28.39 [−30.18, −26.59] | −29.79 [−32.16, 27.41] | −25.55 [−28.26, −22.84] | 4.24 [0.68, 7.80] | 0.020 | 5.67[1.52, 9.82] | 0.007 |
| BISS depression | 37.6 ± 14.1 (478) | 37.7 ± 14.1 (266) | 37.4 ± 14.1 (212) | −18.59 [−19.99, −17.20] | −20.08 [−21.93, 18.23] | −16.28 [−18.39, −14.18] | 3.80 [1.03, 6.56] | 0.007 | 4.66[1.45, 7.87] | 0.005 |
| BISS suicidality | 0.7 ± 1.0 (478) | 0.8 ± 1.1 (266) | 0.6 ± 0.9 (212) | −0.41 [−0.50, −0.32] | −0.55 [−0.66, 0.43] | −0.27 [−0.41, −0.14] | 0.27 [0.10, 0.45] | 0.002 | 0.30[0.12, 0.48] | 0.001 |
| LIFE-RIFT total | 14.2 ± 3.4 (472) | 14.2 ± 3.4 (263) | 14.3 ± 3.3 (209) | −3.62 [−4.02, −3.22] | −4.10 [−4.65, −3.55] | −2.97 [−3.60, −2.35] | 1.13 [0.31, 1.95] | 0.007 | 1.11 [0.17, 2.04] | 0.021 |
| Q-LES-Q overall | 41.5 ± 24.6 (471) | 40.6 ± 24.7 (262) | 42.6 ± 24.5 (209) | 20.09 [17.33, 22.85] | 22.96 [19.15, 26.76] | 16.95 [12.64, 21.26] | −6.01 [−11.68, −0.34] | 0.038 | −6.18[−12.44, 0.08] | 0.053 |
Key: BISS = Bipolar Inventory of Symptoms Scale; CGI-BP Severity = Clinical Global Impression Severity Scale for Bipolar Disorder; CI = confidence interval; LIFE-RIFT = Life-Range of Impaired Functioning Tool; Q-LES-Q = Quality-of-Life Enjoyment and Satisfaction Questionnaire; sIPW = stabilized inverse probability weighting
Table 4.
Effect of baseline BMI on changes in clinical outcomes at six months
| Estimated 6-month difference per 1 unit increase in baseline | |||
|---|---|---|---|
|
| |||
| Variable | Baseline | BMI | |
| Mean ± SD (N) | Mean [95%CI] | P | |
| CGI-BP Severity | 4.5 ± 0.9 (482) | 0.02 [0.00, 0.03] | 0.018 |
| BISS total | 56.1 ± 18.8 (482) | 0.16 [−0.08, 0.40] | 0.201 |
| BISS depression | 37.6 ± 14.0 (482) | 0.16 [−0.03, 0.34] | 0.097 |
| BISS suicidality | 0.7 ± 1.0 (482) | 0.02 [0.01, 0.03] | 0.002 |
| LIFE-RIFT total | 14.2 ± 3.4 (476) | 0.05 [−0.01, 0.10] | 0.102 |
| Q-LES-Q overall | 41.6 ± 24.5 (475) | −0.45 [−0.84, −0.07] | 0.022 |
Key: BISS = Bipolar Inventory of Symptoms Scale; CGI-BP Severity = Clinical Global Impression Severity Scale for Bipolar Disorder; CI = confidence interval; LIFE-RIFT = Life-Range of Impaired Functioning Tool; Q-LES-Q = Quality-of-Life Enjoyment and Satisfaction Questionnaire
Figure 1.
Means of selected clinical outcomes by study week and baseline obesity status
Based on mixed effects regression models, all differences in slopes between obese and non-obese patients were significantly different from zero (all P’s < 0.05 for outcomes in figures above)
Key: BISS = Bipolar Inventory of Symptoms Scale; CGI-BP Severity = Clinical Global Impression Severity Scale for Bipolar Disorder; CI = confidence interval; LIFE-RIFT = Life-Range of Impaired Functioning Tool; Q-LES-Q = Quality-of-Life Enjoyment and Satisfaction Questionnaire
Table 3.
Effect of baseline abdominal obesity status on changes in clinical outcomes at six months
| Variable | Baseline | Estimated change from baseline | Estimated difference in 6-month change | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Overall | No abdominal obesity | Yes abdominal obesity | Overall | No abdominal obesity | Yes abdominal obesity | Yes – No | ||
|
| ||||||||
| Mean ± SD (N) | Mean ± SD (N) | Mean ± SD (N) | Mean[95%CI] | Mean [95%CI] | Mean [95%CI] | Mean [95%CI] | P | |
| CGI-BP Severity | 4.5 ± 0.9 (469) | 4.5 ± 0.9 (244) | 4.5 ± 0.8 (225) | −1.61 [−1.73, −1.50] | −1.72 [−1.88, −1.56] | −1.41 [−1.57, −1.24] | 0.31 [0.08, 0.55] | 0.008 |
| BISS total | 56.0 ± 18.7 (469) | 55.8 ± 17.8 (244) | 56.2 ± 19.7 (225) | −28.39 [−30.18, −26.59] | −29.44 [−31.96, −26.93] | −26.68 [−29.25, −24.12] | 2.76 [−0.82, 6.34] | 0.131 |
| BISS depression | 37.5 ± 14.1 (469) | 37.4 ± 13.9 (244) | 37.6 ± 14.3 (225) | −18.59 [−19.99, −17.20] | −20.10 [−22.07, −18.12] | −16.86 [−18.87, −14.84] | 3.24 [0.43, 6.05] | 0.024 |
| BISS suicidality | 0.7 ± 1.0 (469) | 0.8 ± 1.0 (244) | 0.6 ± 0.9 (225) | −0.41 [−0.50, −0.32] | −0.53 [−0.66, −0.41] | −0.32 [−0.45, −0.20] | 0.21 [0.04, 0.39] | 0.019 |
| LIFE-RIFT | 14.2 ± 3.4 (463) | 14.1 ± 3.4 (241) | 14.4 ± 3.4 (222) | −3.62 [−4.02, −3.22] | −4.08 [−4.67, −3.49] | −3.17 [−3.77, −2.56] | 0.91 [0.08, 1.75] | 0.032 |
| Q-LES-Q overall | 41.5 ± 24.7 (462) | 40.8 ± 24.7 (240) | 42.2 ± 24.7 (222) | 20.09 [17.33, 22.85] | 24.40 [20.33, 28.47] | 16.65 [12.55, 20.76] | −7.75 [−13.48, −2.02] | 0.008 |
Key: BISS = Bipolar Inventory of Symptoms Scale; CGI-BP Severity = Clinical Global Impression Severity Scale for Bipolar Disorder; CI = confidence interval; LIFE-RIFT = Life-Range of Impaired Functioning Tool; Q-LES-Q = Quality-of-Life Enjoyment and Satisfaction Questionnaire
Similar results were found in our sIPW analysis. Each variable identified in our unweighted analysis remained statistically significant (or marginally for Q-LES-Q) even after weighting by the potential confounders (Table 2). Our weighting models seemed to fit the data well and, as expected, the stabilized weights were centered around 1 (analyses not shown).
Discussion
This study is consistent with previous data showing that bipolar disorder is associated with increased adiposity, and extends these data by showing that both total and abdominal obesity are associated with less improvement on a number of treatment outcomes. Obesity was associated with less global improvement and less improvement in depressive and total mood symptoms, suicidality, functioning, and life satisfaction. These associations persisted after accounting for gender, race, marital status, any current anxiety disorder, binge eating, treatment with lithium or quetiapine, and treatment with medications associated with weight gain. Abdominal obesity was similarly associated with less global improvement and less improvement in depressive symptoms, suicidality, and life satisfaction. With increasing baseline BMI, participants showed less global improvement and less improvement in depressive symptoms, suicidality, functioning, and life satisfaction. By contrast, the presence of metabolic syndrome had no effect on outcome.
That adiposity measures, but not metabolic syndrome, were associated with less improvement in clinical outcomes suggests that elevated adiposity may have a stronger predictive effect on the course of bipolar disorder than the presence of metabolic syndrome. A limitation of this conclusion, however, is that although rates of adiposity were comparably high as in other samples of individuals with bipolar disorder (1, 9, 12, 13, 15–17, 19), the rate of metabolic syndrome was relatively low and similar to that of the general population (4). The low baseline rate of metabolic syndrome may have reduced the power of our analyses to detect outcome effects. Nonetheless, our findings that metabolic syndrome did not moderate treatment outcome are consistent with two earlier studies (23, 24).
It is unclear why obesity is associated with poorer outcome in bipolar disorder. One possibility is that obesity is a marker of severity in bipolar disorder. Although this explanation is not supported by the lack of baseline associations of adiposity measures with illness severity in the present sample, neuroimaging and genetic studies suggest bipolar disorder plus obesity may be an important phenotypic subtype characterized by abnormalities in gray and white matter regions involved in generating and regulating emotion (39–41) or sharing a common genetic risk for type 2 diabetes (e.g., TCF7L2) (3). Another possibility is that obesity is an epiphenomenon of another factor associated with a more severe course of bipolar disorder, such as comorbid anxiety disorder or binge eating (19, 42). However, effects of obesity on outcome persisted after accounting for these variables.
Yet another possibility is that the harmful effects of obesity could worsen the course of bipolar disorder (2). Thus, it has been hypothesized that increased adiposity contributes to the inflammation seen in mood disorders, which in turn has deleterious effects on brain structure and function (2, 43–45). This possibility is supported by findings that increased adiposity is associated with central nervous system inflammation (46, 47), poor cognitive function and development of neurodegenerative disorders (48, 49), disruptions in white matter integrity (50), decreased hippocampal n-acetylaspartate (51), and loss of brain volume over time (52); and with findings that long-term inflammation increases the risk for common mental disorders (53).
Several additional limitations of the current study need to be considered. Neither BMI nor waist circumference are ideal measures of total and abdominal obesity, respectively. BMI does not take age, gender, bone structure, fat distribution, or muscle mass into consideration (54) and waist circumference cannot distinguish subcutaneous fat from abdominal fat (6). Thus, findings may have differed if more direct and precise measures, such as dual-energy X-ray absorptiometry or bioelectrical impedence, were used to assess body fat (55). Additionally, as only 43 participants had abdominal obesity without total obesity, we were unable to determine whether abdominal obesity in the absence of total obesity moderated outcome. Finally, data were collected through a clinical trial, potentially limiting the generalizability of the findings. Although the study sample was heterogeneous given the limited entry criteria, the findings may not extend to individuals with more severe symptomatic presentations or those who are not in treatment.
In sum, in a prospective, comparative effectiveness trial, obesity and abdominal obesity, but not metabolic syndrome, were associated with less improvement in response to lithium or quetiapine-based therapy. Further study of the effects of adiposity on outcome in bipolar disorder is greatly needed.
Significant outcomes.
Forty-four % of participants had obesity and 48% had abdominal obesity. Though not associated with illness severity at baseline, both obesity and abdominal obesity were associated with less symptomatic and functional improvement after 6 months of lithium- or quetiapine- based treatment. The difference in improvement between obese and non-obese patients was most apparent in the last 3 months of treatment.
Metabolic syndrome, defined by National Cholesterol Education Program (NCEP) criteria and present in 27.3% of participants, had no effect on outcome after six months of lithium- or quetiapine- based treatment.
Association of obesity with poorer outcome persisted after other correlates of obesity in bipolar disorder, including binge eating and treatment with medications associated with weight gain, were controlled for.
Limitations.
Lack of placebo-control group.
Given the exploratory nature of the statistical analyses, multiple comparison adjustments were not applied.
Low rate of metabolic syndrome.
Acknowledgments
Funding Sources
This study was funded by AHRQ Grant R01 HS019371-01.
Declaration of interests
Dr. McElroy is a consultant to or member of the scientific advisory boards of Alkermes, Corcept, MedAvante, Naurex, Novo Nordisk, Shire, Sunovian, Takeda, and Teva. She is a principal or co-investigator on studies sponsored by the Agency for Healthcare Research & Quality (AHRQ), Alkermes, AstraZeneca, Cephalon, Eli Lilly and Company, Marriott Foundation, National Institute of Mental Health, Orexigen Therapeutics, Inc., Shire, Takeda Pharmaceutical Company Ltd., and Transcept Pharmaceutical, Inc. She is also an inventor on United States Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and along with the patient’s assignee, University of Cincinnati, Cincinnati, Ohio, has received payments from Johnson & Johnson, which has exclusive rights under the patent.
Dr. Kemp serves on the speakers’ bureau for Pfizer and AstraZeneca, and is a consultant for Bristol-Myers Squibb, Teva, Corcept, and Janssen. His spouse is a minor stockholder for Sanofi and Abbott.
Dr. Friedman receives grant support from Novartis, St. Jude Medical, Medtronics, Repligen, Astra-Zeneca, Roche, and Takeda. He receives royalties from Springer.
Dr. Reilly-Harrington receives royalties from Oxford University Press, the American Psychological Association, and New Harbinger. She serves as a consultant for United Biosource Corporation and was a shareholder in Concordant Rater Systems.
Dr. Sylvia was a shareholder in Concordant Rater Systems and serves as a consultant for United Biosource Corporation and Clintara. She receives royalties from New Harbinger.
Dr. Calabrese receives federal funding from the Department of Defense, Health Resources Services Administration, and NIMH; he receives research funding or grants from the following private industries or nonprofit funds: Cleveland Foundation, NARSAD, and Stanley Medical Research Institute; he receives research grants from Abbott, AstraZeneca, Cephalon, GlaxoSmithKline, Janssen, Eli Lilly, and Lundbeck; he serves on the advisory boards of Abbott, AstraZeneca, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Forest, France Foundation, GlaxoSmithKline, Janssen, NeuroSearch, OrthoMcNeil, Repligen, Schering-Plough, Servier, Solvay/Wyeth, Takeda, and Supernus Pharmaceuticals; and he reports CME activities with AstraZeneca, Bristol-Myers Squibb, France Foundation, GlaxoSmithKline, Janssen, Johnson & Johnson, Schering-Plough, and Solvay/Wyeth.
Mr. Rabideau reports no competing interests.
Dr. Ketter, between May 14, 2010 and May 14, 2013, had the following financial interests/arrangements or affiliations that could be perceived as real or apparent conflicts of interest: Grant/Research Support from the AstraZeneca Pharmaceuticals LP, Cephalon Inc., Eli Lilly and Company, Pfizer Inc., and Sunovion Pharmaceuticals; Consultant Fees from Allergan, Inc., Avanir Pharmaceuticals, Bristol-Myers Squibb Company, Cephalon Inc., Forest Pharmaceuticals, Janssen Pharmaceutica Products, LP, Merck & Co., Inc., Sunovion Pharmaceuticals, Teva Pharmaceuticals; Lecture Honoraria from Abbott Laboratories, Inc., AstraZeneca Pharmaceuticals LP, GlaxoSmithKline, and Otsuka Pharmaceuticals; and Publication Royalties from American Psychiatric Publishing, Inc. In addition, Dr. Ketter’s spouse is an employee of and holds stock in Janssen Pharmaceuticals.
Dr. Thase has been an advisor/consultant: to Alkermes, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck, MedAvante, Merck, Mylan, Neuronetics, Otsuka, Pamlab, PharmaNeuroboost, Pfizer, Rexahn, Roche, Shire, Sunovion, Supernus, Takeda, and Teva, as well as the US Food and Drug Administration and the National Institute of Mental Health. During the same time frame, Dr. Thase has received honoraria for talks from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Merck, and Pfizer and he has received research grants from Alkermes, AstraZeneca, Eli Lilly, Forest, GlaxoSmithKline, Otsuka, PharmaNeuroboost, and Roche, as well as the National Institute of Mental Health and the Agency for Healthcare Research and Quality.
Dr. Singh has been a speaker for Merck and Sunovion. He has received research support from Novartis and Astra Zeneca.
Dr. Tohen was an employee of Lilly (1997 to 2008) and has received honoraria from or consulted for Abbott, Alkermes, AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Lilly, Johnson & Johnson, Otsuka, Merck, Sunovion, Forest, Roche, Elan, Lundbeck, Teva, Pamlab, Wyeth and Wiley Publishing. His spouse was a full time employee at Lilly (1998–2013).
Dr. Bowden is a research collaborator with Elan and a consultant with Teva, He has no participation with speaker bureaus, nor does he or his wife hold any equity position in any biomedical or pharmaceutical corporation.
Ms. Bernstein reports no competing interests.
Dr. Brody has received salary support over the past 3 years from grants funded by Forrest, Agency for Healthcare Quality and Research, and Pritzker neuropsychiatric disorders research consortium.
Dr. Deckersbach has received research support from NIMH, NARSAD, TSA, OCF, Tufts University, NIH, NIA, Janssen Pharmaceuticals, the Forest Research Institute, Shire Development Inc., Medtronic, Cyberonics, Northstar. He has received honoraria, consultation fees and/or royalties from the following: Medacorp, MGH Psychiatry Academy, BrainCells Inc., Systems Research and Applications Corporation, Boston University, Tufts University, the Catalan Agency for Health Technology Assessment and Research, the National Association of Social Workers Massachusetts, Massachusetts Medical Society, and Oxford University Press.
Dr. Kocsis has received research grants and contracts from AHRQ, NIMH, NIDA, Burroughs Wellcome Trust, Pritzker Consortium, Takeda, Forest, Astra Zeneca, Roche. He is on the speaker’s bureau at Pfizer and Merck and on the advisory board at Corcept.
Dr. Kinrys has received research support from Astra-Zeneca, Bristol-Myers Squibb Company, Cephalon, Elan Pharmaceuticals, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, Sanofi/Synthelabo, Sepracor Inc., Pfizer Inc, UCB Pharma, and Wyeth-Ayerst Laboratories. He has been an advisor or consultant for Astra-Zeneca, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, Janssen Pharmaceutica, Pfizer Inc, Sepracor Inc., UCB Pharma, and Wyeth-Ayerst Laboratories. Dr. Kinrys has been a speaker for Astra-Zeneca, Forest Pharmaceuticals Inc., GlaxoSmithkline, Sepracor Inc., and Wyeth-Ayerst Laboratories.
Dr. Bobo reports no competing interests in the past three years.
Dr. Kamali has received research support from Janssen.
Dr. McInnis has received grants for research support from NIMH, the Heinz C Prechter Research Fund, and the Michigan Institute for Clinical Health Research (MICHR). He has received consulting income from the Qatar National Research Foundation and Merck Pharmaceuticals.
Dr. Faraone has received consulting income, travel expenses and/or research support from Ironshore, Shire, Akili Interactive Labs, Alcobra, VAYA Pharma, and SynapDx and research support from the National Institutes of Health (NIH) (over the past year). His institution is seeking a patent for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by: Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health and Oxford University Press: Schizophrenia: The Facts.
Dr. Nierenberg is a consultant for Abbott Laboratories, Astra Zeneca, Basilea, BrainCells Inc., Brandeis University, Bristol-Myers Squibb, Cephalon, Corcept, Eli Lilly & Co., Forest, Genaissance, GlaxoSmithKline, Innapharma, Janssen Pharmaceutica, Jazz Pharmaceuticals, Lundbeck, Merck, Novartis, PamLabs, PGx Health, Pfizer, Ridge Diagnostics, Roche, Sepracor, Schering-Plough, Shire, Somerset, Sunovion, Takeda, Targacept, and Teva. He is a stakeholder in Appliance Computing, Inc. (MindSite); Brain Cells, Inc., InfoMed (potential share of income). He receives research support from AHRQ, Bristol-Myers Squibb, Cederroth, Cyberonics, Elan, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, LichtwerPharma, Eli Lilly, Mylin (formerly Dey Pharmaceuticals), NARSAD, NIMH, Pamlabs, Pfizer, Shire, Stanley Foundation, and Wyeth-Ayerst. Honoraria include MGH Psychiatry Academy in the past 3 years (Prior to 3 years ago, honoraria from Bristol-Myers Squibb, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Eli Lilly, Shire, Wyeth-Ayerst). Dr. Nierenberg receives other income from legal case reviews for CRICO, MBL Publishing for past services as Editor-in-chief of CNS Spectrums, Slack Inc. for services as Associate Editor of Psychiatric Annals, and Editorial Board, Mind Mood Memory, Belvior Publications. He has copyright joint ownership with MGH for Structured Clinical Interview for MADRS and Clinical Positive Affect Scale and additional honoraria from ADURS, American Society for Clinical Psychopharmacology and Zucker Hillside Hospital and Forest and Janssen, Biomedical Development, Boston Center for the Arts, University of Pisa, University of Wisconsin at Madison, University Texas Southwest at Dallas, Health New England and Harold Grinspoon Charitable Foundation and Eli Lilly and AstraZeneca, Brandeis University, International Society for Bipolar Disorder, 2nd East Asian Bipolar Forum, Mid-Atlantic Permanente Research Institute, Up-to-Date.
Dr. Shelton has been a consultant for Bristol-Myers Squibb Company; Cyberonics, Inc.; Eli Lilly and Company; Janssen Pharmaceutica; Medtronic, Inc.; Pamlab, Inc.; Pfizer, Inc.; Ridge Diagnostics; and Takeda Pharmaceuticals. He has grant support from Bristol-Myers Squibb; Eli Lilly and Company; Elan, Corp.; Euthymics Bioscience; Forest Pharmaceuticals; Janssen Pharmaceutica; Naurex, Inc.; Novartis Pharmaceuticals; Otsuka Pharmaceuticals; Pamlab, Inc.; Pfizer, Inc.; Repligen, Corp.; Ridge Diagnostics; St. Jude Medical, Inc.; Takeda Pharmaceuticals.
References
- 1.McElroy SL, Keck PE., Jr Obesity in bipolar disorder: An overview. Curr Psychiatry Rep. 2012;14:650–658. doi: 10.1007/s11920-012-0313-8. [DOI] [PubMed] [Google Scholar]
- 2.Liu CS, Carvalho AF, Mansur RB, McIntyre RS. Obesity and bipolar disorder: synergistic neurotoxic effects? Adv Ther. 2013;30:987–1006. doi: 10.1007/s12325-013-0067-7. [DOI] [PubMed] [Google Scholar]
- 3.Winham SJ, Cuellar-Barboza AB, Oliveros A, et al. Genome-wide association study of bipolar disorder accounting for effect of body mass index identifies a new risk allele in TCF7L2. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.159. [DOI] [PubMed] [Google Scholar]
- 4.Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170:265–274. doi: 10.1176/appi.ajp.2012.12050620. [DOI] [PubMed] [Google Scholar]
- 5.McElroy SL, Keck PE. Metabolic syndrome in bipolar disorder: a review with a focus on bipolar depression. J Clin Psychiatry. 2014;75:46–61. doi: 10.4088/JCP.13r08634. [DOI] [PubMed] [Google Scholar]
- 6.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 7.Vannucchi G, Toni C, Maremmani I, Perugi G. Does obesity predict bipolarity in major depressive patients? J Affect Disord. 2014;155:118–122. doi: 10.1016/j.jad.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Hayes JF, Miles J, Walters K, King M, Osborn DP. A systematic review and meta-analysis of premature mortality in bipolar affective disorder. Acta Psychiatr Scand. 2015;131:417–425. doi: 10.1111/acps.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63:207–213. doi: 10.4088/jcp.v63n0306. [DOI] [PubMed] [Google Scholar]
- 10.Jerrell JM, McIntyre RS, Tripathi A. A cohort study of the prevalence and impact of comorbid medical conditions in pediatric bipolar disorder. J Clin Psychiatry. 2010;71:1518–1525. doi: 10.4088/JCP.09m05585ora. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BI, Liu SM, Schaffer A, Sala R, Blanco C. Obesity and the three-year longitudinal course of bipolar disorder. Bipolar disorders. 2013;15(3):284–293. doi: 10.1111/bdi.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagiolini A, Kupfer DJ, Houck PR, Novick DM, Frank E. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160:112–117. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]
- 13.Fagiolini A, Kupfer DJ, Rucci P, Scott JA, Novick DM, Frank E. Suicide attempts and ideation in patients with bipolar I disorder. J Clin Psychiatry. 2004;65:509–514. doi: 10.4088/jcp.v65n0409. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein BI, Birmaher B, Axelson DA, et al. Preliminary findings regarding overweight and obesity in pediatric bipolar disorder. J Clin Psychiatry. 2008;69:1953–1959. doi: 10.4088/jcp.v69n1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calkin C, van de Velde C, Ruzickova M, et al. Can body mass index help predict outcome in patients with bipolar disorder? Bipolar disorders. 2009;11:650–656. doi: 10.1111/j.1399-5618.2009.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemp DE, Gao K, Chan PK, Ganocy SJ, Findling RL, Calabrese JR. Medical comorbidity in bipolar disorder: relationship between illnesses of the endocrine/metabolic system and treatment outcome. Bipolar disorders. 2010;12:404–413. doi: 10.1111/j.1399-5618.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein BI, Liu SM, Zivkovic N, Schaffer A, Chien LC, Blanco C. The burden of obesity among adults with bipolar disorder in the United States. Bipolar disorders. 2011;13:387–395. doi: 10.1111/j.1399-5618.2011.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yim CY, Soczynska JK, Kennedy SH, Woldeyohannes HO, Brietzke E, McIntyre RS. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. Eur Psychiatry. 2012;27:223–228. doi: 10.1016/j.eurpsy.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 19.McElroy SL, Crow S, Biernacka JM, et al. Clinical phenotype of bipolar disorder with comorbid binge eating disorder. J Affect Disord. 2013;150:981–986. doi: 10.1016/j.jad.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemp DE, Sylvia LG, Calabrese JR, et al. General medical burden in bipolar disorder: findings from the LiTMUS comparative effectiveness trial. Acta Psychiatr Scand. 2014;129:24–34. doi: 10.1111/acps.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitiello B, Riddle MA, Yenokyan G, et al. Treatment moderators and predictors of outcome in the Treatment of Early Age Mania (TEAM) study. J Am Acad Child Adolesc Psychiatry. 2012;51:867–878. doi: 10.1016/j.jaac.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller S, Ittasakul P, Wang PW, et al. Enhanced ziprasidone combination therapy effectiveness in obese compared to nonobese patients with bipolar disorder. J Clin Psychopharmacol. 2012;32:814–819. doi: 10.1097/JCP.0b013e318270dea9. [DOI] [PubMed] [Google Scholar]
- 23.Kemp DE, Eudicone JM, McQuade RD, Chambers JS, Baker RA. Metabolic syndrome and its potential effect on treatment response to aripiprazole: a post hoc analysis of the stabilization phase of a long-term, double-blind study in patients with bipolar disorder (CN138-010) J Clin Psychopharmacol. 2010;30:631–634. doi: 10.1097/JCP.0b013e3181f0569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp DE, Karayal ON, Calabrese JR, et al. Ziprasidone with adjunctive mood stabilizer in the maintenance treatment of bipolar I disorder: long-term changes in weight and metabolic profiles. Eur Neuropsychopharmacol. 2012;22:123–131. doi: 10.1016/j.euroneuro.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nierenberg AA, Sylvia LG, Leon AC, et al. Clinical and Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE): A pragmatic trial of complex treatment for a complex disorder. Clinical Trials. 2014;11:114–127. doi: 10.1177/1740774513512184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): A pragmatic six month trial of lithium vs. quetiapine for bipolar disorder. J Clin Psychiatry. doi: 10.4088/JCP.14m09349. in press. [DOI] [PubMed] [Google Scholar]
- 27.Sylvia LG, Shelton RC, Kemp DE, et al. Medical burden in bipolar disorder: findings from the Clinical and Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder study (Bipolar CHOICE) Bipolar disorders. 2015;17:212–223. doi: 10.1111/bdi.12243. [DOI] [PubMed] [Google Scholar]
- 28.Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66:870–886. doi: 10.4088/jcp.v66n0710. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 30.Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 31.Bowden CL, Singh V, Thompson P, et al. Development of the bipolar inventory of symptoms scale. Acta Psychiatr Scand. 2007;116:189–194. doi: 10.1111/j.1600-0447.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez JM, Bowden CL, Katz MM, et al. Development of the Bipolar Inventory of Symptoms Scale: concurrent validity, discriminant validity and retest reliability. Int J Methods Psychiatr Res. 2008;17:198–209. doi: 10.1002/mpr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leon AC, Solomon DA, Mueller TI, et al. A brief assessment of psychosocial functioning of subjects with bipolar I disorder: the LIFE-RIFT. Longitudinal Interval Follow-up Evaluation-Range Impaired Functioning. Tool J Nerv Ment Dis. 2000;188:805–812. doi: 10.1097/00005053-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 35.National Institutes of Health. National Heart, Lung and Blood Institute: Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight in Adults: The Evidence Report. Bethesda, MD: National Institutes of Health; 1998. [Google Scholar]
- 36.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Bond DJ, Lang DJ, Noronha MM, et al. The association of elevated body mass index with reduced brain volumes in first-episode mania. Biol Psychiatry. 2011;70(4):381–387. doi: 10.1016/j.biopsych.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Bond DJ, Ha TH, Lang DJ, et al. Body mass index-related regional gray and white matter volume reductions in first-episode mania patients. Biol Psychiatry. 2014;76:138–145. doi: 10.1016/j.biopsych.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Kuswanto CN, Sum MY, Yang GL, Nowinski WL, McIntyre RS, Sim K. Increased body mass index makes an impact on brain white-matter integrity in adults with remitted first-episode mania. Psychol Med. 2014;44:533–541. doi: 10.1017/S0033291713000858. [DOI] [PubMed] [Google Scholar]
- 42.Otto MW, Simon NM, Wisniewski SR, et al. Prospective 12-month course of bipolar disorder in out-patients with and without comorbid anxiety disorders. Br J Psychiatry. 2006;189:20–25. doi: 10.1192/bjp.bp.104.007773. [DOI] [PubMed] [Google Scholar]
- 43.Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shelton RC, Miller AH. Inflammation in depression: is adiposity a cause? Dialogues Clin Neurosci. 2011;13:41–53. doi: 10.31887/DCNS.2011.13.1/rshelton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reininghaus EZ, McIntyre RS, Reininghaus B, et al. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder: a preliminary report. Bipolar disorders. 2014;16:432–440. doi: 10.1111/bdi.12166. [DOI] [PubMed] [Google Scholar]
- 46.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab. 2013;24:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckman LB, Hasty AH, Flaherty DK, et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong SK, Nam HS, Son MH, Son EJ, Cho KH. Interactive effect of obesity indexes on cognition. Dement Geriatr Cogn Disord. 2005;19:91–96. doi: 10.1159/000082659. [DOI] [PubMed] [Google Scholar]
- 49.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med. 2012;74:682–690. doi: 10.1097/PSY.0b013e318261909c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coplan JD, Fathy HM, Abdallah CG, et al. Reduced hippocampal N-acetyl-aspartate (NAA) as a biomarker for overweight. Neuroimage Clin. 2014;4:326–335. doi: 10.1016/j.nicl.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond) 2012;36:656–664. doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kivimaki M, Shipley MJ, Batty GD, et al. Long-term inflammation increases risk of common mental disorder: a cohort study. Mol Psychiatry. 2014;19:149–150. doi: 10.1038/mp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond) 2008;32(Suppl 3):S56–59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 55.Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]