Abstract
During past decades, the formation and storage principle of memory have received much attention in the neuroscience field. Although some studies have attempted to demonstrate the nature of the engram, elucidating the memory engram allocation mechanism was not possible because of the limitations of existing methods, which cannot specifically modulate the candidate neuronal population. Recently, the development of new techniques, which offer ways to mark and control specific populations of neurons, may accelerate solving this issue. Here, we review the recent advances, which have provided substantial evidence showing that both candidates (neuronal population that is activated by learning, and that has increased CREB level/excitability at learning) satisfy the criteria of the engram, which are necessary and sufficient for memory expression.
Keywords: Memory engram, excitability, CREB, Memory allocation, Review
INTRODUCTION
The nature of memory, including its formation, expression, and maintenance processes, has received much attention in the neuroscience field. During past decades, molecular and cellular biology demonstrated that several encoded proteins and molecules have important roles in these processes. In addition, the mechanism of synaptic plasticity such as long-term potentiation (LTP) and its significance as a functional module of memory storage have been identified. Furthermore, it is widely demonstrated that each brain region or circuit has crucial function(s) in specific types of memory such as the hippocampus encoding contextual information and the amygdala encoding fear memory.
Along with these discoveries, many attempts to determine the physical substance of memory have been conducted. In 1949, Donald O. Hebb proposed that memory would be represented by sparsely distributed neuronal ensembles, which are co-activated during learning or experience, but not by all neurons in the whole brain or memory-related regions [1]. Based on these discerning hypothesis and other pioneers' suggestions, it is broadly accepted that the memory engram, which is defined as the physical substrate of memory in the brain [2], is formed by learning, and reactivated by retrieval.
For investigating the memory engram, some defining characteristics are usually used rather than an exact definition of the engram. For instance, one of the commonly used defining characteristics is the necessity and sufficiency for memory expression [3,4]. Although 'encoding' is the key point of the engram definition, this criterion is generally accepted because memory storage is not verifiable without memory expression. In addition, according to Semon, who introduced the term 'engram' almost 100 years ago [5,6,7], four defining characteristics (persistence, ecphory, content, and dormancy) are also used to identify the engram [7]. Here, we will regard the neuronal population that is necessary and sufficient for memory expression as the engram.
If the engram were formed by learning, the activated neurons by learning would be recruited into the memory engram. Therefore, many researchers have focused on the neuronal population, which is activated by learning, to search for the engram. Recently, the firing pattern comparison between the populations of neurons marked by neuronal activation during learning and the populations of neurons that express immediate early genes (IEG) during retrieval revealed that neurons, which were activated by learning, showed relatively higher firing probability at memory retrieval [8,9]. In addition to these results, it was shown that neurons whose synaptic plasticity was changed by learning have higher firing rate during retrieval as seen in electrophysiological experiments [10]. These results have shown a possibility that neuronal ensembles, which were activated by learning, could be preferentially recruited into the memory-encoding population and eventually become the memory engram.
Meanwhile, more and more compelling evidence suggest that transcription factor cAMP responsive element binding protein (CREB) plays a significant role in governing which LA neurons will participate in a given memory trace [11]. CREB research first started from revealing that CREB overexpression in small portion (~20%) of amygdala neurons could enhance fear memory [12]. The same group of researchers further developed the study by showing LA neurons with increased CREB levels are favorably recruited to involve in the memory trace. Sheena Josselyn and her colleagues brilliantly hypothesized that neurons overexpressing CREB at the time of learning shall win the "competition" to become part of the memory trace [13], and proved this right through biological imaging technique. In other words, neuronal population which contains relatively high level of CREB at the time of the learning also has been proposed as a candidate of the engram [12,13]. Indeed there are many other protein molecules that may be crucial for the memory allocation process. So far CREB is the most well studied and understood factor in governing the selection of neurons into the memory trace, but considering the complex and dynamic nature of memory formation and storage, it is unquestionable that there are many other unknown protein candidates to be resolved by future studies.
Although previous studies have tried to offer good evidence that neuronal population, which is activated by learning or which has increased CREB level, will be forming the engram, it is difficult to confirm whether these neuronal populations are the engram by showing their necessity and sufficiency for retrieval. Until recently, the necessity and sufficiency for memory encoding and expression could not be verifiable because of the limitations of the existing molecular biological and electrophysiological methods, which could not separate the important neurons from the residual neurons. However, the improvement and development of new techniques like optogenetics and transgenic mouse (BOX) accelerate solving these problems. Here, based on the engram defining characteristics (necessity and sufficiency for memory expression), we discuss the recent studies that have provided good evidence that both engram candidates (neuronal population that is activated by learning and that has high CREB level) satisfy the criteria for the engram. In addition, remaining questions in the engram research field are suggested in the discussion section.
Optogenetics
Optogenetics is a neuronal activity controlling technique using ion channels/pumps derived from microbial opsin whose opening and closing are regulated by light. In 2005, Karl Deisseroth's group reported for the first time that the activity of hippocampal neurons expressing the channelrhodopsin, which is the blue lightgated non-specific cation channel, could be regulated by light with millisecond precision control [14].
Before the development of optogenetics, activity control (neuronal firing activation and silencing) of a neuronal population was conducted through non-specific lesion and/or electric stimulation, because targeting a specific neuronal population was impracticable. However, for engram research, a technique that could control neuronal activity of a specific population during a specific time point was clearly necessary because neurons comprising an engram are sparsely distributed.
This powerful technique has provided a way to control neuronal activity at precise temporal and spatial conditions as long as the channelrhodopsin is expressed in that specific neuronal population. Nowadays, archaerhodopsin (ArchT), which is widely used light-driven proton pump to inhibit neuronal activation, and other modified ion channels (e.g., halorhodopsin) are available to control neuronal activity [15]. With the advances in optogenetics, not only artificial synchronous firing and silencing but also asynchronous enhancement of excitability has become possible [16,17].
TetTag system
The gene expression control techniques have been developed to limit the gene expression to the neuronal populations of interest. In the engram research field, the combination between Tet expression system and immediate early gene (IEG) promoter is widely used to mark neurons that are activated at a specific time point [8,18]. In this technique, tetracycline transactivator (tTA), which is a key activator of doxycycline (DOX) mediated inducible gene expression system, is driven by the well-known IEG promoter, such as c-fos or Arc. In the presence of DOX in the diet, the transcription of the gene of interest is effectively suppressed by interrupting the binding of tTA to the tetracyclineresponsive element (TRE) site, although tTA has been expressed by neuronal activity. When DOX is cleared from the diet, tTA, driven by neuronal activity, is able to bind to the TRE site and activate transcription of the gene of interest without any hindrance. Therefore, the IEG promoter-controlled tTA expression functions as neuronal activity specific marker, and DOX control confers temporal specificity. Using this system, the populations of neurons that fire at a specific time point (e.g. at the time of learning) could be selectively tagged by channelrhodopsin or other controllable membrane proteins.
NEURONAL POPULATION THAT IS ACTIVATED BY LEARNIN G IS NECESSARY FOR MEMORY EXPRESSION
To examine the necessity of the neuronal population that is activated by learning, it should be checked whether the memory is lost when this population was inactivated (Fig. 1A). The main difficulty of testing the necessity for storage or representation is the inhibition of the specific neuronal population without any perturbation of the other nearby neurons [19].
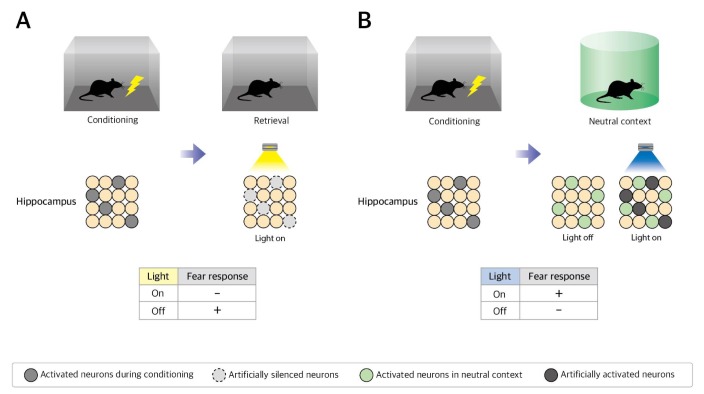
Fig. 1. Strategies and results for testing the necessity and sufficiency of the neuronal population, which is activated by learning. (A) Activated neurons during conditioning are marked by ArchT through gene expression control techniques. If this population is necessary for memory, the expression of fear memory associated with a context will be reduced by optogenetic silencing of this population. A reduced fear response by yellow light shows the necessity of the activation of this activated neuronal population for memory expression. (B) Activated neurons during conditioning are marked by ChR2 through gene expression control techniques. If this population is sufficient for memory, an artificial activation of this neuronal population will elicit a fear response in the neutral context. An induced fear response by blue light in neutral context shows the sufficiency of this activated neuronal population for memory expression.
In 2014, two independent papers have shown that a hippocampal neuronal population that is activated by learning is necessary for the retrieval of stored memory [20,21]. To implement the limited expression of the ArchT only in the activated neurons during fear conditioning, adeno-associative virus (AAV) encoding doublefloxed inverted ArchT expressed in a Cre recombinase dependent manner was injected into the dorsal hippocampus CA1 region of the Fos-tTA/tetO-Cre/tetO-H2B-GFP mouse, which could express Cre recombinase specifically in recently activated neurons (BOX). These mice were conditioned to associate the context and electric shock in the OFF-DOX condition and 2 days later, memory was tested while the marked neurons were inactivated by ArchT and light control. The lower freezing level of the inhibition group demonstrated that the CA1 neuronal population that is activated by learning is required to elicit the conditioned fear responses (Fig. 1B) [20]. Moreover, other research groups have revealed that the inhibition of the dentate gyrus and CA3 neuronal population that is activated by learning reduced the freezing level at retrieval 2 weeks after conditioning with similar methods [21]. Using the newly developed techniques, the necessity of the neuronal population that is activated by learning has been clearly verified at least in the hippocampal fear memory engram.
NEURONAL POPULATION THAT IS ACTIVATED BY LEARNIN G IS SUFFICIENT FOR MEMORY EXPRESSION
To verify the sufficiency of the activated neurons during learning, it should be checked whether the memory could be retrieved successfully by the artificial activation of these neurons without any natural and environmental cue about the memory (Fig. 1C). In 2012, by demonstrating that contextual fear memory could be retrieved only via optogenetic stimulation without natural conditioned stimulus, Tonegawa's group has reported that the artificial activation of the population of neurons, which was activated by contextual fear conditioning in the dentate gyrus, resulted in recall of the fear memory (Fig. 1D) [18]. This pioneering experiment has clearly shown that the activation of a hippocampal neuronal population that is activated by learning is sufficient to elicit contextual information representation.
Consistent with this, the optogenetic activation of a neuronal population that is activated by learning in the retrosplenial cortex is also sufficient for contextual memory reactivation [22]. In this case, pharmacological inhibition of the hippocampus during the optogenetic activation of the retrosplenial cortex engram did not show any disturbance in recalling the fear memory. In addition, these two studies have revealed the context specificity of these neuronal populations [18,22]. This specificity could be a strong piece of evidence that a sparse neuronal population, which fires during learning, is capable of information-specific memory encoding and that the activation of these populations leads to the retrieval of a specific memory.
In addition to the results that the artificial activation of the neuronal population that is activated by learning could reactivate a memory, several memory engineering results have offered strong evidence for the sufficiency of the neuronal population, which is fired by learning, for memory expression. In most memory engineering studies, it is assumed that the artificial activation of the neuronal population, which is activated by learning, would represent the encoding information. 'Creating false memory', 'emotional valence change', and 'artificial association' have been reported in memory engineering studies.
To create a false memory, electric shock was delivered in the 'fear context', but during this conditioning process, the neuronal population, which was already activated and marked with ChR2 in a 'safe' context, was artificially activated [23]. Because of this "memory inception" protocol, the fear emotion was associated with the "safe" context where electric shock was never delivered [23,24]. It changes the emotional valence already associated with the specific context. The subject male mouse was trained to prefer 'positive' context where female mouse was involved, and to avoid 'negative' context where electric shock was delivered [25]. After the "memory induction" protocol, which is the activation of the 'positive' context encoding neuronal population during electric shock delivered in 'negative-context' and vice versa, the 'positivecontext' was not preferred and the 'negative-context' was not avoided anymore. Moreover, it has been shown that the artificial association between the hippocampal contextual information considered to be encoded in the neuronal population which was activated by context habituation, and amygdala fear information considered to be encoded in the neuronal population which was activated by foot shock using optogenetic co-activation, generated a qualitatively new context-fear associative memory [26]. The evidence of memory representation by the artificial activation and memory manipulation studies described above strongly implies that a neuronal population, which is activated by learning, is sufficient for memory expression.
In summary, despite the limited region and memory task, definitive evidence revealed that a neuronal population, which is activated by learning, is necessary and sufficient for memory retrieval. Therefore, such a neuronal population, which is activated by learning, satisfies the criteria of the engram, and could be considered as the memory engram.
IMPORTANCE OF CREB/EXCITABILITY TO ALLOCATION
CREB is a transcription factor activated by cAMP level, protein kinase A (PKA), and other signaling pathways [27]. The evidence from numerous researches has shown that CREB is important for long-term memory consolidation and neuronal excitability [28,29,30,31,32,33]. In 2007, Han et al. published a pioneering paper suggesting that neuronal competition and selection are the major mechanism for determining which amygdala neurons would be recruited into the conditioned fear memory trace [12]. When wildtype CREB vector is overexpressed in LA neurons, these neurons showed a higher probability of being recruited into the fear memory trace, demonstrated by higher percentage of Arc (molecular marker of recently active neuron) induction in CREB neurons compared to neighboring neurons. Research went further to find it is the function of CREB that is relevant to the memory process than the protein level itself. Han et al. used a dominant-negative form of CREB to confirm that LA neurons expressing nonfunctional CREB resulted in a lower probability of detecting Arc + nuclei.
More recently, it has been demonstrated that the neuronal excitability, which is regulated by CREB [30,32,33], is the actual factor for neuronal selection mechanism taking advantage of the new techniques, which can increase the neuronal excitability without manipulating the CREB level. The results that neurons with artificially increased excitability via voltage-dependent K+ channels or designer receptors exclusively activated by designer drugs (DREADDs) hM3Dq showed a higher firing probability at retrieval have demonstrated the importance of neuronal excitability to the memory allocation process [34].
In addition to this result showing that a neuronal population having high CREB level/excitability at learning is likely to participate more in the memory retrieval process, the necessity and sufficiency of this high CREB/excitability neuronal population for memory expression have been verified using the up-to-date techniques (BOX). Recently, many attempts confirmed that the high CREB level/excitability neurons are going to be the memory engram especially in the amygdala, which is an important region for fear memory, by demonstrating the necessity and sufficiency of these populations of neurons for fear memory retrieval.
HIGH CREB/HIGH EXCITABILITY NEURONS ARE NECESSARY FOR MEMORY RETRIEVAL
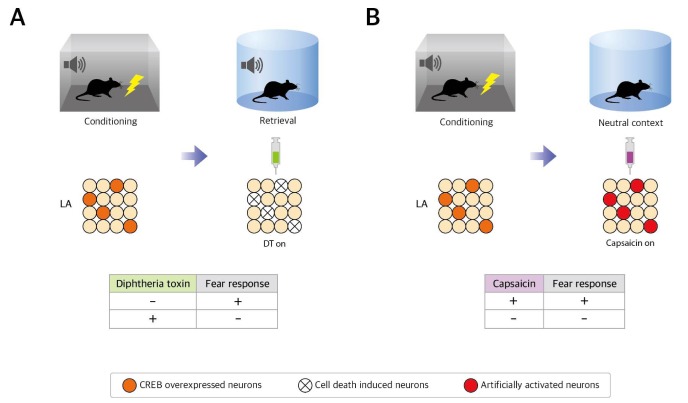
The necessity of the high CREB level neuronal population for memory was reported for the first time in 2009 [35]. By injecting the CREB encoding herpes simplex virus (HSV) into the amygdala, several neurons (roughly 20%) gained high CREB levels. After fear conditioning, selective ablation of these neurons using inducible diphtheria toxin (DT) strategy lead to deficit of memory expression (Fig. 2A, B). DT strategy involves binding of the DT to DT receptor (DTR) to induce apoptosis. The transgenic mice used in this particular study express DTR in a Cre-recombinase-dependent manner, so when LA neurons are infected by CREB-Cre HSV virus, later injection of DT selectively ablates these CREB-overexpressing cells. This observed memory loss by cell death of the high CREB level neuron population suggests that these neurons are required to retrieve the memory, and the memory was allocated in the CREB overexpressed neurons. Similarly, another group has also shown that selective inactivation of CREB overexpressing neurons by AlstR/ligand system disrupts fear memory retrieval [36]. In addition, based on the wholecell recording experiments showing that CREB overexpressing neurons had increased neuronal excitability [36], it has been suggested that neurons, which have relatively higher excitability, are required for memory expression.
Fig. 2. Strategies and results for testing the necessity and sufficiency of the neuronal population, which have high CREB level/excitability during learning. (A) If the neurons having a high CREB level and excitability are required to express the encoded memory, the expression of the fear memory associated with tone will be suppressed by induced cell death of these high CREB/excitability neurons. A reduced fear response after the targeted cell death of these high CREB neurons by inducible diphtheria-toxin strategy shows the necessity of the high CREB level neurons. (B) If the neurons having a high CREB level and excitability are sufficient to express the encoded memory, the activation of these neurons will be sufficient to elicit the fear response. An induced fear response using TRPV1/capsaicin system shows the sufficiency of the high CREB level neurons for the expression of fear memory.
In addition to an amygdala dependent cued fear conditioning scheme, evidence shows memory allocation to high CREB/excitability neurons; hence, the necessity of such neurons for expression of a particular memory also applies to other brain regions and corresponding memory tasks. It has been revealed that LA neurons having high CREB level at the time of learning are necessary to activate the "cocaine engram" of a cocaine-cue association memory [37]. This study has shown that when the neurons overexpressing CREB were silenced before a memory test, mice no longer elicited cocaine-conditioned place preference behavior. Furthermore, neurons in the insular cortex with high CREB levels during taste aversion training were demonstrated to be essential for later performance of the conditioned taste aversion (CTA) task [38]. Here, researchers used the chemogenetic DREADD system of manipulating neuronal activity. The hM4Di form of DREADD is an engineered G-protein coupled receptor activated by inert drug-like molecule clozapine-N-oxide (CNO). CNO-activation of the receptor induces membrane hyperpolarization and neuronal silencing [39]. Using the hM4Di DREADD receptor to specifically inhibit the CREB-positive neurons, it was demonstrated that without the activity of these neurons, mice were unable to retrieve the CTA memory. These results imply that the relative level of CREB and/or neuronal excitability is more than essential for the expression of diverse types of memories in broad brain areas. Reports mentioned above have provided evidence supporting the requirement of neurons with relatively high CREB levels and, in turn, increased excitability at the time of memory formation for retrieval of that memory.
HIGH CREB/HIGH EXCITABILITY NEURONS ARE SUFFICIENT FOR MEMORY RETRIEVAL
Studies have verified the sufficiency of high CREB/excitability neurons as well as their necessity. One study has confirmed this sufficiency by selectively activating CREB-overexpressing neurons using ectopic rat vanilloid receptor (TRPV1)/Capsaicin system (Fig. 2C) [40]. LA neurons were infected with HSV that coexpresses CREB and TRPV1, which allowed the neurons with high CREB levels during auditory fear conditioning to be manipulated by capsaicin injection. Because of capsaicin microinjection into the LA after fear learning, the freezing level increased even without the actual conditioned stimulus. Furthermore, the capsaicin-recalled memory was not only rendered vulnerable to anisomycin, but was also strengthened with repeated reactivation. Induction of fear response and reconsolidation-like processes by TRPV1 activation are two convincing evidence for the sufficiency of high CREB neurons for memory expression (Fig. 2D).
More recently, in line with the finding that CREB regulates excitability of neurons, research has been shifted to investigating the allocation of memory to neurons with higher excitability. One profound study has revealed the sufficiency of increased-excitability neurons in memory retrieval using various techniques to manipulate neuronal excitability [34]. Instead of modulating the CREB levels, voltage-dependent K+ channels, hM3Dq DREADD, and light-driven ChR2 opsin were used to decrease or increase neuronal excitability prior to training. When neurons of increased excitability immediately before conditioning were reactivated at a later time point, this acted as a sufficient retrieval cue such that animals showed the conditioned response even in the absence of the conditioned stimulus. These results have emphasized the importance of neuronal excitability over CREB level for sufficiency of memory expression.
In summary, a number of research clearly demonstrated that high CREB/excitability property of neurons during learning makes it a necessary and sufficient component in expressing an encoded memory. In other words and by our definition stated earlier, neurons that have high CREB level and/or increased excitability during learning could be considered to constitute the memory engram.
DISCUSSION
Here, we discussed the evidence showing that two candidates (neuronal population that is activated by learning and that has high CREB level/excitability) are both qualified to be considered as the engram by showing the necessity and sufficiency of these populations for memory expression. However, there are some limitations in the current knowledge and certain questions remain to be resolved. In this part, remaining questions and future direction are discussed and suggested.
It has been demonstrated that the neuronal populations, which are inferred to be the engram, are necessary and sufficient for later retrieval of the memory. In addition, neuronal excitability is regulated by the CREB level and neuronal activation is easily influenced by neuronal excitability [16,17]. For this reason, it is possible that these two populations represent the same or at least largely overlapping neuronal population. However, most of the CREB/excitability-related studies were conducted by artificial modification of the CREB level or excitability conditions with CREB-encoding HSV or other techniques. Therefore, to determine whether these two engram candidates represent the same neuronal population, it should be confirmed whether the neuronal populations, which have endogenously high CREB/excitability at the time of learning, are indeed activated by learning procedures, and therefore, these endogenously selected neurons are necessary and sufficient for memory encoding and expression. Until this experiment and results are reported, it is hard to say is the real engram between these two candidates described above.
Additionally, there are some interesting questions to be resolved in the engram research field. Artificial activation of the neuronal population, which is activated by learning, using new techniques does not mimic the natural temporal firing pattern of these neurons. In fact, similar study using the DREADD system to activate the hippocampal engram instead of optogenetics has shown that electric shock might be associated with mixed contextual information (natural context and activated context) [41] unlike suggested by previous research. While optogenetics provides precise temporal control by light, DREADD system can activate or inhibit neuronal firing for relatively long periods by drug injection. This major discrepancy might highlight the difference between artificial memory recall results using optogenetics and the DREADD system [24].
In many studies described here, the memory engram was considered as the population of neurons which fire during learning. However, the memory engram could include the whole neuronal population and even non-neuronal components that encode the specific memory. Though most studies confined the engram to the sub-region of the hippocampus or amygdala, research that encompasses the whole-brain using connectomics technique to examine the undiscovered functions and characteristics of the engram according to the brain region should be conducted [42].
Despite the necessity and sufficiency of the neuronal population, which is activated by learning, the engram might be more specific because not all neurons, which are activated by learning, are reactivated by retrieval. In fact, only a small portion of neurons (<10%) activated by learning are re-activated during retrieval [9,21]. Although the numerical value is significantly higher than random probability, low reactivation ratio implies that neurons activated by learning might include other non-engram neurons such as those related to the encoding process and/or to the various incoming stimuli [7]. Therefore, the real-engram could be more specifically representing neurons that are active during both learning and retrieval.
In addition, the results that the memory engram allocation is dependent on the activation and/or high CREB level/excitability during learning procedure, and that the CREB level and neuronal excitability could be increased after neuronal activation [43,44,45] suggests that there might be an engram-integrating phenomenon of temporally close or qualitatively related memories in the memory-encoding network. In other words, two engrams encoding temporally close events or similar objects might share more neurons in common than unrelated engrams because neurons, that have been active a moment ago or have been reactivated by a retrieval process have increased CREB level, excitability, and activation probability for memory formation or retrieval, respectively.
Research has started to reveal the identity of the memory engram. In the near future further analysis of the memory engram will not only lead to discovery of new therapies to alleviate psychiatric diseases associated with memory deficit, but also make artificial memory encoding and decoding possible.
ACKNOWLEDGEMENTS
B.-K.K. was supported by the National Honor Scientist Program (2012R1A3A1050385) from the National Research Foundation of the Ministry of Science, Information, Communication and Technology. J.-i. K. was supported by the TJ Park Foundation. J.-H. H. was supported by grants from the National Research Foundation of Korea (NRF) funded by the Korea government (MSIP) (2014R1A2A1A10053821).
References
- 1.Hebb DO. The organization of behavior: a neuropsychological theory. New York, NY: Wiley; 1949. [Google Scholar]
- 2.Franz SI, Lashley KS. The retention of habits by the rat after destruction of the frontal portion of the cerebrum. Psychobiology. 1917;1:3–18. [Google Scholar]
- 3.Tonegawa S, Liu X, Ramirez S, Redondo R. Memory engram cells have come of age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P. Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron. 2016;89:1074–1085. doi: 10.1016/j.neuron.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Semon RW. Mnemic psychology. London: Allen & Unwin; 1923. [Google Scholar]
- 6.Semon RW. The mneme. London: Allen & Unwin; 1921. [Google Scholar]
- 7.Josselyn SA, Köhler S, Frankland PW. Finding the engram. Nat Rev Neurosci. 2015;16:521–534. doi: 10.1038/nrn4000. [DOI] [PubMed] [Google Scholar]
- 8.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 9.Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23:99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Nonaka A, Toyoda T, Miura Y, Hitora-Imamura N, Naka M, Eguchi M, Yamaguchi S, Ikegaya Y, Matsuki N, Nomura H. Synaptic plasticity associated with a memory engram in the basolateral amygdala. J Neurosci. 2014;34:9305–9309. doi: 10.1523/JNEUROSCI.4233-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Kwon JT, Kim HS, Han JH. CREB and neuronal selection for memory trace. Front Neural Circuits. 2013;7:44. doi: 10.3389/fncir.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 13.Josselyn SA. Continuing the search for the engram: examining the mechanism of fear memories. J Psychiatry Neurosci. 2010;35:221–228. doi: 10.1503/jpn.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 15.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, Wiltgen BJ. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 2014;84:347–354. doi: 10.1016/j.neuron.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84:432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez S, Tonegawa S, Liu X. Identification and optogenetic manipulation of memory engrams in the hippocampus. Front Behav Neurosci. 2014;7:226. doi: 10.3389/fnbeh.2013.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513:426–430. doi: 10.1038/nature13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohkawa N, Saitoh Y, Suzuki A, Tsujimura S, Murayama E, Kosugi S, Nishizono H, Matsuo M, Takahashi Y, Nagase M, Sugimura YK, Watabe AM, Kato F, Inokuchi K. Artificial association of pre-stored information to generate a qualitatively new memory. Cell Reports. 2015;11:261–269. doi: 10.1016/j.celrep.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 28.Sekeres MJ, Neve RL, Frankland PW, Josselyn SA. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn Mem. 2010;17:280–283. doi: 10.1101/lm.1785510. [DOI] [PubMed] [Google Scholar]
- 29.Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- 31.Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez de Armentia M, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J Neurosci. 2007;27:13909–13918. doi: 10.1523/JNEUROSCI.3850-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viosca J, Lopez de Armentia M, Jancic D, Barco A. Enhanced CREB-dependent gene expression increases the excitability of neurons in the basal amygdala and primes the consolidation of contextual and cued fear memory. Learn Mem. 2009;16:193–197. doi: 10.1101/lm.1254209. [DOI] [PubMed] [Google Scholar]
- 34.Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, Woodin MA, Frankland PW, Josselyn SA. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiang HL, Epp JR, van den Oever MC, Yan C, Rashid AJ, Insel N, Ye L, Niibori Y, Deisseroth K, Frankland PW, Josselyn SA. Manipulating a "cocaine engram" in mice. J Neurosci. 2014;34:14115–14127. doi: 10.1523/JNEUROSCI.3327-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano Y, Shobe JL, Zhou M, Huang S, Shuman T, Cai DJ, Golshani P, Kamata M, Silva AJ. CREB regulates memory allocation in the insular cortex. Curr Biol. 2014;24:2833–2837. doi: 10.1016/j.cub.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G proteincoupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Kwon JT, Kim HS, Josselyn SA, Han JH. Memory recall and modifications by activating neurons with elevated CREB. Nat Neurosci. 2014;17:65–72. doi: 10.1038/nn.3592. [DOI] [PubMed] [Google Scholar]
- 41.Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. Generation of a synthetic memory trace. Science. 2012;335:1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler AL, Teixeira CM, Wang AH, Xiong X, Kovacevic N, Lerch JP, McIntosh AR, Parkinson J, Frankland PW. Identification of a functional connectome for long-term fear memory in mice. PLoS Comput Biol. 2013;9:e1002853. doi: 10.1371/journal.pcbi.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Disterhoft JF, Oh MM. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci. 2006;29:587–599. doi: 10.1016/j.tins.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]