Abstract
Regulation of cell volume is an important aspect of cellular homeostasis during neural activity. This volume regulation is thought to be mediated by activation of specific transporters, aquaporin, and volume regulated anion channels (VRAC). In cultured astrocytes, it was reported that swelling-induced mitogen-activated protein (MAP) kinase activation is required to open VRAC, which are thought to be important in regulatory volume decrease and in the response of CNS to trauma and excitotoxicity. It has been also described that sodium fluoride (NaF), a recognized G-protein activator and protein phosphatase inhibitor, leads to a significant MAP kinase activation in endothelial cells. However, NaF's effect in volume regulation in the brain is not known yet. Here, we investigated the mechanism of NaF-induced volume change in rat and mouse hippocampal slices using intrinsic optical signal (IOS) recording, in which we measured relative changes in intracellular and extracellular volume as changes in light transmittance through brain slices. We found that NaF (1~5 mM) application induced a reduction in light transmittance (decreased volume) in CA1 hippocampus, which was completely reversed by MAP kinase inhibitor U0126 (10 µM). We also observed that NaF-induced volume reduction was blocked by anion channel blockers, suggesting that NaF-induced volume reduction could be mediated by VRAC. Overall, our results propose a novel molecular mechanism of NaF-induced volume reduction via MAP kinase signaling pathway by activation of VRAC.
Keywords: Sodium Fluoride, Hippocampus, Volume regulation, VRAC
INTRODUCTION
Fluoride is an effective and safe method for prevention of dental caries, and is used for public health intervention in several countries through water fluoridation [1]. NaF has been used to fluoridate water: as an industry standard for fluoridation and as an additive in toothpastes to prevent cavities [2,3]. It has also been used in laundry, metallurgy and organic synthesis of many chemicals including fluorocarbons. NaF is also used in medicine in some diagnostic procedures employing radiolabeled fluoride. Despite its broad usage, in recent years fluoride has raised a substantial concerns regarding cellular toxicity in the brain. A recent report from the National Research Council (NRC 2006) in US concluded that adverse effects of high fluoride concentrations in drinking water might be of concern. Fluoride may cause neurotoxicity in laboratory animals with behavioral consequences including adverse effects on learning and memory [4,5,6,7]. Exposure of chronic or sub-chronic dose of fluoride into animal or human in developmental stage causes lack of recognition or decrease of IQ [8,9]. Hippocampus has been postulated to be one of the target sites attacked by fluoride. Previous reports showed that fluorosis might impair hippocampus synaptic interface structure [10,11]. And the changes in the synaptic interface structure would necessarily affect the transmission of neural information [12]. A recent study reported that fluoride could induce oxidative damage in hippocampal neurons in vitro [13]. However, the precise molecular mechanism of how fluoride affects hippocampal physiology is unknown.
Regulation of cell volume including baseline volume and volume dynamics during synaptic activity is an important aspect of cellular homeostasis during neural activity [14]. Evoked synaptic activity is followed by a rapid shrinkage of the extracellular volume, due to swelling of cell volume [15,16,17]. Cell swelling is substantial, ranging from 4 to 30%, depending on various conditions, such as the mode of stimulation, type of preparation, and area under investigation [18,19,20]. The volume regulation has a significant impact on the concentration of extracellular solutes and ions, and strongly influences subsequent neuronal activity [21,22]. It is thought to be mediated by activation of volume regulatory mechanism, such as co-transporter and/or anion channels, which are known to be dependent on the activation of MAP kinase [10]. For example, synaptic stimulation causes release of a number of solutes that are removed through a co-transporter, NKCC1, via MAP kinase activation [23,24].
In many biological studies, NaF has been used as an activator of the stimulus-response signaling cascade involving mitogenactivated protein (MAP) kinases, Erk-1 and Erk-2 [25]. Because Ras is a direct target of fluoride [26], the Ras/MEKK/MEK-mediated pathway activates Erk. This provides a useful mechanism of NaF-induced Erk activation [23,26,27,28]. MAP kinases can be activated by growth factors, cytokines, and some G-protein-coupled receptors, which are involved in the regulation of gene expression [29,30,31]. MAP kinases are also known to regulate several ion channel activity; in particular, the VRAC of astrocytes was reported to be a cytoplasmic target for the MAP kinase signaling pathway [32]. For this reason, the activity of regulatory volume system seems to be connected with MAP kinase signaling pathway. Therefore, NaF can potentially serve as a useful tool to activate VRAC. So far, the effect of NaF in volume regulation in the brain has not been determined yet.
In this study, we investigated the involvement of MAP kinase in NaF-induced changes of volume and neural activity in hippocampal slices, using IOS recording for volume change for neural activity. We utilized U0126, highly selective inhibitor of both MEK1 and MEK2 (a type of MAPK/ERK kinase) to inhibit MAP kinase. We report that NaF induced a reduction of baseline volume and volume dynamics during electrical stimulation, which was dependent on the activity of MAP kinase. Furthermore, we suggest that VRAC might be responsible for the NaF-induced hippocampal volume regulation.
MATERIALS AND METHODS
Experiments were performed in accordance with National Institutes of Health guidelines for animal care and welfare. Protocols were approved in advance by the UNC and KIST (Seoul, Korea) Institutional Animal Care and Use Committee. Subjects were young adult male rats and mice (21~35 days; 50~150 g; Sprague-Dawley and C57BL6, Charles River).
Slice preparation
Transverse slices of the hippocampus (400 µm thickness) were cut using an oscillating tissue slicer (OTS-4000, Electron Microscopy Sciences, Vibratome 3000) and placed in a reservoir containing ACSF warmed (30℃) and oxygenated (using a 95% O2 and 5% CO2 gas mix). Slices remained in the reservoir (never less than 1 hr) until transferred by pipette to a submerged position in a recording chamber perfused with warmed (28~30.8℃) and oxygenated ACSF (perfusion rate 2~3 ml/min). Composition (in mM) of the ACSF delivered to the slice prior to treatment with a drug or drug combination was 124 NaCl, 3.0 KCl, 2.5 CaCl2, 25 NaHCO3, 1 MgSO4, 1.25 NaH2PO4, and 10 glucose. After warming and oxygenating ACSF was delivered to the submerged slice via the chamber perfusion system.
IOS imaging
The slice was transilluminated and images obtained at ×2 or ×4 magnification using an inverted microscope (Diaphot 200, Nikon) and a cooled, slow-scan CCD camera (Photometrics Inc.). An intrinsic optical signal (IOS) was evoked within the hippocampi by application of a repetitive constant-current stimulus to the Schaffer collateral pathway using a glass insulated, 50 µm diameter, metal bipolar stimulating electrode connected to an isolation unit, and programmable pulse generator (Master-8, AMPI). Stimulation parameters were: pulse duration - 0.2 ms; intensity - 2~4× the threshold current for evoking an optical response; train duration - 1.0 s; frequency - 20 Hz; intertrain interval - 5 min. Each image included all of the hippocampus on the same side. 30~60 images were obtained in association with each repetitive stimulus (a "trial"). The 1st and 2nd images ("reference" images) in each trial were obtained at 1,000 ms and at 500 ms, respectively, prior to stimulus onset; 2 "poststimulus onset" images were acquired during delivery of the repetitive stimulus, and the remaining 26 images after stimulus termination (image acquisition rate=2/s). Trial duration was 9s. An average (across-frame) difference image was generated from the optical response to each repetitive Shaffer collateral stimulus by (1) subtracting the reference image obtained at 500 ms before stimulus onset from each image obtained in the same trial between 2.0~7.5 s after stimulus onset (images 6~25; total of 20), and (2) at each pixel location by dividing the sum of the differences between the post-stimulus and reference images (same-trial) by the number of frames. An intensity value was calculated for each pixel in a difference image using the formula Σ(Tij-Ti,ref)/Ti,ref, where Tij is the intensity of the ith pixel in the jth image, and Ti,ref is the intensity of the ith pixel in the reference image. Mean intensity (ΔT/T) of the IOS was determined by computing the average intensity of all pixels within the responding region of the striatum radiaum on the IOS of bath-applied drug (either 5 mM NaF or µM U0126, or 50 µM Acetazolamide, or 50 µM Niflumic acid, or 50 µM NPPB, or 10 µM Bumetanide and 10 µM Furosemide in combination) was expressed as a percentage of the mean intensity of the response observed prior to treatment: i.e., ΔT/Ttreatment/ΔT/Tcontrol×100=%.
Drugs
All chemicals for ACSF were purchased from Sigma Aldrich. U0126 (MAPK inhibitor), and Bumetanide / Furosemide (potent NKCC1 inhibitor) were purchased from Sigma. , NPPB, Anion channel blockers, and and DCPIB, VRAC blocker, were obtained form Tocris.
RESULTS
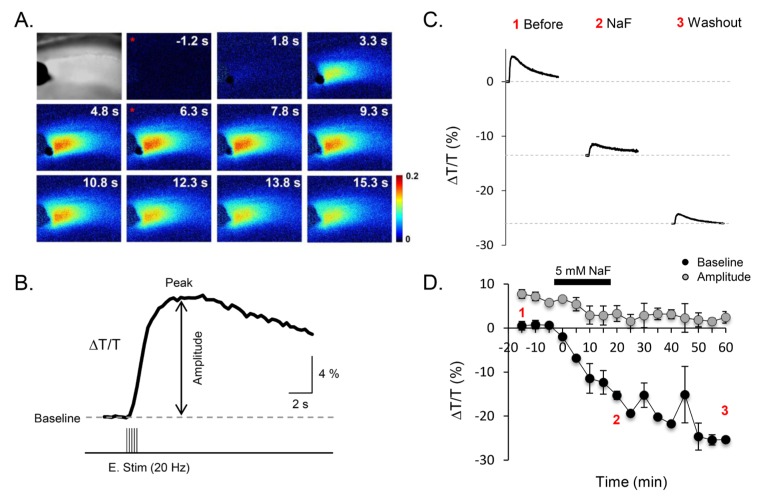
The neuronal activity-dependent transient volume change along with baseline volume change was detected and visualized as an IOS upon intense electrical stimulation of Schaffer collateral fibers for 1 s at 20 Hz (Fig. 1A) with a rise (4~6 s) and decay (70~80 s) in light transmittance through stratum radiatum of CA1 hippocampal slices (Fig. 1B), as previously described [15,19]. This stimulation-induced IOS was recorded repetitively at every 5 min (Fig. 1C and D). For analysis, we measured the baseline change and amplitude that was the maximal difference of light transmittance, which reached at 4~5 sec after electrical stimulation (Fig. 1B). Using this IOS technique, we tested the effect of NaF on volume regulation. We found that upon application of 5 mM NaF the baseline and amplitude of stimulation-induced IOS dropped dramatically and remained decreased even after washout with normal ACSF (Baseline; % of ΔT/T, Control, 0.64 ± 0.15; NaF, -15.30±0.93; Washout, -25.34±0.61, n=9) (Peak; % of ΔT/T, Control, 6.44±0.16; NaF, -12.34±2.65; Washout, -22.90±2.03, n=9) (Amplitude=Peak - Baseline, % of ΔT/T, Control, 5.80±; NaF, 2.96±1.79; Washout, 2.44±1.32) (Fig. 1C and D). These results suggest that fluoride has a potent volume-reducing effect on cellular volume in hippocampal slices.
Fig. 1. NaF decreased peak amplitude and baseline of intrinsic optical signal (IOS) in hippocampal slices. (A) Series of pseudo color images at different time with electrical stimulation was shown for change of light transmittance. (B) Example of light transmittance change of CA1 SR area and measurement of transmittance intensity. (C) Representative example of IOS before and during NaF application. Numbers with red color indicate where IOS recorded. Dotted line indicated that shifting of baseline with application of NaF. (D) Time course of IOS for baseline and amplitude during application of 5 mM NaF. Baseline, peak, and amplitude of IOS are indicated with black circle (●), and gray circle ( ) respectively.
) respectively.
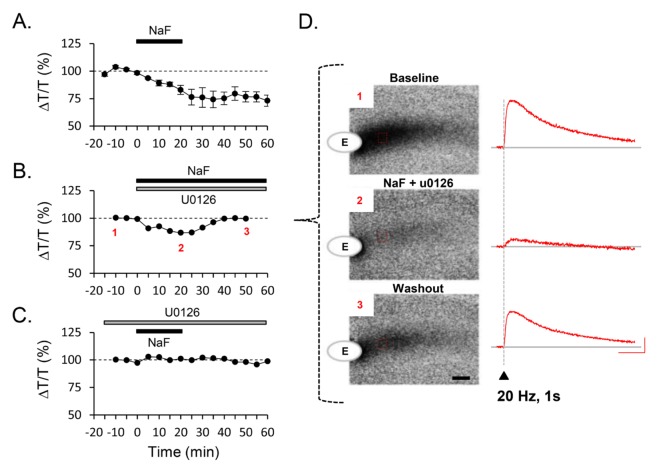
Because NaF is known as G-protein activator and activated MAP kinase [27,28,33], we tested whether NaF-induced volume reduction is dependent on the activity of MAP kinase. To investigate MAP kinase dependency, we monitored NaF-induced light transmittance change from hippocampal slices with MAP kinase inhibitor. We found that co-treatment of NaF and 10 µM U0126, MAP kinase inhibitor, initially reduced the baseline of light transmittance slightly (Fig. 2B and D). However, the baseline gradually recovered to the control level (Fig. 2A, middle panel, inset 3; % of baseline, 99.56±4.75, n=9), compared to NaF alone (Fig. 2A; % of baseline, 76.71±4.71, n=9). More importantly, we found that pre-treatment of U0126 completely prevented NaF-induced volume reduction (Fig. 2C; % of baseline, 98.06±4.75, n=3). These results indicate that fluoride shows a potent volume-reducing effect on brain tissue that is dependent on MAP kinase activity.
Fig. 2. MAPK inhibitor blocked decrease of ΔT/T baseline. (A-C) Time course of baseline change by NaF (A) and coapplication with U0126 (B, C) was shown. Difference of light transmittance was normalized by drug-free condition - the baseline before NaF treatment. (D) Example of IOS image at different time followed by NaF and U0126 coapplication was shown. Red traces in images indicated the change of light intensity evoked by 20 Hz stimulation measured from the area of hippocampus (indicated with dotted line box). Scale bar is 3 s and 1%.
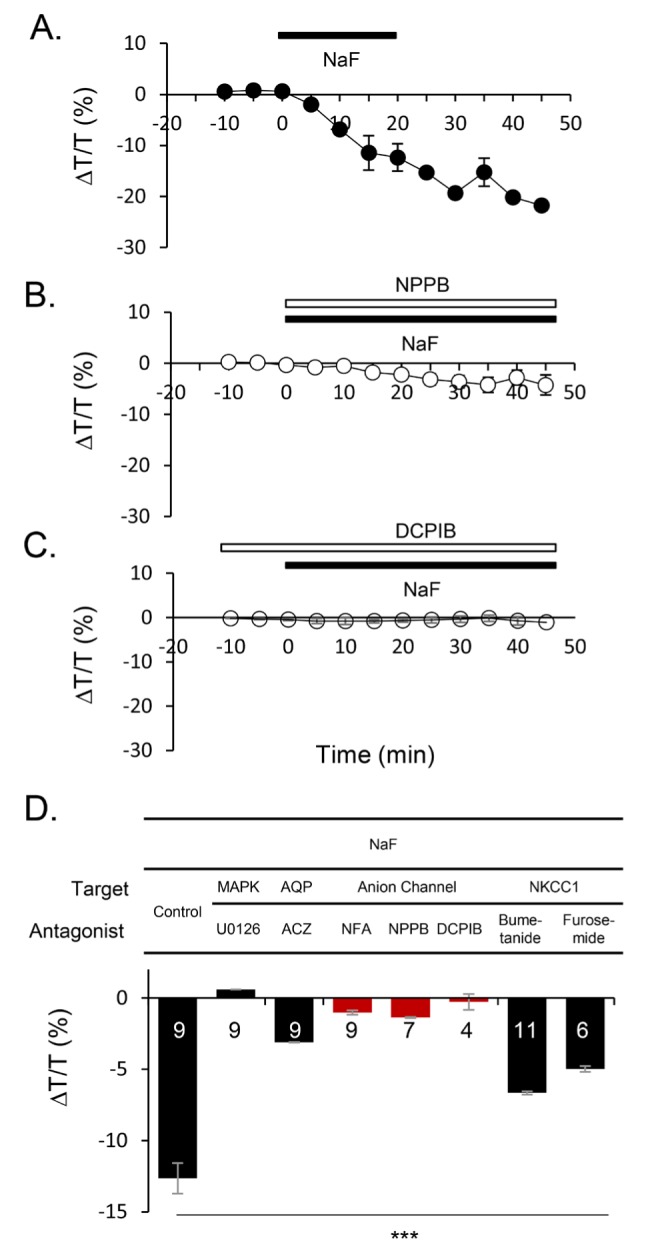
Next, we investigated the downstream target of NaF-activated MAP kinase pathway. We selected candidate targets that are likely influenced by MAP kinase and potentially involved in volume regulation and pharmacologically tested three candidates: aquaporin, anion channels, and NKCC1. We found that although all three candidates show some degree of blocking effect, anion channel blockers, niflumic acid (NFA), NPPB, and DCPIB have the most significant blocking effect on NaF-induced volume reduction (Fig. 3A and B; % of ΔT/T, 5 mM NaF, -12.66±1.07, n=9; NaF+10 µM U0126, 0.60±0.05, n=9; 20 µM Acetazolimide for aquaporin blocker, -3.11±0.02, n=9; 100 µM NFA, 200 µM NPPB, and 10 µM DCPIB for anion channel blockers, -1.03±0.14, n=9, -1.37±0.03, n=7, and -0.23±0.56, n=4 respectively; 25 µM Bumetanide and 3 mM Furosemide for NKCC1 blockers, -6.67±0.12, n=11 and -4.98±β0.21, n=6 respectively). Among the anion channel blockers, DCPIB, a known selective blocker for VRAC [34,35], showed most inhibitory effect of NaF-induced volume reduction. This result indicates that NaF-induced volume reduction is mostly mediated by opening of VRAC.
Fig. 3. Reduction of baseline for IOS induced by NaF shows anion channel(s) dependency. (A-C) Exemplary time course of the effect of NPPB and DCPIB on NaF-induced volume reduction was shown. (D) Summary bar graph of reduction of light transmittance for various antagonists compared with NaF effect.
DISCUSSION
The present report describes NaF, a well-known G-protein activator and protein phosphatase inhibitor, leads to a significant activation of MAP kinase, followed by a substantial volume reduction in hippocampal slices. Our study demonstrates that acute application of sub-chronic dose of NaF causes a critical effect on volume regulation and leads to suppression of neural activity. We found that NaF-induced reduction of hippocampal volume as well as neural activity was critically dependent on MAP kinase activity in hippocampal slices.
Regulation of cell volume is an important aspect of cellular homeostasis during neural activity. In the brain, concentration gradient of osmolytes and ions in between cell membrane is known to change following neural activity [16,36,37]. As ions and water move from plasma through ion channels, transporters, and water channels, the brain cell volume must be tightly controlled to avoid significant changes in cell size during subsequent neural activity. Thus it is expected that cells have fairly well developed regulatory cell volume mechanisms in the brain. In current study, anion channel blockers have the most inhibitory effect on NaF-induced volume reduction (Fig. 3), which strongly suggests that flouride ion induced opening of anion channels to enhance efflux of Cl- and osmolytes. It has been suggested that astrocytes are the major cell types in the brain to maintain homeostasis of cellular environment. Although we have not provided detailed analysis of cell types, NaF-induced reduction of light transmittance following the shrinkage of tissue volume is probably mediated by an efflux of Cl- and osmolytes from astrocytes. Future study is needed to address this important question.
Our study provides an explanation for the negative aspect of NaF in that an exposure of the chemical to hippocampal slices induces a dramatic volume reduction. It might be related with an activity of VRAC through MAP kinase signaling pathway. These findings imply that NaF-induced toxicity might be related with volume regulatory system in the brain, which could alter normal brain function. To extend our knowledge of the effect of NaF in the brain, the identification of specific cell type and the molecular identity of anion channel for the regulation of cellular volume need to be investigated in the future study.
In summary, despite its broad industrial and health applications, fluoride's effect in the brain has remained unclear. In the present study, we pharmacologically addressed the molecular mechanism of NaF-induced volume reduction. We propose that NaF can be a useful tool to activate VRAC via activation of MAP kinase pathway. We also raise an important public concern that chronic use of fluoride can exert toxic effect on brain function through the negative influence on brain volume dynamics.
ACKNOWLEDGEMENTS
This study was supported by Creative Research Initiative Program, Korean National Research Foundation (2015R1A3A2066619), and KIST Institutional Grant (2E26662) (C. Justin Lee, P.I.) and by NIH grant NS037501 (B. Whitsel, P.I.). M. Tommerdahl was supported, in part, by NIH grant NS050587 (M. Tommerdahl, P.I.).
References
- 1.Padilla O, Davis MJ. Fluorides in the new millennium. N Y State Dent J. 2001;67:34–38. [PubMed] [Google Scholar]
- 2.van Loveren C, Duggal MS. The role of diet in caries prevention. Int Dent J. 2001;51:399–406. doi: 10.1111/j.1875-595x.2001.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 3.Buzalaf MA, de Almeida BS, Cardoso VE, Olympio KP, Furlani Tde A. Total and acid-soluble fluoride content of infant cereals, beverages and biscuits from Brazil. Food Addit Contam. 2004;21:210–215. doi: 10.1080/02652030310001637128. [DOI] [PubMed] [Google Scholar]
- 4.Mullenix PJ, Denbesten PK, Schunior A, Kernan WJ. Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol. 1995;17:169–177. doi: 10.1016/0892-0362(94)00070-t. [DOI] [PubMed] [Google Scholar]
- 5.Borasio PG, Cervellati F, Pavan B, Pareschi MC. "Low" concentrations of sodium fluoride inhibit neurotransmitter release from the guinea-pig superior cervical ganglion. Neurosci Lett. 2004;364:86–89. doi: 10.1016/j.neulet.2004.03.089. [DOI] [PubMed] [Google Scholar]
- 6.Chioca LR, Raupp IM, Da Cunha C, Losso EM, Andreatini R. Subchronic fluoride intake induces impairment in habituation and active avoidance tasks in rats. Eur J Pharmacol. 2008;579:196–201. doi: 10.1016/j.ejphar.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Choi AL, Sun G, Zhang Y, Grandjean P. Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environ Health Perspect. 2012;120:1362–1368. doi: 10.1289/ehp.1104912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira M, Dombrowski PA, Losso EM, Chioca LR, Da Cunha C, Andreatini R. Memory impairment induced by sodium fluoride is associated with changes in brain monoamine levels. Neurotox Res. 2011;19:55–62. doi: 10.1007/s12640-009-9139-5. [DOI] [PubMed] [Google Scholar]
- 9.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen AK, Galtung HK. Aquaporin expression and cell volume regulation in the SV40 immortalized rat submandibular acinar cell line. Pflugers Arch. 2007;453:787–796. doi: 10.1007/s00424-006-0158-2. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Xu X, Shen X, Xu X. Effect of fluoride exposure on synaptic structure of brain areas related to learning-memory in mice. Fluoride. 2008;41:139–143. [PubMed] [Google Scholar]
- 12.Shivarajashankara YM, Shivashankara AR, Bhat PG, Rao SM, Rao SH. Histological changes in the brain of young fluoride-intoxicated rats. Fluoride. 2002;35:12–21. [Google Scholar]
- 13.Zhang M, Wang A, He W, He P, Xu B, Xia T, Chen X, Yang K. Effects of fluoride on the expression of NCAM, oxidative stress, and apoptosis in primary cultured hippocampal neurons. Toxicology. 2007;236:208–216. doi: 10.1016/j.tox.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Kimelberg HK, Macvicar BA, Sontheimer H. Anion channels in astrocytes: biophysics, pharmacology, and function. Glia. 2006;54:747–757. doi: 10.1002/glia.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacVicar BA, Hochman D. Imaging of synaptically evoked intrinsic optical signals in hippocampal slices. J Neurosci. 1991;11:1458–1469. doi: 10.1523/JNEUROSCI.11-05-01458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Østby I, Øyehaug L, Einevoll GT, Nagelhus EA, Plahte E, Zeuthen T, Lloyd CM, Ottersen OP, Omholt SW. Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Comput Biol. 2009;5:e1000272. doi: 10.1371/journal.pcbi.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin BJ, Zhang H, Binder DK, Verkman AS. Aquaporin-4-dependent K(+) and water transport modeled in brain extracellular space following neuroexcitation. J Gen Physiol. 2013;141:119–132. doi: 10.1085/jgp.201210883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietzel I, Heinemann U, Hofmeier G, Lux HD. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp Brain Res. 1980;40:432–439. doi: 10.1007/BF00236151. [DOI] [PubMed] [Google Scholar]
- 19.Holthoff K, Witte OW. Intrinsic optical signals in rat neocortical slices measured with near-infrared dark-field microscopy reveal changes in extracellular space. J Neurosci. 1996;16:2740–2749. doi: 10.1523/JNEUROSCI.16-08-02740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syková E, Vargová L, Kubinová S, Jendelová P, Chvátal A. The relationship between changes in intrinsic optical signals and cell swelling in rat spinal cord slices. Neuroimage. 2003;18:214–230. doi: 10.1016/s1053-8119(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 21.MacAulay N, Zeuthen T. Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience. 2010;168:941–956. doi: 10.1016/j.neuroscience.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Zeuthen T. Water-transporting proteins. J Membr Biol. 2010;234:57–73. doi: 10.1007/s00232-009-9216-y. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadian MR, Mittal R, Hall A, Wittinghofer A. Aluminum fluoride associates with the small guanine nucleotide binding proteins. FEBS Lett. 1997;408:315–318. doi: 10.1016/s0014-5793(97)00422-5. [DOI] [PubMed] [Google Scholar]
- 24.Liedtke CM, Cole TS. Activation of NKCC1 by hyperosmotic stress in human tracheal epithelial cells involves PKC-delta and ERK. Biochim Biophys Acta. 2002;1589:77–88. doi: 10.1016/s0167-4889(01)00189-6. [DOI] [PubMed] [Google Scholar]
- 25.Paul A, Wilson S, Belham CM, Robinson CJ, Scott PH, Gould GW, Plevin R. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403–410. doi: 10.1016/s0898-6568(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 26.Mittal R, Ahmadian MR, Goody RS, Wittinghofer A. Formation of a transition-state analog of the Ras GTPase reaction by Ras-GDP, tetrafluoroaluminate, and GTPase-activating proteins. Science. 1996;273:115–117. doi: 10.1126/science.273.5271.115. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, Verin AD, Birukova A, Gilbert-McClain LI, Jacobs K, Garcia JG. Mechanisms of sodium fluoride-induced endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1472–L1483. doi: 10.1152/ajplung.2001.281.6.L1472. [DOI] [PubMed] [Google Scholar]
- 28.Bogatcheva NV, Wang P, Birukova AA, Verin AD, Garcia JG. Mechanism of fluoride-induced MAP kinase activation in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1139–L1145. doi: 10.1152/ajplung.00161.2005. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann SR, Kubasch AS, Ioannidis C, Rösen-Wolff A, Girschick HJ, Morbach H, Hedrich CM. Altered expression of IL-10 family cytokines in monocytes from CRMO patients result in enhanced IL-1beta expression and release. Clin Immunol. 2015;161:300–307. doi: 10.1016/j.clim.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Korneeva NL, Song A, Gram H, Edens MA, Rhoads RE. Inhibition of mitogen-activated protein kinase (MAPK)-interacting kinase (MNK) preferentially affects translation of mRNAs containing both a 5'-terminal cap and hairpin. J Biol Chem. 2016;291:3455–3467. doi: 10.1074/jbc.M115.694190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamato D, Thach L, Getachew R, Burch M, Hollenberg MD, Zheng W, Little PJ, Osman N. Protease activated receptor-1 mediated dual kinase receptor transactivation stimulates the expression of glycosaminoglycan synthesizing genes. Cell Signal. 2016;28:110–119. doi: 10.1016/j.cellsig.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Crépel V, Panenka W, Kelly ME, MacVicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J Neurosci. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonny B, Sukumar M, Bigay J, Chabre M, Higashijima T. The mechanism of aluminum-independent G-protein activation by fluoride and magnesium. 31P NMR spectroscopy and fluorescence kinetic studies. J Biol Chem. 1993;268:2393–2402. [PubMed] [Google Scholar]
- 34.Decher N, Lang HJ, Nilius B, Brüggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol. 2001;134:1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in cultured rat astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Tommerdahl M, Favorov OV, Whitsel BL. Optically recorded response of the superficial dorsal horn: dissociation from neuronal activity, sensitivity to formalin-evoked skin nociceptor activation. J Neurophysiol. 2005;94:852–864. doi: 10.1152/jn.00976.2004. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Favorov OV, Tommerdahl M, Lee CJ, Whitsel BL. Attenuated Glial K(+) clearance contributes to long-term synaptic potentiation via depolarizing GABA in dorsal horn neurons of rat spinal cord. Exp Neurobiol. 2014;23:53–64. doi: 10.5607/en.2014.23.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]