Abstract
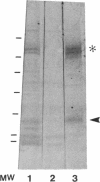
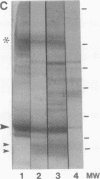
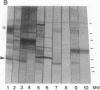
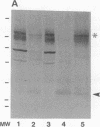
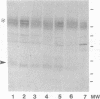
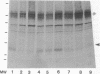
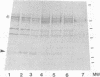
Altered proteolysis of the beta-amyloid precursor protein (beta APP) resulting in release of the approximately 40-residue amyloid beta-protein (A beta P) may be a seminal pathogenetic event in Alzheimer disease. Using region-specific beta APP antibodies, we searched for stable proteolytic intermediates containing the intact A beta P region in brain tissue. A 22-kDa beta APP fragment was selectively detected in microvessels purified from cerebral cortex and other brain regions. On immunoblots, the 22-kDa band is labeled by five distinct antisera to beta APP carboxyl-terminal peptides and by affinity-purified antibodies to the recombinant proteins beta APP444-592 and beta APP592-695, which flank the A beta P region. The protein is virtually undetectable in whole-brain homogenates or microvessel-free fractions of brain. The protein is extractable from microvessels in Triton X-100 and other detergents, indicating its membrane association. In comparison with cortical microvessels, microvessels purified from white matter, cerebellum, and nonneural tissues contain lower amounts of the 22-kDa protein. The protein is found in microvessels of both normal and Alzheimer disease brains and occurs in low amounts in microvessels from fresh bovine brain. The size and specific immunoreactivity of the 22-kDa protein indicate that it is a stable fragment of beta APP containing the intact A beta P. The occurrence of this potentially amyloidogenic intermediate in microvessels is consistent with a vascular or hematogenous origin for some A beta P deposits in Alzheimer disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bush A. I., Martins R. N., Rumble B., Moir R., Fuller S., Milward E., Currie J., Ames D., Weidemann A., Fischer P. The amyloid precursor protein of Alzheimer's disease is released by human platelets. J Biol Chem. 1990 Sep 15;265(26):15977–15983. [PubMed] [Google Scholar]
- Cole G. M., Galasko D., Shapiro I. P., Saitoh T. Stimulated platelets release amyloid beta-protein precursor. Biochem Biophys Res Commun. 1990 Jul 16;170(1):288–295. doi: 10.1016/0006-291x(90)91272-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Henriksson T., Barbour R. M., Braa S., Ward P., Fritz L. C., Johnson-Wood K., Chung H. D., Burke W., Reinikainen K. J., Riekkinen P. Analysis and quantitation of the beta-amyloid precursor protein in the cerebrospinal fluid of Alzheimer's disease patients with a monoclonal antibody-based immunoassay. J Neurochem. 1991 Mar;56(3):1037–1042. doi: 10.1111/j.1471-4159.1991.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Ishii T., Kametani F., Haga S., Sato M. The immunohistochemical demonstration of subsequences of the precursor of the amyloid A4 protein in senile plaques in Alzheimer's disease. Neuropathol Appl Neurobiol. 1989 Mar-Apr;15(2):135–147. doi: 10.1111/j.1365-2990.1989.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Mori H., Selkoe D. J. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989 Sep 21;341(6239):226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Harik S. I. Adenosine receptors and the nucleoside transporter in human brain vasculature. J Cereb Blood Flow Metab. 1988 Feb;8(1):32–39. doi: 10.1038/jcbfm.1988.5. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaire H. G., Salbaum J. M., Multhaup G., Kang J., Bayney R. M., Unterbeck A., Beyreuther K., Müller-Hill B. The PreA4(695) precursor protein of Alzheimer's disease A4 amyloid is encoded by 16 exons. Nucleic Acids Res. 1989 Jan 25;17(2):517–522. doi: 10.1093/nar/17.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T., Shimoji A., Kuramoto R., Higuchi Y. The relationship between senile plaques and cerebral blood vessels in Alzheimer's disease and senile dementia. Morphological mechanism of senile plaque production. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982 Aug;40(2):121–129. doi: 10.1007/BF02932857. [DOI] [PubMed] [Google Scholar]
- Mönning U., König G., Prior R., Mechler H., Schreiter-Gasser U., Masters C. L., Beyreuther K. Synthesis and secretion of Alzheimer amyloid beta A4 precursor protein by stimulated human peripheral blood leucocytes. FEBS Lett. 1990 Dec 17;277(1-2):261–266. doi: 10.1016/0014-5793(90)80861-c. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., Sinha S. The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989 Sep 14;341(6238):144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. The beta-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny M. B., Tolan D. R., Selkoe D. J. Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer's disease. Am J Pathol. 1991 Jun;138(6):1423–1435. [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Bell D. S., Podlisny M. B., Price D. L., Cork L. C. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987 Feb 20;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Molecular pathology of amyloidogenic proteins and the role of vascular amyloidosis in Alzheimer's disease. Neurobiol Aging. 1989 Sep-Oct;10(5):387–395. doi: 10.1016/0197-4580(89)90072-9. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Hirai S., Harigaya Y., Kawarabayashi T., Yamaguchi H. The amyloid beta-protein precursor is localized in smooth muscle cells of leptomeningeal vessels. Brain Res. 1990 Oct 15;530(1):113–116. doi: 10.1016/0006-8993(90)90665-x. [DOI] [PubMed] [Google Scholar]
- Störkel S., Bohl J., Schneider H. M. Senile amyloidosis: principles of localization in a heterogeneous form of amyloidosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(2):145–161. doi: 10.1007/BF02890166. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Ghiso J., Timmers W. F., Giaccone G., Bugiani O., Frangione B. Coexistence of Alzheimer's amyloid precursor protein and amyloid protein in cerebral vessel walls. Lab Invest. 1990 Jun;62(6):761–767. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T., Mimori Y., Nakamura S., Kameyama M. A micromethod for the isolation of large and small microvessels from frozen autopsied human brain. J Neurochem. 1987 Dec;49(6):1796–1800. doi: 10.1111/j.1471-4159.1987.tb02438.x. [DOI] [PubMed] [Google Scholar]
- Yang G. C., Gallo G. R. Protein A-gold immunoelectron microscopic study of amyloid fibrils, granular deposits, and fibrillar luminal aggregates in renal amyloidosis. Am J Pathol. 1990 Nov;137(5):1223–1231. [PMC free article] [PubMed] [Google Scholar]