LETTER
Despite multiple efforts at drafting a general guideline or consensus document, the major international orthopedic centers and the orthopedic community in general are still searching for a definitive, comprehensive, and robust approach for the diagnosis and management of prosthetic joint infections (PJIs), although in the recent past, many efforts have been made to establish uniform criteria and a worldwide accepted definition (1–3). Based on an initiative from the International Society of Orthopaedic Centers (ISOC) group during the meeting in Hamburg, Germany, in 2013, it was decided to send out a questionnaire in order to document the present microbiological routine used for the diagnosis of PJIs.
In 2013, orthopedics departments at each of the 20 centers within the ISOC were invited to participate in a self-administered questionnaire in order to define the present microbiological routine used for the diagnosis of PJIs. Answers collected from each ISOC unit have been aggregated and presented in Mexico during the 6th ISOC meeting in October 2014.
Contact persons of the survey were members of the ISOC. An item-based questionnaire was constructed on the bases of a literature search and according to the last Musculoskeletal Infection Society suggestions after the meeting in Philadelphia in 2013 (3).
The items were grouped into the following four main areas: (i) infection microbiological guideline adherence, (ii) sample collection, (iii) methodology, and (iv) internal/external lab.
The final questionnaire for this study contained a total of 21 items, with 11 closed questions and 10 questions for free-text answers (Table 1).
TABLE 1.
General information about questionnaire provisiona
| Question | ISOC member reply | No. (%) |
|---|---|---|
| 1.What cutoff levels of ESRb and CRPc do you consider? | ESR, >40 mm/h; CRP, >1 mg/dl | 5 (31.3) |
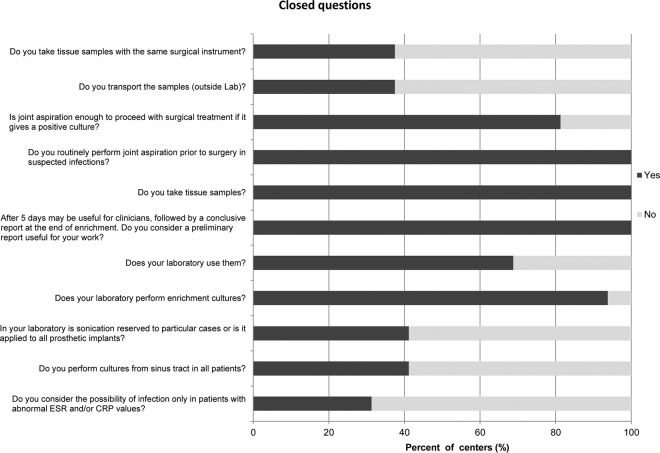
| 2.Do you consider the possibility of infection only in patients with abnormal ESR and/or CRP values? | Yes | 5 (31.3) |
| 3.What cutoff value for WBCd count and neutrophils percentage in joint aspirates do you consider suggestive for PJIs? | >1,700 cells/μl, >65% | 4 (23.5) |
| 4.Do you perform cultures from sinus tract in all patients? | Yes | 7 (41.2) |
| 5.How many samples do you usually send for cultures? | 3–5 | 8 (50.0) |
| 6.In your laboratory, is sonication reserved for particular cases, or is it applied to all prosthetic implants? | Yes | 7 (41.2) |
| 7.If yes, for which particular samples is sonication reserved? | ||
| 8.Does your laboratory perform enrichment cultures? | Yes | 15 (93.8) |
| 9.If yes, for how many days? | 15 | 9 (52.9) |
| 10.What is, in your opinion, an acceptable turnaround time? | 2–3 days | 9 (35.2) |
| 11.What is your opinion about the use of molecular methods? | ||
| 12.Does your laboratory use them? | Yes | 11 (68.8) |
| 13.If yes, what kind of molecular methods are used? | Real-time PCR | 7 (43.8) |
| 14.It is advisable to report isolation of each microorganism as soon as possible, also indicating if microbial growth has been observed on agar plates or after broth enrichment. A preliminary report for negative samples after 5 days may be useful for clinicians, followed by a conclusive report at the end of enrichment (after 15 days). Do you consider a preliminary report useful for your work? | Yes | 16 (100) |
| 15.Do you take tissue samples? | Yes | 16 (100) |
| 16.Do you routinely perform joint aspiration prior to surgery in suspected infections? | Yes | 11 (68.8) |
| 17.If yes, when? | 2–4 wk | 4 (25.0) |
| 18.Is joint aspiration enough to proceed with surgical treatment if it gives a positive culture? | Yes | 13 (81.3) |
| 19.Do you transport the samples (outside lab)? | Yes | 6 (37.5) |
| 20.Do you take tissue samples with the same surgical instrument? | Yes | 6 (37.5) |
| 21.When are samples cultured? | Within 2 h | 9 (52.9) |
n = 21; results are expressed as a percentage of the total number of answers per item.
ESR, erythrocyte sedimentation rate.
CRP, C-reactive protein.
WBC, white blood cell.
The survey presented here showed that concordance has reached a low-medium level in the centers analyzed by the ISOC (>80% concordance in only 4 [19%] of the surveys) and that the methodology, approaches, and strategies used are quite different at the different centers. Sample collection, transportation, and, in many cases, processing methodology are quite different. Biases of this survey can, of course, intrinsically occur when data are collected by this methodology (Table 1 and Fig. 1). In addition, the questions used can provide only a brief representation of the different situations in individual disciplines at the various hospitals. Further, centers answered questions regarding the microbiological routines they use for the diagnosis of PJIs, but within the study, there was no assessment of how closely centers follow their own guidelines. It is recognized that knowledge of guidelines does not necessarily always match actual practice.
FIG 1.
Closed questions. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
The aims for the next years should include fostering a survey with a constant dialog between the medical centers for addressing common problems and the development of a database with different quality-tested tools that can be accessed by all centers.
This study shows that the survey has gained a firm place in the ISOC meeting. Many institutions should grasp the potential and value of a good infrastructure in this field. However, the distribution and promotion of the survey are inhomogeneous. This survey should be repeated to document further developments and even extended internationally to compare more countries and to discover potential for future cooperation.
(These data were presented at the 6th meeting of the ISOC, Mexico City, Mexico, 23 to 25 October 2014.).
REFERENCES
- 1.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 2.Parvizi J, Della Valle CJ. 2010. AAOS clinical practice guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg 18:771–772. [DOI] [PubMed] [Google Scholar]
- 3.Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Drago L, Marston SB, Meermans G, Perez J, Ploegmakers JJ, Rosenberg A, Simpendorfer C, Thomas P, Tohtz S, Villafuerte JA, Wahl P, Wagenaar FC, Witzo E. 2014. Diagnosis of periprosthetic joint infection. J Arthroplasty 29(2 Suppl):77–83. doi: 10.1016/j.arth.2013.09.040. [DOI] [PubMed] [Google Scholar]