Abstract
Lyme disease prevails as the most commonly transmitted tick-borne infection in the United States, and serologic evaluation for antibodies to Borrelia burgdorferi remains the recommended modality for diagnosis. This review presents a brief historical perspective on the evolution of serologic assays for Lyme disease and provides a summary of the performance characteristics for the currently recommended two-tiered testing algorithm (TTTA). Additionally, a recently proposed alternative to the traditional TTTA is discussed, and novel methodologies, including immuno-PCR and metabolic profiling for Lyme disease, are outlined.
INTRODUCTION
The 2014 statistics for Lyme disease (LD) in the United States are staggering; more than 30,000 confirmed or probable cases were reported to the Centers for Disease Control and Prevention (CDC), nearly 300,000 presumed cases went unreported, and approximately 2.4 million specimens were submitted for LD testing with an associated cost of approximately $492 million (1, 2). Since endorsement of the two-tiered testing algorithm (TTTA) for diagnosing LD by the CDC, the National Institutes of Health, the Infectious Diseases Society of America, and other health agencies in 1995, 51 assays from more than 20 manufacturers have received FDA clearance for this purpose. Notably, while the detection of numerous infectious agents and syndromes has improved with the advent of molecular-based assays, many of which have received FDA clearance, and as the field of clinical microbiology enters the “-omics” realm of diagnostic testing, all of the available in vitro diagnostic products for LD remain based on serologic detection of antibodies to Borrelia burgdorferi sensu stricto (herein referred to as B. burgdorferi).
This review provides a brief historical perspective on the evolution of serologic methods for LD diagnosis, discusses performance characteristics of the recommended TTTA, presents possible amendments to the current format, and concludes with a summary of recently described, alternative methods for the diagnosis of LD. For detailed discussion of other serologic assays and nonserologic techniques, including molecular methods and culture for B. burgdorferi, see previous reviews (3, 4).
FACTORS AFFECTING SEROLOGIC TEST PERFORMANCE FOR LYME DISEASE
The diagnostic accuracy of serologic assays is dependent on multiple factors, including the timing of specimen collection with respect to disease state, the kinetics of antibody expansion to the particular infectious agent, the selection of appropriate immunodominant target peptides or antigens, and the assay methodology, although this is not an exhaustive list.
Following transmission of B. burgdorferi by an infected Ixodes species tick, the innate and adaptive immune branches are stimulated. Early localized LD (stage 1) is classically defined as the presence of an expanding erythema migrans (EM) rash appearing at the tick bite site an average of 7 to 14 days (range, 3 to 32 days) after infection in up to 80% of individuals (5). EM is a direct result of released proinflammatory markers, inoculum dose, and strain pathogenicity. While humoral immunity is likewise stimulated at this stage, only 10% to 50% of patients with culture-confirmed early LD (i.e., EM rash of <7 days' duration) will have a detectable antibody response (3, 6). For this reason, serologic evaluation for antibodies to B. burgdorferi following removal of an attached tick or soon after an EM rash is noticed is not recommended; results are often negative and therefore of limited clinical utility. While convalescent testing following the completion of antimicrobial therapy may be performed to demonstrate seroconversion, some individuals may remain seronegative, presumably due to insufficient exposure of the humoral immune system to the spirochete (5, 7).
In the absence of treatment, infection with B. burgdorferi can progress to early disseminated disease (stage 2) weeks to months following transmission and is characterized most commonly by neurologic manifestations (e.g., meningitis, cranial neuropathy, and radiculoneuropathy) or, rarely, carditis (e.g., atrioventricular heart block) (3). Late LD (stage 3) typically occurs months following infection, and in the United States, patients most often present with intermittent or persistent arthritis in one or more large joints. Importantly, the humoral immune response progressively matures as the infection develops, and as a result, the clinical sensitivity of serologic assays during these later stages of disease is improved over that of testing at earlier time points.
SEROLOGIC TESTING FOR LYME DISEASE: A HISTORICAL PERSPECTIVE
The TTTA for LD emerged from a need to standardize the testing methods used and the interpretive criteria applied toward those tests. Before 1995, the methods used to detect B. burgdorferi-specific antibodies were quite varied and included enzyme-linked immunosorbent assays (ELISAs) based on spirochete whole-cell sonicate (WCS) material, partially purified or recombinant proteins from different B. burgdorferi strains, indirect immunofluorescence assays (IFAs), and Western blot (WB) analysis to detect total or individual IgM and IgG class antibodies. Each of these assays had their own unique interpretive criteria. Despite FDA clearance of many of these assays, published proficiency testing studies revealed significant result heterogeneity between the different commercially available kits and, perhaps most worrisome, appreciable intralaboratory variability for duplicate samples. One such study demonstrated that, among the 45 participating laboratories, up to 55% failed to detect antibodies to B. burgdorferi in sera from patients with clinically characterized LD who were seropositive according to a reference IFA (8). Precision was likewise shown to be poor; one laboratory documented a coefficient of variation of more than 120% for a sample tested in triplicate, and another proficiency sample tested across the 45 laboratories had a reproducibility rate of only 27%.
A number of studies were subsequently undertaken to better understand the dominant antigenic determinants of B. burgdorferi and to better define the kinetics of the humoral immune response. Two seminal studies emerged during this period. These studies evaluated ELISA methodologies using WCS material, but from different B. burgdorferi strains, and proposed specific IgM and IgG WB interpretive criteria. The first of these studies was by Dressler et al. (9), who used sera from patients with clinically characterized LD and control patients (e.g., diagnosed with infections or syndromes resembling LD) to determine the optimal WB banding patterns for IgM and IgG class antibodies to B. burgdorferi. They established that, for early LD, IgM reactivity to at least two of eight B. burgdorferi antigens led to a sensitivity and specificity of 32% and 100%, respectively, and that IgG reactivity to at least 5 of 10 antigens provided high diagnostic accuracy for LD in patients with at least 1 week of symptoms (sensitivity, 83%; specificity, 95%). The second study, by Engstrom et al., involved 55 patients on antibiotic therapy for clinically characterized LD who were serially sampled and monitored for antibody expansion over a 1-year period. This group determined that reactivity at two of three antigens in the IgM WB analysis improved sensitivity to 58.5% during early LD, and IgG seroreactivity at two of five bands provided optimal sensitivity during early (54.6%) and late (100%) LD (10). The Dressler et al. and Engstrom et al. publications also documented higher specificities in the IgM and IgG WB analyses compared with those in their respective WCS ELISAs, and they both agreed that, despite optimization of WB banding patterns, false-positive results can still occur in healthy individuals and in patients with diseases mimicking LD (e.g., syphilis, rheumatoid arthritis, systemic lupus erythematosus, etc.).
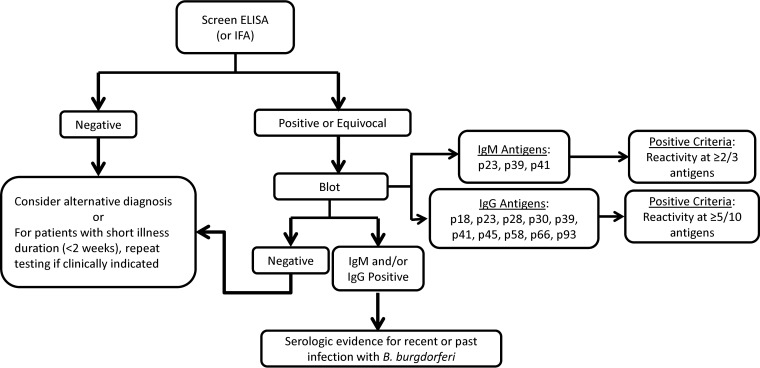
Collectively, however, these two studies laid the foundation for the currently recommended TTTA for detection of antibodies to B. burgdorferi (Fig. 1). The TTTA begins with a first-tier screening ELISA for IgM and IgG class antibodies (either separately or together) or IFA (uncommonly used). Unless testing was performed soon after symptom onset or exposure, no further testing is warranted for patients with negative screening results. Specimens with a positive or equivocal result in the first-tier assay require confirmatory testing by B. burgdorferi-specific IgM and IgG WB analysis to ensure specificity. An important caveat to this algorithm is that, for patients with symptom duration of more than 30 days, IgM WB testing should not be performed or, if performed, the result should not be considered, because IgM seroreactivity can remain detectable for months to years following disease resolution (11). Interpretive criteria for results of WB analysis derive from a combination of recommendations from Dressler et al. and Engstrom et al.; IgM WB positivity adheres to the Engstrom et al. criteria, which require antibody reactivity to at least two of three antigens (i.e., p23 [OspC], p39 [BmpA], and p41 [FlaB]), whereas IgG positivity is based on the Dressler et al. criteria, which require antibody reactivity to at least five of 10 bands (i.e., p18, p23, p28, p30, p39, p41, p45, p58, p66, and p93). Finally, although offered through certain Lyme disease specialty laboratories, Western blot analysis for IgA class antibodies to B. burgdorferi has not been well studied, and the clinical utility of testing for this analyte remains unclear.
FIG 1.
Diagram of the two-tiered testing algorithm including interpretation and IgM/IgG blot positivity criteria for the United States. For individuals with more than 30 days of symptoms, IgM Western blot analysis should not be performed, or, if performed, the results should not be used to guide clinical decisions.
While the following discussion focuses on the diagnosis of systemic Lyme disease, it is important to note that for patients with suspected Lyme neuroborreliosis, the TTTA does not apply to cerebrospinal fluid (CSF). Instead, diagnosis is based on the presence of compatible clinical features and demonstration of B. burgdorferi-specific intrathecal antibody synthesis (12, 13). Briefly, CSF and normalized serum are tested by a B. burgdorferi-specific ELISA, and intrathecal antibody production is established by calculating an antibody index (AI) from the ELISA results. The AI value allows for discrimination between true intrathecal antibody production and passive transfer of B. burgdorferi-specific antibodies across the blood-brain barrier or blood contamination of CSF due to a traumatic lumbar puncture.
PERFORMANCE CHARACTERISTICS OF THE CURRENT TWO-TIERED TESTING ALGORITHM
First- and second-tier serologic assays for the detection of antibodies to LD have undergone significant modification over the past 10 to 15 years that led to improved clinical performance compared with that of the previous methods.
ELISAs using B. burgdorferi WCS material continue to be performed in many clinical laboratories today. A recent study by Wormser et al. evaluated more than 500 patients with clinically characterized LD and more than 2,000 control subjects and showed that, despite high overall sensitivity for LD (75% for stage 1 and 98% for stages 2/3), when used alone, the WCS ELISA can lead to falsely reactive results in approximately 4% of healthy individuals and in nearly 11% of patients presenting with symptoms similar to those of LD (Table 1) (14). This lack of specificity is due primarily to antibody cross-reactivity with proteins in the WCS ELISA that are conserved between B. burgdorferi and other, more commonly encountered bacteria (e.g., heat shock [p66] and flagellar [p41] proteins). While supplemental testing of positive and equivocal WCS ELISA results by WB analysis, as recommended by the TTTA, improves specificity to greater than 99%, sensitivity decreases to 35.2% and 77.3% to 96% for early and later stages of LD, respectively.
TABLE 1.
Select studies that evaluated the performance characteristics of the WCS, VlsE, C6, and pepC10 immunoassays alone or in combination with supplemental Western blot testing
| Assaya | Sensitivity (%) |
Specificity (%) |
Reference | ||
|---|---|---|---|---|---|
| Early (stage 1) | Late (stages 2, 3) | Healthy donorsb | Patients with non-LD infections or conditions | ||
| WCS ELISA | 74.9 | 97.7, 98.4 | 96.4 | 89.3 | 14 |
| WCS ELISA + WB | 35.2 | 77.3, 95.9 | 99.5 | 99.2 | 14 |
| C6 ELISA | 66.5 | 88.6, 98.4 | 98.8 | 99.5 | 14 |
| C6 ELISA + WB | 34.5 | 75, 95.1 | 99.5 | 99.5 | 14 |
| VlsE CIAc | 69.8 | 100 | 99.5 | 93.7 | 16 |
| pepC10 kELISA | 47.3 | 46.1, 10.3 | 100 | 98.0 | 20 |
| VlsE/pepC10 kELISA | 67.2 | 88.5, 94.1 | 99.2 | 96.7 | 20 |
WCS, whole-cell sonicate; VlsE, variable major protein (Vmp)-like sequence, expressed; WB, Western blot; ELISA, enzyme linked immunosorbent assay; CIA, chemiluminescent immunoassay; kELISA, kinetic ELISA.
Data from healthy donors from regions in which Lyme disease is endemic and from those in which it is not endemic were combined.
Lyme disease stages 2 and 3 were not separated out in this study.
In an effort to improve performance characteristics of the first-tier screening assay and the TTTA overall, contemporary ELISAs were developed using select recombinant proteins and/or select synthetic peptides from immunodominant regions within those proteins that are specific to and conserved among the B. burgdorferi sensu lato (sl) complex members (e.g., B. burgdorferi, Borrelia garinii, and Borrelia afzelii). While many different proteins have been investigated, the two most commonly targeted ones are both expressed on the surface of B. burgdorferi and include the VlsE (variable major protein [Vmp]-like sequence expressed; 35 kDa) and OspC (outer-surface protein C; 23 kDa) proteins.
VlsE is composed of alternating variable (VR1 to VR6) and invariable (IR1 to IR6) regions, flanked by conserved domains at both the amino and carboxy termini (15). This lipoprotein undergoes extensive recombination at the variable regions, likely as a means of immune evasion, is expressed by the spirochete only after transmission to the mammalian host, and induces a strong and specific immune response during the course of infection. A commercially available, FDA-cleared chemiluminescent immunoassay (CLI) for total IgM and IgG antibodies to recombinant VlsE (rVlsE) was evaluated by Ledue et al. using two CDC panels of well-characterized sera from patients with LD (n = 102) and separate, matched controls (n = 807). While the authors reported a high sensitivity (70% to 100%) for each stage of LD and improved specificity in sera from healthy blood donors (99.5%) compared with that in the WCS ELISA alone, the accuracy of the rVlsE CIA approached only 94% among individuals with other bacterial or viral infections (Table 1) (16).
Among the different VlsE IRs, IR6 was identified as an immunodominant epitope for B. burgdorferi sl, and an ELISA using a recombinant 26-amino acid peptide of this region, referred to as C6, was cleared by the FDA for use in 2003. The C6 peptide primarily elicits an IgG class antibody response, but when used as a standalone assay and compared with the TTTA using a WCS ELISA (WCS TTTA), the C6 ELISA showed significantly improved sensitivity for early LD (66.5% versus 35.2%, respectively; P < 0.001) and high sensitivity for later stages of LD (Table 1). Although the specificity of the C6 ELISA alone is similar to that of the WCS TTTA for patients with a non-LD infection or condition (99.5% versus 99.2%, respectively), the WCS TTTA significantly outperforms the C6 ELISA among healthy blood donors (99.5% versus 98.8%, respectively; P = 0.002) (14, 17). While a difference in specificity of 0.8% may appear trivial, when considering the volume of serologic tests that are performed annually, the use of the C6 ELISA alone would lead to a large number of inaccurate results in a field that is already infiltrated with diagnostic assays of questionable validity (18). Use of the C6 ELISA as part of a TTTA provides a specificity that is similar to that of the WCS TTTA, but not surprisingly, sensitivity, particularly for early LD, is lost (34.5%) (Table 1).
The second commonly targeted antigen, OspC, is an immunodominant protein required for the transmission of LD-associated Borrelia from the tick vector to the mammalian host. Because OspC is expressed on the surface of B. burgdorferi before infection, it is available for immune stimulation sooner than VlsE (19). OspC is often used for B. burgdorferi strain typing due to the high level of sequence variability, but it also exhibits a well-conserved 10-amino acid peptide sequence at the carboxy terminus (pepC10) (20). Evaluation of a kinetic ELISA using pepC10 as the sole antigen showed low sensitivity for early (47.3%) and later (10.3% to 46.1%) stages of LD, likely because only IgM class antibodies were targeted by this assay. Notably, however, combination of pepC10 with rVlsE in a single ELISA significantly improved performance to 67.2% and 88.5% to 94.1% for early and later stages of Lyme disease, respectively (Table 1) (20). The specificities of the WCS TTTA and the VlsE/pepC10 kinetic ELISA were similar (>98%), and a commercially available ELISA using these two antigens (rVlsE1/pepC10) was cleared by the FDA in 2013. Due to its ability to stimulate an early immune response, OspC continues to be a protein of interest with respect to LD diagnostics. A novel peptide region of OspC, OspC1, was recently identified, and preliminary data suggest it results in improved performance for the detection of early LD compared with that of pepC10 alone (62.1% versus 49.0%, respectively) (21). Further studies are needed, however, to confirm these findings and to better define a role for these antigens as markers of LD.
Despite the development and optimization of new first-tiered screening assays, the specificities of these contemporary ELISAs do not reach that of the TTTA; therefore, supplemental WB analysis is still required. However, these second-tiered assays are associated with a number of limitations, the most important of which is the decrease in sensitivity observed for patients with early LD, an issue that is especially problematic for individuals who present with an atypical EM rash or lack dermatologic evidence of LD entirely. This phenomenon can be attributed in part to differential protein expression between B. burgdorferi isolated from a mammalian host and in vitro cultured B. burgdorferi spirochetes, which are the form used for WB production. For example, VlsE is largely suppressed in cultured B. burgdorferi, and therefore, antibodies to VlsE detected by a screening ELISA may be unconfirmed by WB analysis (22). The reliance on WBs is further complicated by the subjectivity associated with visual interpretation of band intensities, which is particularly challenging for IgM WBs. Due to the requirement that only two of three bands be present for an IgM WB to be considered positive, and one of these bands can be the well-conserved flagellar FlaB (p41) protein, IgM blots are often overread, which leads to a high false-positivity rate (23). These limitations, alongside the generally laborious nature of the methodology, have led to nearly 75% of laboratories that perform LD testing to use a reference laboratory for supplemental WB analysis (17). Some of these challenges have been overcome with the implementation of automated instruments for the blotting process, the availability of software for densitometric measurement of band intensity to provide more objective band interpretation, and the development of B. burgdorferi immunoblots (i.e., nitrocellulose membranes onto which recombinant antigens are “stamped”) to minimize the background and nonspecific reactivity associated with classic WBs (24). However, these improvements are associated with significant costs that may be insurmountable by local hospital laboratories.
AN ALTERNATIVE TTTA?
In an effort to overcome these obstacles, a modified TTTA based on two sequential ELISAs, rather than supplemental testing by immunoblot analysis, was proposed by multiple groups. Most recently, Branda et al. evaluated a TTTA using a WCS ELISA and the C6 ELISA as the first- and second-tier assays, respectively (2-ELISA TTTA). Compared with the traditional TTTA (WCS ELISA and WB analysis), confirmatory testing of positive or equivocal WCS ELISA results by the C6 assay yielded improved sensitivities in patients with stage 1 LD (42.1% versus 52.6%, respectively) and in those with stage 2 LD (73% versus 100%, respectively) and equivalent performance in patients with late LD (Table 2) (17). Intriguingly, the authors reported identical specificities for the 2-ELISA and the traditional TTTAs in healthy blood donors (99.4%) and among patients with a non-LD associated condition (100%) (Table 2). Finally, using hypothetical LD prevalence rates ranging from 0.5% to 2%, the authors showed that the 2-ELISA algorithm had positive predictive values (41% to 74%) that were consistently higher than those of either the traditional TTTA (36% to 70%) or the C6 ELISA alone (18% to 47%) (17). This same group subsequently showed that this 2-ELISA approach (WCS/C6) is 27.1% to 44.0% less expensive than TTTAs using a supplemental immunoblot assay (25). Collectively, these data suggest that a 2-ELISA testing algorithm provides improved clinical performance and is a more cost-effective alternative to the traditional TTTA for the diagnosis of LD.
TABLE 2.
Comparison of the traditional TTTA to a 2-EIA TTTA and the C6 ELISA alone in sera from patients with well-characterized Lyme diseasea
| Testing algorithm | Sensitivity (%) |
Specificity (%) |
|||
|---|---|---|---|---|---|
| Stage 1 (n = 114) | Stage 2 (n = 26) | Stage 3 (n = 29) | Healthy donorsb (n = 1,246) | Patients with a non-LD infection or condition (n = 54) | |
| Traditionalc | 42.1 | 73.1 | 100 | 99.4 | 100 |
| C6 ELISA alone | 56.1 | 100 | 100 | 98.4 | 98.1 |
| 2-ELISAd | 52.6 | 100 | 100 | 99.4 | 100 |
Adapted from reference 17.
Data from healthy donors from regions in which Lyme disease is endemic and from those in which it is not endemic were combined.
Traditional TTTA, WCS ELISA followed by Western blot analysis.
2-ELISA, WCS ELISA followed by C6-ELISA.
NOVEL APPROACHES FOR LD DETECTION
Improvements in the diagnostic accuracy of immunoassays for the detection of LD continue to be pursued through the development of multiplex assays for “broad-range” antibody detection, the combination of serologic and molecular techniques, and the identification of specific metabolic biosignatures. For some of these methods, promising proof-of-principle studies have been published, and their results are reviewed briefly here.
Over the past decade, it has become apparent that antibody detection of a single or even a few B. burgdorferi proteins by means of a standard ELISA does not provide sufficient sensitivity for the diagnosis of early LD. To overcome this, multiple groups have identified additional B. burgdorferi sl antigens and used them as part of larger multiplex panels on contemporary immunoassay platforms for antibody detection. Lahey et al. identified 10 unique B. burgdorferi antigens, including previously described (e.g., OspC and FlaB) and novel peptides, and developed a multiplex panel using Luminex technology (26). With the requirement that reactivity be present for at least two of the 10 antigens for a sample to be considered positive, this group showed equivalent specificity (100%) among healthy subjects and significantly improved sensitivity for early LD compared with that of the traditional TTTA (87.5% versus 67.5%, respectively; P < 0.05).
In an effort to improve the limit of detection for ELISAs, an immuno-PCR (iPCR) assay was recently developed and evaluated for the detection of antibodies to the B. burgdorferi C6 peptide (27). iPCR harnesses the amplification property of PCR to increase the sensitivity of standard ELISAs by 100- to 10,000-fold; this technique has already been developed for the detection of multiple bacterial and viral antigens (28). Briefly, the methodology for the C6 iPCR study is as follows. Synthetic C6 peptide is coupled to magnetic beads, which are sequentially incubated with human serum and a secondary, reporter antibody. The reporter antibody is conjugated to an oligonucleotide tag via a streptavidin-biotin bond, and the detection of host antibody to C6 is achieved by real-time PCR of the oligonucleotide tag directly on this immune complex (i.e., magnetic bead-C6 antigen-host antibody-reporter antibody-oligonucleotide tag). The authors reported strong correlation between the C6 ELISA and the iPCR assay and higher sensitivity of the iPCR among 18 patients with early LD (58% versus 75%, respectively). This novel approach and encouraging preliminary data warrant further analysis with studies in larger cohorts.
Finally, as an alternative to classic antibody detection for LD, the CDC, along with multiple academic and clinical collaborators, recently evaluated the applicability of metabolomics, defined as the evaluation of low-molecular-weight (<1,500-Da) biomolecules, for the diagnosis of early LD (29). An individual's baseline metabolic profile can be altered by a variety of environmental stressors, and the detection of changes to this baseline may lay the foundation for the development of a novel diagnostic tool for infections, including LD. Using liquid chromatography mass spectrometry and statistical modeling, Molins et al. defined a metabolic profile of 44 biosignatures, primarily lipid and lipophilic molecules, present in patients with early LD and compared the performance of this panel with that of the traditional and 2-ELISA TTTAs. The metabolomics assay outperformed both TTTAs with respect to sensitivity (88% versus 43% to 48%, respectively; P < 0.0001) and showed statistically equivalent specificities among healthy controls and individuals with a non-LD condition (29). Although certain challenges with the methodology exist, on the basis of the preliminary data, metabolomic profiling appears to hold promise as a future tool for the diagnosis of LD.
Biography

Elitza (Elli) S. Theel graduated with a B.S. in biology from the University of Notre Dame in Notre Dame, IN, and earned her Ph.D. in medical microbiology and immunology at the University of Wisconsin-Madison in Madison, WI, in 2010. She completed a postdoctoral clinical microbiology fellowship in 2012 at the Mayo Clinic in Rochester, MN, and remained on staff as the director of the Infectious Disease Serology Laboratory and as an assistant professor of laboratory medicine and pathology at the Mayo Clinic. Her research interests revolve around developing and evaluating serologic markers for the detection of difficult-to-cultivate or otherwise detecting infectious agents, including Borrelia burgdorferi and common and uncommon arboviruses.
REFERENCES
- 1.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis 21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. 2005. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alby K, Capraro GA. 2015. Alternatives to serologic testing for diagnosis of Lyme. Dis Clin Lab Med 35:815–825. doi: 10.1016/j.cll.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro ED. 2014. Clinical practice. Lyme disease. N Engl J Med 370:1724–1731. doi: 10.1056/NEJMcp1314325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguero-Rosenfeld ME, Nowakowski J, Bittker S, Cooper D, Nadelman RB, Wormser GP. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol 34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steere AC, McHugh G, Damle N, Sikand VK. 2008. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 47:188–195. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakken LL, Case KL, Callister SM, Bourdeau NJ, Schell RF. 1992. Performance of 45 laboratories participating in a proficiency testing program for Lyme disease serology. JAMA 268:891–895. doi: 10.1001/jama.1992.03490070073045. [DOI] [PubMed] [Google Scholar]
- 9.Dressler F, Whalen JA, Reinhardt BN, Steere AC. 1993. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 10.Engstrom SM, Shoop E, Johnson RC. 1995. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol 33:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference of Serologic Diagnosis of Lyme. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 12.Koedel U, Fingerle V, Pfister HW. 2015. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol 11:446–456. doi: 10.1038/nrneurol.2015.121. [DOI] [PubMed] [Google Scholar]
- 13.Halperin JJ. 2015. Nervous system Lyme. Dis Clin Lab Med 35:779–795. doi: 10.1016/j.cll.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Wormser GP, Schriefer M, Aguero-Rosenfeld ME, Levin A, Steere AC, Nadelman RB, Nowakowski J, Marques A, Johnson BJ, Dumler JS. 2013. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis 75:9–15. doi: 10.1016/j.diagmicrobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra A, Latov N, Wormser GP, Marques AR, Alaedini A. 2011. Epitope mapping of antibodies to VlsE protein of Borrelia burgdorferi in post-Lyme disease syndrome. Clin Immunol 141:103–110. doi: 10.1016/j.clim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledue TB, Collins MF, Young J, Schriefer ME. 2008. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin Vaccine Immunol 15:1796–1804. doi: 10.1128/CVI.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. 2011. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 53:541–547. doi: 10.1093/cid/cir464. [DOI] [PubMed] [Google Scholar]
- 18.Fallon BA, Pavlicova M, Coffino SW, Brenner C. 2014. A comparison of Lyme disease serologic test results from 4 laboratories in patients with persistent symptoms after antibiotic treatment. Clin Infect Dis 59:1705–1710. doi: 10.1093/cid/ciu703. [DOI] [PubMed] [Google Scholar]
- 19.Padula SJ, Dias F, Sampieri A, Craven RB, Ryan RW. 1994. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J Clin Microbiol 32:1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacon RM, Biggerstaff BJ, Schriefer ME, Gilmore RD Jr, Philipp MT, Steere AC, Wormser GP, Marques AR, Johnson BJ. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis 187:1187–1199. doi: 10.1086/374395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnaboldi PM, Seedarnee R, Sambir M, Callister SM, Imparato JA, Dattwyler RJ. 2013. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early Lyme disease. Clin Vaccine Immunol 20:474–481. doi: 10.1128/CVI.00608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norris SJ, Carter CJ, Howell JK, Barbour AG. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun 60:4662–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. 2012. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 18:1236–1240. doi: 10.1111/j.1469-0691.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 24.Binnicker MJ, Jespersen DJ, Harring JA, Rollins LO, Bryant SC, Beito EM. 2008. Evaluation of two commercial systems for automated processing, reading, and interpretation of Lyme borreliosis Western blots. J Clin Microbiol 46:2216–2221. doi: 10.1128/JCM.00200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wormser GP, Levin A, Soman S, Adenikinju O, Longo MV, Branda JA. 2013. Comparative cost-effectiveness of two-tiered testing strategies for serodiagnosis of Lyme disease with noncutaneous manifestations. J Clin Microbiol 51:4045–4049. doi: 10.1128/JCM.01853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahey LJ, Panas MW, Mao R, Delanoy M, Flanagan JJ, Binder SR, Rebman AW, Montoya JG, Soloski MJ, Steere AC, Dattwyler RJ, Arnaboldi PM, Aucott JN, Robinson WH. 2015. Development of a multiantigen panel for improved detection of Borrelia burgdorferi infection in early Lyme disease. J Clin Microbiol 53:3834–3841. doi: 10.1128/JCM.02111-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halpern MD, Jain S, Jewett MW. 2013. Enhanced detection of host response antibodies to Borrelia burgdorferi using immuno-PCR. Clin Vaccine Immunol 20:350–357. doi: 10.1128/CVI.00630-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niemeyer CM, Adler M, Wacker R. 2005. Immuno-PCR: high sensitivity detection of proteins by nucleic acid amplification. Trends Biotechnol 23:208–216. doi: 10.1016/j.tibtech.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Molins CR, Ashton LV, Wormser GP, Hess AM, Delorey MJ, Mahapatra S, Schriefer ME, Belisle JT. 2015. Development of a metabolic biosignature for detection of early Lyme disease. Clin Infect Dis 60:1767–1775. doi: 10.1093/cid/civ185. [DOI] [PMC free article] [PubMed] [Google Scholar]