Abstract
Human cytomegalovirus (CMV) infection is a major cause of congenital infection leading to birth defects and sensorineural anomalies, including deafness. Recently, cell-mediated immunity (CMI) in pregnant women has been shown to correlate with congenital CMV transmission. In this study, two interferon gamma release assays (IGRA), the CMV enzyme-linked immunosorbent spot (ELISPOT) and CMV QuantiFERON assays, detecting CMV-specific CMI were compared. These assays were performed for 80 CMV-infected (57 primarily and 23 nonprimarily) pregnant women and 115 controls, including 89 healthy CMV-seropositive pregnant women without active CMV infection, 15 CMV-seronegative pregnant women, and 11 seropositive or seronegative nonpregnant women. Statistical tests, including frequency distribution analysis, nonparametric Kruskal-Wallis equality-of-populations rank test, Wilcoxon rank sum test for equality on unmatched data, and lowess smoothing local regression, were employed to determine statistical differences between groups and correlation between the assays. The CMV ELISPOT and CMV QuantiFERON assay data were not normally distributed and did not display equal variance. The CMV ELISPOT but not CMV QuantiFERON assay displayed significant higher values for primarily CMV-infected women than for the healthy seropositive pregnant and nonpregnant groups (P = 0.0057 and 0.0379, respectively) and those with nonprimary infections (P = 0.0104). The lowess local regression model comparing the assays on an individual basis showed a value bandwidth of 0.8. Both assays were highly accurate in discriminating CMV-seronegative pregnant women. The CMV ELISPOT assay was more effective than CMV-QuantiFERON in differentiating primary from the nonprimary infections. A substantial degree of variability exists between CMV ELISPOT and CMV QuantiFERON assay results for CMV-seropositive pregnant women.
INTRODUCTION
Cytomegalovirus (CMV) represents a leading cause of congenital infections affecting about 0.7% live births (1–3). The clinical outcome of the congenital infection is variable and is associated with the maternal serostatus and time of onset of infection during pregnancy (4–6). Whenever clinically evident, CMV-induced damages include sensorineural hearing loss (SNHL), visual impairment, delayed psycho-motorial development, and retardation (7–9). Therapeutic interventions may include CMV hyperimmune immunoglobulin infusion (10–12); however, controversial effectiveness and safety issues have been suggested (13).
It has been recently shown that cell-mediated immunity (CMI) is involved in augmented risk of congenital CMV transmission, particularly when high maternal CMI responses are associated with low maternal CMV IgG avidity (14). In this study, two interferon gamma (IFN-γ) release assays (IGRA), the CMV enzyme-linked immunosorbent spot (ELISPOT) and CMV QuantiFERON assays, widely used to detect pathogen-specific CMI (15–21), were compared in a group of primarily and nonprimarily CMV-infected pregnant women and in a control group of healthy seropositive and seronegative pregnant and nonpregnant women without evidence of active CMV infection. Several characteristics differ between the CMV ELISPOT and CMV QuantiFERON assays. The CMV ELISPOT assay is made on a given number of peripheral blood mononuclear cells (PBMCs) (∼2 × 105), while the CMV QuantiFERON assay is performed on a defined volume of blood (∼1 ml). Moreover, the CMV ELISPOT assay detects both CD4+ and CD8+ T-cell responses, while the CMV QuantiFERON assay detects only CD8+ T-cell responses. The two assays may also differ on the antigen stimulus used: CMV pp65 (ppUL83) and/or IE1 peptide pools are widely used for their high immunogenicity (22–27). At present, the stimulus compositions of both assays are patent protected, and thus, the use of different peptide pool combinations may influence the interassay variability. Moreover, the HLA type may affect the efficacy of peptide pool antigen presentation (28) and thus the detection of pathogen specific immune response.
(The data in this article were presented in part at the Congenital CMV Conference, Brisbane, Australia, 2015.)
MATERIALS AND METHODS
Patients.
This study was conducted with 195 Caucasian women, including 57 primarily CMV-infected pregnant women, 23 nonprimarily (relapse or reinfection) CMV-infected pregnant women, 15 seronegative pregnant women, 4 seronegative nonpregnant women, 7 seropositive nonpregnant women, and 89 CMV-seropositive women without evidence of active infection. Active CMV infection is defined as the presence of detectable CMV DNA in blood or urine. The median age of the group was 32 years (range, 21 to 42). The patients' exclusion criteria were (i) any existing or acquired immunodeficiency and (ii) exhibition of primary CMV infection after the 20th week of gestation. Primary and nonprimary CMV infections were previously defined (14). For primarily infected pregnant women, the estimated timing of CMV infection occurred within a median of 6 weeks of gestation (range, 0 to 20) and the CMV ELISPOT and CMV QuantiFERON assays were performed within a median of 8 weeks (range, 2 to 17) after the CMV infection. For the nonprimary infections, it was not possible to determine the timing of reactivation or reinfection, and the two assays were performed within a median of 1 week (range, 1 to 4) after the detection of CMV DNA in maternal urine. The Padua General Hospital Ethical Committee approved the study.
CMV serology, CMV QuantiFERON and CMV ELISPOT tests, and detection of CMV DNA in blood and urine.
For the CMV serostatus determination, CMV IgM and CMV IgG (Siemens Immulite) were evaluated according to the manufacturer's instructions. Blood draws were performed at the same time for both assays. For the CMV ELISPOT assay, 10 ml of blood was collected in tubes containing sodium citrate, while for the CMV QuantiFERON assay, 3 ml of blood was collected in 3 tubes (positive and negative controls, CMV, ∼1 ml in each tube, according to the manufacturer's instructions). For the CMV ELISPOT assay (Autimmun Diagnostika), peripheral blood mononuclear cells (PBMCs) were Ficoll purified and used as previously described (29) and stimulated with CMV pp65 antigen (Autimmun Diagnostika) to assess the specific CMI. CMV QuantiFERON (Qiagen) tubes were kept overnight at 37°C, and samples were then processed according to the manufacturer's instructions.
The range of CMV ELISPOT assay responses was chosen to be 0 to 1,000 spots/2 × 105 PBMCs, since 1,000 spots saturated the ELISPOT well. The CMV QuantiFERON assay range is 0 to 10 IU of IFN-γ/ml of whole blood.
Statistical analysis.
The frequency analysis was used to assess the data distribution. The nonparametric Kruskal-Wallis equality-of-populations rank statistical test was employed to analyze the CMV ELISPOT and QuantiFERON assay groups. The Wilcoxon rank sum test for equality on unmatched data was used to analyze CMV ELISPOT and CMV QuantiFERON assay results under six conditions (pregnancy and nonpregnancy, CMV seropositivit and seronegativity, and primary and nonprimary infections). The nonparametric lowess local regression model was employed to compare the CMV ELISPOT and CMV QuantiFERON assays. P values of <0.05 were considered statistically significant. The software Stata 14.1 (StataCorp) was used for statistical analysis.
RESULTS
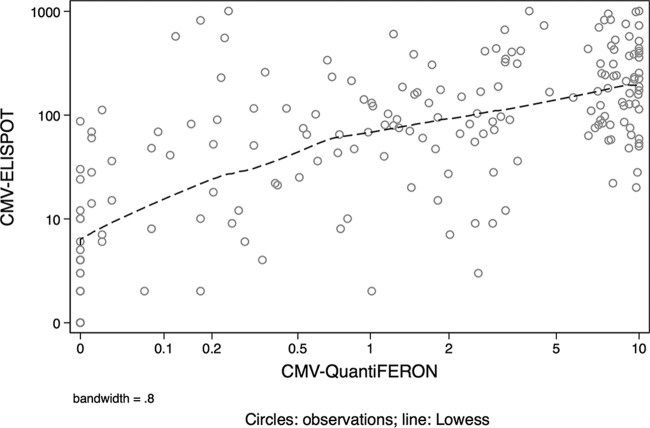
The CMV ELISPOT and CMV QuantiFERON assays were used to detect the CMI under six different conditions; subjects included 57 primarily and 23 nonprimarily CMV-infected pregnant women in a control group represented by 89 CMV-seropositive pregnant women, 15 CMV-seronegative pregnant women, 7 CMV-seropositive nonpregnant women, and 4 CMV-seronegative nonpregnant women (Table 1). The immune profile of each group is shown in a box-and-whisker graph reporting the median and interquartile range for the CMV ELISPOT and CMV QuantiFERON assays (Fig. 1A and B, respectively). The frequency distribution analysis revealed that the results of the two assays were not normally distributed and did not display equal variance (Fig. 2A and B, respectively). In particular, the CMV QuantiFERON assay results displayed a bimodal pattern of CMI. In order to compare the data, the nonparametric Kruskal-Wallis equality-of-populations rank statistical test was applied separately to CMV ELISPOT and CMV QuantiFERON groups. A statistically significant heterogeneity between the six clinical types was found (P = 0.0001 for both assays). Then a pairwise analysis using the Wilcoxon rank sum test for equality on unmatched data was performed between the six groups for both assays (Table 2). Interestingly, the CMV ELISPOT results were statistically significant for the following pairs: (i) primarily CMV-infected and nonpregnant seropositive subjects (P = 0.0379), (ii) primarily CMV-infected and CMV-seropositive pregnant subjects (P = 0.0057), and (iii) subjects with primary and nonprimary infections (P = 0.0104). When the same pairs were analyzed using the CMV QuantiFERON assay, there were no statistical differences. In order to explore the correlation of CMV ELISPOT with CMV QuantiFERON assay results on individual basis, a lowess local regression model was applied to the assays (Fig. 3). The graph bandwidth was 0.8.
TABLE 1.
Comparison of CMV-ELISPOT and CMV-QuantiFERON assays under different conditionsa
| Type | IGRA | No. of samples | Mean | SD | p25 | p50 | p75 |
|---|---|---|---|---|---|---|---|
| npreg, sero+ | CMV ELISPOT | 7 | 86.86 | 105.50 | 11 | 58 | 127 |
| CMV QuantiFERON | 7 | 4.71 | 4.40 | 0.61 | 3.25 | 9.72 | |
| npreg, sero− | CMV ELISPOT | 4 | 9 | 10.03328 | 2 | 6.5 | 16 |
| CMV QuantiFERON | 4 | 0 | 0 | 0 | 0 | 0 | |
| preg, sero+ | CMV ELISPOT | 89 | 168.17 | 227.95 | 35 | 81 | 187 |
| CMV QuantiFERON | 89 | 3.39 | 3.44 | 0.51 | 2.4 | 6.93 | |
| preg, sero− | CMV ELISPOT | 15 | 1.87 | 2.77 | 0 | 1 | 3 |
| CMV QuantiFERON | 14b | 0 | 0 | 0 | 0 | 0 | |
| Primary CMV infection | CMV ELISPOT | 57 | 273.28 | 264.72 | 65 | 170 | 411 |
| CMV QuantiFERON | 57 | 4.56 | 3.88 | 0.67 | 3.43 | 8.12 | |
| Nonprimary CMV infection | CMV ELISPOT | 23 | 108.70 | 111.07 | 20 | 79 | 129 |
| CMV QuantiFERON | 23 | 4.42 | 4.32 | 0.41 | 1.82 | 9.63 |
Conditions include pregnancy (preg), nonpregnancy (npreg), and CMV serostatus (sero+, seropositivity; sero−, seronegativity). p25, p50, and p75 indicate the 25th, 50th, and 75th percentiles, respectively.
One CMV-QuantiFERON sample is missing due to tube breakage during the processing step.
FIG 1.
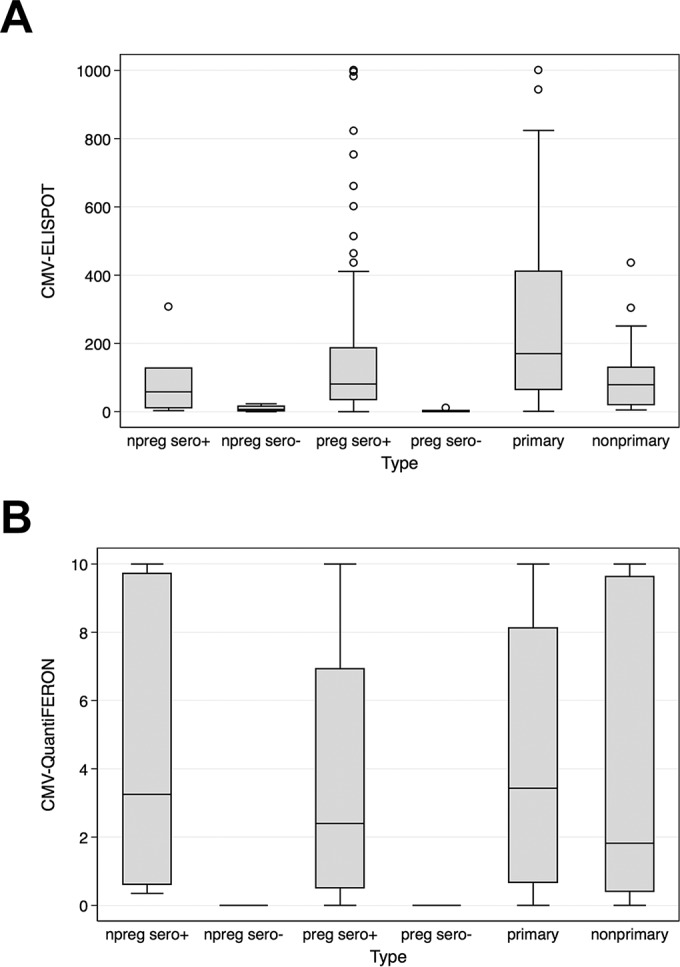
Box-and-whisker distribution of CMV ELISPOT (A) and CMV QuantiFERON (B) assay values under different conditions, including pregnancy (preg), nonpregnancy (npreg), and CMV seropositivity (sero+ or sero−). “Primary” and “nonprimary” refer to CMV infection. CMV ELISPOT assay values were limited to 1,000 spots/2 × 105 PBMCs, while CMV QuantiFERON assay values were limited to 10 IU of IFN-γ/ml. CMV viremia occurred prior to CMV ELISPOT and CMV QuantiFERON assay determination.
FIG 2.
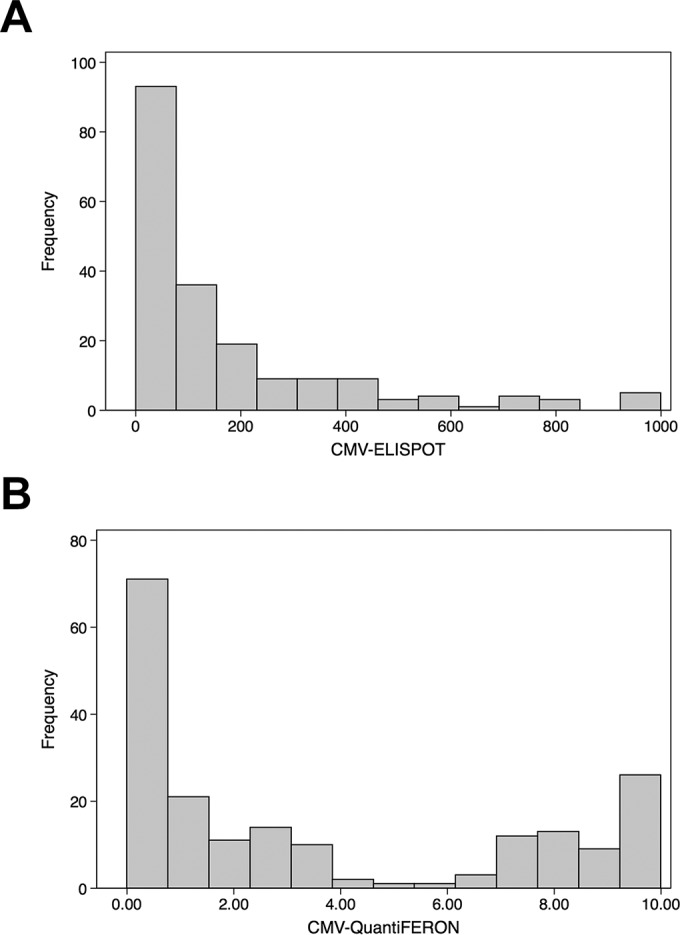
Frequency distribution of CMV ELISPOT (A) and CMV QuantiFERON (B).
TABLE 2.
Pairwise comparison between CMV-ELISPOT and CMV-QuantiFERON assays under different conditionsa
| Type pair |
P value for assay |
|
|---|---|---|
| CMV ELISPOT | CMV QuantiFERON | |
| npreg, sero− vs npreg, sero+ | NS | NS |
| preg, sero+ vs npreg, sero+ | NS | NS |
| preg, sero− vs npreg, sero+ | 0.0004 | 0.0000 |
| Primary infection vs npreg, sero+ | 0.0379 | NS |
| Nonprimary infection vs npreg sero+ | NS | NS |
| preg, sero+ vs npreg, sero− | 0.0070 | 0.0017 |
| preg, sero− vs npreg, sero− | NS | NA |
| Primary infection vs npreg, sero− | 0.0039 | 0.0011 |
| Nonprimary infection vs npreg, sero− | 0.0115 | 0.0026 |
| preg, sero− vs preg, sero+ | 0.0000 | 0.0000 |
| Primary infection vs preg, sero+ | 0.0057 | NS |
| Nonprimary infection vs preg, sero+ | NS | NS |
| Primary infection vs preg, sero− | 0.0000 | 0.0000 |
| Nonprimary infection vs preg, sero− | 0.0000 | 0.0000 |
| Nonprimary infection vs primary infection | 0.0104 | NS |
Conditions included pregnancy (preg), nonpregnancy (npreg), and CMV serostatus (sero+, seropositivity; sero−, seronegativity). P value is indicated. NS, not significant (P > 0.05); NA, not assessable.
FIG 3.
Comparison of CMV ELISPOT and QuantiFERON in 195 pregnant and nonpregnant CMV-seropositive and -seronegative women. CMV ELISPOT values were limited to 1,000 spots/2 × 105 PBMCs while CMV QuantiFERON was limited to 10 IU of IFN-γ/ml. The dashed line indicates the lowess local regression-smoothing model. The bandwidth is 0.8.
DISCUSSION
It has been recently shown that there is an association between cell-mediated immunity and the increased risk of congenital infection in primarily infected pregnant women (14). In this study, CMV QuantiFERON and CMV ELISPOT assays were compared by testing a group of pregnant women with active CMV infection and a control group represented by healthy pregnant and nonpregnant CMV-seropositive and -seronegative women. The study showed that both of these assays are highly accurate in discriminating CMV-seronegative pregnant and nonpregnant women. Statistically significant differences between the two assays were found when primarily infected pregnant women were compared to CMV-seropositive pregnant and nonpregnant women and with nonprimarily infected pregnant women, with the CMV ELISPOT assay having the ability to discriminate primary from nonprimary infections. A substantial degree of variability between the assays emerged in the results for CMV-seropositive pregnant and nonpregnant, primarily and nonprimarily infected women. The interassay heterogeneity was also observed for healthy subjects and kidney transplant recipients (30). Potential sources of heterogeneity in the CMV-infected and CMV-seropositive group may be ascribed to the interassay differences, such as the comparison of CMI in a volume of blood (CMV QuantiFERON) versus a given number of cells (CMV ELISPOT). Other important sources of interassay heterogeneity may be the differences in the CMV antigen stimulus used. The antigen composition may affect the HLA recognition and the magnitude of the immune response and may contribute to the interassay differences observed in this study.
ACKNOWLEDGMENTS
We thank the Padua General Hospital-Azienda Ospedaliera di Padova for providing us with the IFN-γ ELISPOT plates and Cellestis and A.D.A., Italy, for providing QuantiFERON control tubes and reagents. We are deeply indebted to the nurses Gelinda Chies and Alessandra degli Agostini for their help with sample collection and Federica Franceschi for technical aid.
D.A., A.S., G.F., and C.M. analyzed the data and wrote the manuscript. A.S., L.F., D.T., and M.P. collected and analyzed the CMV ELISPOT and CMV QuantiFERON assay data. G.F. and N.G. collected the clinical data. C.M. performed the statistical analysis. G.P. and N.G. supervised the study.
We declare no conflict of interest.
This study was supported by the University of Padua (60A07-8071/14 and 60A07-4239/15).
REFERENCES
- 1.Dollard SC, Grosse SD, Ross DS. 2007. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. 2013. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boppana SB, Ross SA, Fowler KB. 2013. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 57(Suppl 4):S178–S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler KB, Stagno S, Pass RF. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289:1008–1011. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 6.Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. 2006. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol 35:216–220. doi: 10.1016/j.jcv.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Grosse SD, Ross DS, Dollard SC. 2008. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol 41:57–62. doi: 10.1016/j.jcv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Rivera LB, Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. 2002. Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection. Pediatrics 110:762–767. doi: 10.1542/peds.110.4.762. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal LS, Fowler KB, Boppana SB, Britt WJ, Pass RF, Schmid SD, Stagno S, Cannon MJ. 2009. Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J 28:515–520. doi: 10.1097/INF.0b013e318198c724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler SP, Nigro G. 2009. Findings and conclusions from CMV hyperimmune globulin treatment trials. J Clin Virol 46(Suppl 4):S54–S57. [DOI] [PubMed] [Google Scholar]
- 11.Nigro G, Adler SP. 2013. Hyperimmunoglobulin for prevention of congenital cytomegalovirus disease. Clin Infect Dis 57(Suppl 4):S193–S195. [DOI] [PubMed] [Google Scholar]
- 12.Visentin S, Manara R, Milanese L, Da Roit A, Forner G, Salviato E, Citton V, Magno FM, Orzan E, Morando C, Cusinato R, Mengoli C, Palu G, Ermani M, Rinaldi R, Cosmi E, Gussetti N. 2012. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin Infect Dis 55:497–503. doi: 10.1093/cid/cis423. [DOI] [PubMed] [Google Scholar]
- 13.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G. 2014. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 14.Saldan A, Forner G, Mengoli C, Gussetti N, Palu G, Abate D. 2015. Strong cell-mediated immune response to human cytomegalovirus is associated with increased risk of fetal infection in primarily infected pregnant women. Clin Infect Dis 61:1228–1234. doi: 10.1093/cid/civ561. [DOI] [PubMed] [Google Scholar]
- 15.Abate D, Cesaro S, Cofano S, Fiscon M, Saldan A, Varotto S, Mengoli C, Pillon M, Calore E, Biasolo MA, Cusinato R, Barzon L, Messina C, Carli M, Palu G. 2012. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation 93:536–542. doi: 10.1097/TP.0b013e31824215db. [DOI] [PubMed] [Google Scholar]
- 16.Abate D, Fiscon M, Saldan A, Cofano S, Mengoli C, Sgarabotto D, d'Agostino C, Barzon L, Cusinato R, Toscano G, Feltrin G, Gambino A, Gerosa G, Palu G. 2012. Human cytomegalovirus-specific T-cell immune reconstitution in preemptively treated heart transplant recipients identifies subjects at critical risk for infection. J Clin Microbiol 50:1974–1980. doi: 10.1128/JCM.06406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abate D, Saldan A, Fiscon M, Cofano S, Paciolla A, Furian L, Ekser B, Biasolo MA, Cusinato R, Mengoli C, Bonfante L, Rossi B, Rigotti P, Sgarabotto D, Barzon L, Palu G. 2010. Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J Infect Dis 202:585–594. doi: 10.1086/654931. [DOI] [PubMed] [Google Scholar]
- 18.Gerna G, Lilleri D, Fornara C, Comolli G, Lozza L, Campana C, Pellegrini C, Meloni F, Rampino T. 2006. Monitoring of human cytomegalovirus-specific CD4 and CD8 T-cell immunity in patients receiving solid organ transplantation. Am J Transplant 6:2356–2364. doi: 10.1111/j.1600-6143.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- 19.Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, Kalpoe J, Lisboa L, Ely L, Kaul DR, Schwartz BS, Morris MI, Ison MG, Yen-Lieberman B, Sebastian A, Assi M, Humar A. 2013. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis 56:817–824. [DOI] [PubMed] [Google Scholar]
- 20.Nebbia G, Mattes FM, Smith C, Hainsworth E, Kopycinski J, Burroughs A, Griffiths PD, Klenerman P, Emery VC. 2008. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am J Transplant 8:2590–2599. doi: 10.1111/j.1600-6143.2008.02425.x. [DOI] [PubMed] [Google Scholar]
- 21.Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, Bell S, Gailbraith A, McNeil K, Jones S, Khanna R. 2007. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis 9:165–170. doi: 10.1111/j.1399-3062.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 22.Gibson L, Dooley S, Trzmielina S, Somasundaran M, Fisher D, Revello MG, Luzuriaga K. 2007. Cytomegalovirus (CMV) IE1- and pp65-specific CD8+ T cell responses broaden over time after primary CMV infection in infants. J Infect Dis 195:1789–1798. doi: 10.1086/518042. [DOI] [PubMed] [Google Scholar]
- 23.Khan N, Best D, Bruton R, Nayak L, Rickinson AB, Moss PA. 2007. T cell recognition patterns of immunodominant cytomegalovirus antigens in primary and persistent infection. J Immunol 178:4455–4465. doi: 10.4049/jimmunol.178.7.4455. [DOI] [PubMed] [Google Scholar]
- 24.Maecker HT, Dunn HS, Suni MA, Khatamzas E, Pitcher CJ, Bunde T, Persaud N, Trigona W, Fu TM, Sinclair E, Bredt BM, McCune JM, Maino VC, Kern F, Picker LJ. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods 255:27–40. doi: 10.1016/S0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 25.Maecker HT, Ghanekar SA, Suni MA, He XS, Picker LJ, Maino VC. 2001. Factors affecting the efficiency of CD8+ T cell cross-priming with exogenous antigens. J Immunol 166:7268–7275. doi: 10.4049/jimmunol.166.12.7268. [DOI] [PubMed] [Google Scholar]
- 26.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, Sissons JG. 1996. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol 70:7569–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelte C, Cherepnev G, Wang Y, Schoenemann C, Volk HD, Kern F. 2004. Random screening of proteins for HLA-A*0201-binding nine-amino acid peptides is not sufficient for identifying CD8 T cell epitopes recognized in the context of HLA-A*0201. J Immunol 172:6783–6789. doi: 10.4049/jimmunol.172.11.6783. [DOI] [PubMed] [Google Scholar]
- 29.Abate D, Saldan A, Forner G, Tinto D, Bianchin A, Palu G. 2014. Optimization of interferon gamma ELISPOT assay to detect human cytomegalovirus specific T-cell responses in solid organ transplants. J Virol Methods 196:157–162. doi: 10.1016/j.jviromet.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 30.Abate D, Saldan A, Mengoli C, Fiscon M, Silvestre C, Fallico L, Peracchi M, Furian L, Cusinato R, Bonfante L, Rossi B, Marchini F, Sgarabotto D, Rigotti P, Palu G. 2013. Comparison of cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV Quantiferon gamma interferon-releasing assays in assessing risk of CMV infection in kidney transplant recipients. J Clin Microbiol 51:2501–2507. doi: 10.1128/JCM.00563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]