Abstract
The notochord is the structure which defines chordates. It is a rod-like mesodermal structure that runs the anterior-posterior length of the embryo, adjacent to the ventral neural tube. The notochord plays a critical role in embryonic tissue patterning, for example the dorsal-ventral patterning of the neural tube. The cells that will come to form the notochord are specified at gastrulation. Axial mesodermal cells arising at the anterior primitive streak migrate anteriorly as the precursors of the notochord and populate the notochordal plate. Interestingly, even though a lot of interest has centered on investigating the functional and structural roles of the notochord, we still have a very rudimentary understanding of notochord morphogenesis. The events driving the formation of the notochord are rapid, taking place over the period of approximately a day in mice. In this commentary we provide an overview of our current understanding of mouse notochord morphogenesis, from the initial specification of axial mesendodermal cells at the primitive streak, the emergence of these cells at the midline on the surface of the embryo, to their submergence and organization of the stereotypically positioned notochord. We will also discuss some key open questions.
Keywords: Mouse development, gastrulation, endoderm, mesoderm, midline, notochord, axial mesoderm, node, actomyosin network
Introduction: overview of notochord morphogenesis and function
Embryogenesis consists of a series of coordinated cell fate specification and morphogenetic events. Following implantation, the pluripotent epiblast cell population undergoes gastrulation, a process during which cells ingress through a structure called the primitive streak to determine the three definitive germ layers, the endoderm, mesoderm and ectoderm, and elaborate the embryo’s anterior-posterior, dorsal-ventral and left-right axes. This transition comprises cell fate specification and major cellular rearrangements and leads to the formation of tissue anlagen necessary for organ morphogenesis (Nowotschin et al., 2010; Kojima et al., 2014; Posfai et al., 2014).
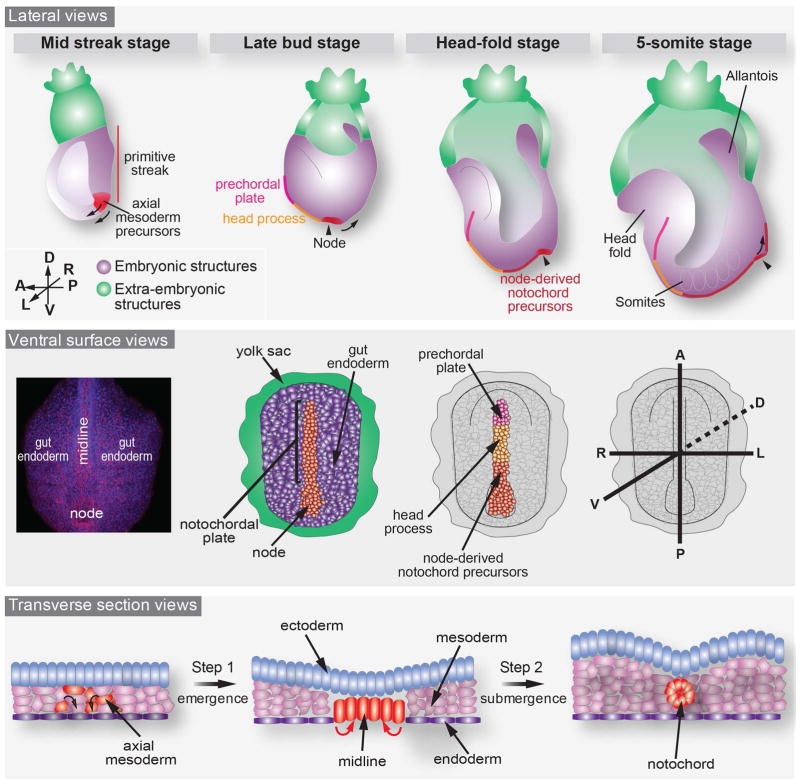
The notochord is a conserved and critical structure present in all chordate embryos (Stemple, 2005). The first description of mouse notochord morphogenesis was based on light and electron microscopy experiments. In 1974, Jurand first described how presumptive notochordal cells localize at the surface of the mouse embryo around embryonic day “E” 8 with their basal lamina contiguous with that of the endoderm. Notochordal cells then invaginate from the embryo’s ventral surface, first at the most anterior region and further progressively in a rostro-caudal direction, still connected by their basement membranes to the endoderm until around E9.5. Around E10.5 endoderm and notochord basement membranes separate and the notochord adheres to the ventral surface of the neural tube. Finally by E12, the notochord also detaches from the neural tube as mesenchymal cells penetrate between the two structures encapsulating the notochord (Figure 1) (Jurand, 1974). The improved resolution brought by scanning electron microscopy further described the changes in cell shape during the emergence of midline structures and notochord formation (Sulik et al., 1994). Mono-ciliated, columnar node and notochordal plate cells are distinguishable at the surface of the cup-shaped mouse embryo from their neighboring endoderm cells, a squamous epithelium, because of their small ventral surface. They first appear around E7 at the distal tip of the mouse embryo to form the node and the notochordal plate, which further elongates as embryo development proceeds. Altogether, the formation of the notochord requires at least two steps. In the first step, a distinct population of mesodermal cells emerges and a columnar epithelium comprising the notochordal plate, which is positioned at the axial midline, forms. In the second step, these cells submerge into the embryo to form the notochord, such that they leave the surface epithelium, which also comprises the endoderm (Figure 1). Thus, cells that eventually come to form the notochord transiently reside on the embryo’s ventral surface in an epithelium contiguous with the gut endoderm.
Figure 1. Schematic representation of notochord morphogenesis in the mouse embryo from mid-gastrula (E7.0) to 5-somite stage (E8.5).
The axial mesodermal (or mesendodermal, in red) cells are specified at the anterior primitive streak during gastrulation and migrate towards the anterior part of the embryo to populate the midline and the node. As shown in a lateral view (upper panel) and ventral surface view (middle panel), the midline is composed of three different cell types: the prechordal plate (in pink, in the most anterior part), followed by cells of the head process (orange) and the node-derived notochord precursors (red, at the posterior part). The former two appear at the surface of the embryo concurrently with the node while the latter is derived from the node through convergent/extension movements. The lower panel depicts in transverse view the two steps necessary for notochord morphogenesis: cells of the axial mesoderm initially emerge at the surface of the embryo (step 1) and subsequently detach and round off to exit the endodermal layer and form the notochord (step 2). Mouse embryonic ages are classified as the embryonic day (E) post coitus, while embryonic stages are classified by morphological landmarks (Downs and Davies, 1993).
Additional studies on the node and notochordal plate precursors established that these cells originate from the population of cells emerging at the most anterior part of the primitive streak at the early-streak stage (E6.25) (Tam and Beddington, 1987). They become morphologically distinct from the rest of the mesoderm and have been referred to as axial mesoderm, chordamesoderm or mesendoderm (Figure 1). Axial mesoderm cells produce three distinct cell populations along the anterior-posterior axis: the prechordal plate, the anterior head process and the node-derived notochordal precursors (Figure 1, see Table 1 for definition and nomenclature) (Tam and Beddington, 1987; Kimelman and Griffin, 2000; Kinder et al., 2001). Together they form the notochordal plate at the surface of the mouse embryo contiguous to the gut endoderm. The prechordal plate gives rise to a population of cells in the forebrain and rostral hindbrain, while cells of the anterior head process participate in the formation of the most anterior part of the notochord (Tam and Behringer, 1997; Camus et al., 2000).
Table 1.
Glossary of structures located at the axial midline of the mouse embryo.
| floor plate | Region of developing neural tube at ventral midline composed of cells that are involved in patterning the ventral part of neural tube. |
| head process | Anterior end of notochord that projects into the head of mammalian and avian embryo and has its origin from cells of the midgastrula organizer (MGO) (Kinder et al., 2001) |
| midline | Refers to tissues with an axial position within the embryo. The node, notochordal plate, notochord, as well as the neural tube are midline structures. |
| node | A morphologically-distinct teardrop-shaped concave structure comprised of apically constricted epithelial cells, present at the anterior extremity of the primitive streak. Functionally it contains motile cilia, whose unidirectional rotation is involved in establishing left-right symmetry breaking. |
| notochord | Transient rod-like mesodermal structure located beneath the neural tube and running the anterior-posterior length of the vertebrate embryo. |
| notochordal plate | Notochord precursor cells before the formation of the rod like structure |
| prechordal plate | Anterior-most mesoderm in the vertebrate embryo, located anterior to the notochord, that gives rise to various ventral tissues of the head and has its origin in cells of the early gastrula organizer (EGO) (Kinder et al., 2001) |
| posterior notochord (PNC) | The indentation at the posterior end of the notochord. The PNC is characterized by features known to be involved in laterality determination. The gastrocoel roof plate (GRP) in Xenopus is equivalent to the mammalian PNC. (Blum et al., 2007) |
The node plays a crucial role in left-right asymmetry determination as well as patterning and organizing the axial midline (Lee and Anderson, 2008). It has therefore been suggested that the region encompassing the node acts as the mammalian organizer (Beddington, 1994), functionally comparable to the blastopore lip or Spemann organizer in Xenopus, and Hensen’s node in the chick, and Kupffer’s vesicle in zebrafish (Harland and Gerhart, 1997; Essner et al., 2005; Meinhardt, 2011). Given, however, the existing ambiguities and non-standardization of terminology across species, it has recently been proposed that a common terminology be adopted across all chordates (Blum et al., 2007; Blum et al., 2014). Blum and colleagues have suggested that the indentation at the posterior end of the notochord, referred to as the posterior notochord (or PNC), is functionally and anatomically distinct from the node, which lies to its posterior and is the only structure to have been shown to exhibit organizer activity. By contrast, the PNC is required for laterality determination (Blum et al., 2007). Since in this review we focus on notochord morphogenesis, we will refer to these two populations as the node region.
As the embryo grows and elongates, trunk notochord precursors emerge from the node and extend the notochordal plate alongside the axial midline (Yamanaka et al., 2007; Ybot-Gonzalez et al., 2007). As somitogenesis begins (~E8.25), the node disappears, while the notochord precursor cells separate and leave the endoderm on the ventral surface of the embryo, and eventually acquire the stereotypical arrangement of the notochord, an epithelial-like rod of cells wrapped around inner vacuolated cells (Jurand, 1974; Sulik et al., 1994; Corallo et al., 2015) which is surrounded by mesenchymal cells. In higher vertebrates, the notochord is a transient structure, often referred to as dorsal mesoderm, which is localized adjacent to the ventral side of the neural tube. The notochord has two functions: it serves as transient axial structural support (Corallo et al., 2015) and as a signaling hub, which patterns surrounding tissues (Placzek, 1995). Its best-characterized function is in patterning of the neural tube, in which the notochord signals to the most ventral part of the neural tube, the floor plate, through several signaling molecules such as the Hedgehog family of protein ligands (Ribes and Briscoe, 2009). These signals promote ventral determination through a gradient that also antagonizes dorsal cell fate (Martí and Bovolenta, 2002; Wilson and Maden, 2005). The notochord has also been described as being critical in the patterning of the gut endoderm, and its gastrointestinal and respiratory derivative tissues, such as the lungs, liver, pancreas and intestine (Cleaver and Krieg, 2001). As development proceeds, the notochord also acquires an essential structural function; it serves as the axial skeleton until formation of the vertebrae. Fate mapping studies have revealed that once the vertebral column forms, the notochord becomes ossified and gives rise to the nucleus pulposis, the central region of the intervertebral discs, whose main function is in shock absorbance (Dahia et al., 2009; McCann et al., 2012; Sivakamasundari and Lufkin, 2012). Thus, defects in the formation of the notochord are associated with severe embryonic defects (Davidson et al., 1999).
Surprisingly, although the role of the notochord in tissue patterning has been extensively studied, the events underlying its formation are not well understood. Axial mesodermal cells are readily identified morphologically at the anterior end of the primitive streak and a few hours later, they are found at the surface of the embryo embedded within the gut endoderm epithelium. At present, the signals and cell fate changes necessary for axial mesoderm and node cells to emerge at the surface and form a furrow contiguous to endodermal cells are largely unknown. Similarly, the mechanisms leading to the submergence of presumptive notochordal cells and a definition of which morphogenetic forces are necessary for these cells to round up and leave the surface of the embryo are in crucial need for more investigation. Until recently, the technical limitations in using the mouse embryo to study such morphogenetic events have reduced the scope of our understanding and lack specific time-points to provide a clear overview of the mechanisms leading to the formation of the notochord. Additionally, many inferences have been made from observations in other vertebrate models, such as the chick, but need to be clarified or confirmed in a mammalian model system. Here, we will review our current understanding while focusing on the mouse embryo and consider several hypotheses regards the mechanisms driving the morphogenesis of the notochord.
The emergence of axial mesodermal cells
By the time the three definitive germ layers have been specified and appropriately positioned, the embryo has transformed from comprising two epithelial layers (the pluripotent epiblast being encapsulated by the visceral endoderm, VE), to an embryo composed of three layers: two epithelia, the squamous gut endoderm and the columnar ectoderm, framing the mesoderm. The only distinction is the region at the midline, which runs the anterior-posterior length of the embryo emanating from the anterior extremity of the node. The midline is composed of two epithelial layers (the ectoderm on the dorsal side and the node or notochordal plate on the ventral side) possessing reverse apical-basal polarity and separated by a basement membrane where their basal sides meet (Jurand, 1974; Sulik et al., 1994). However, it remains unclear whether the dorsal epiblast layer only acquires an ectodermal cell fate or if it participates in the node’s structure and notochordal plate formation. Indeed labeling of cells of the dorsal epithelia adjacent to the node found these cells localized later in the notochord (Beddington, 1981). Thus suggesting that the node structure could be a bilayered epithelium. Moreover the two epithelia might be more tightly associated in comparison with the three germ layers of the embryo that are separated by two basement membranes as they can not be separated by enzymatic digestion (Harrison et al., 1995; Wells and Melton, 2000).
The detailed events leading to the appearance of node and notochordal plate cells on the surface of the embryo have not been described. When do these cells start expressing markers differentiating them from the rest of mesoderm population and which morphological changes are necessary for their emergence? During gastrulation, presumptive mesodermal cells ingress from the epiblast layer, transition through the primitive streak, and as they do so, they undergo epithelial-to-mesenchymal transition (EMT) and subsequently migrate toward the anterior part of the embryo (Viotti et al., 2012). Before these axial mesoderm precursor cells appear at the surface of the embryo, they resume epithelial properties in order to egress and form a columnar epithelium that will give rise to the notochord that is contiguous with the squamous endodermal epithelium (Figure 2) (Lee et al., 2010). Therefore, axial mesoderm precursors are thought to undergo a mesenchymal-to-epithelial transition or MET. To date, it remains unclear if, as these cells acquire axial mesoderm fate, they go through complete or partial EMT as they exit the primitive streak, followed by complete or partial MET. Moreover, axial mesoderm cells are a distinct population of cells emanating from the primitive streak. However, given their transient location on the embryo’s ventral surface, in an epithelium that is contiguous with the endoderm which is positioned laterally, they have been commonly been referred to as mesendoderm cells.
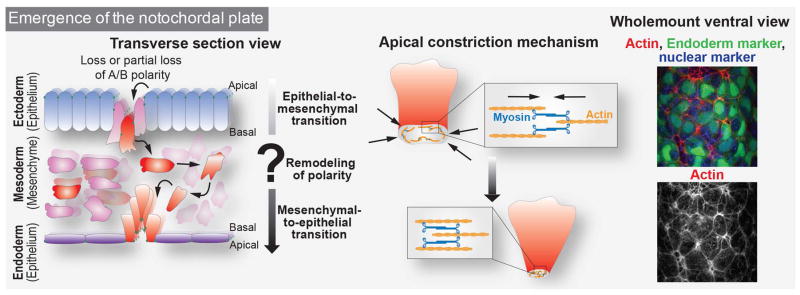
Figure 2. Hypothetical mechanisms driving midline emergence.
During gastrulation, cells emerge from the epiblast layer and go through EMT to form the mesodermal layer. Emanating from the primitive streak, the axial mesodermal cell population (in red) goes through complete or partial EMT and rapidly becomes morphologically distinct from the rest of the mesoderm (in pink). Cells might redefine or invert their polarity and go through complete or partial MET transition to invade the surface of the embryo. As their apical surface is relatively small compare to adjacent endodermal cells, it is likely that apical constriction plays a role in their emergence. The contraction of actin cables by the motor non-muscle myosin II is generally the main mechanism to generate the forces necessary for apical constriction (center). Shown on the right hand side is a wholemount ventral view of the mouse embryo at E7.0–7.25 displaying F-actin staining (in red and monochrome) of axial mesoderm cells emerging at the surface of the embryo together with a green endoderm marker and blue nuclear marker. A/B = Apical/Basal
During embryogenesis, as cells transition between epithelial and mesenchymal states, several transitional stages have been described (Baum et al., 2008; Ferrer-Vaquer et al., 2010; Nakaya and Sheng, 2013). The minimal molecular repertoire used to classify epithelial states is the expression of markers of apical-basal polarity. Cells that do not express these markers are considered to be mesenchymal. However, to be considered a full epithelium, the expression of apical-basal markers is not sufficient and additional features need to be taken into account: epithelia are organized as a continuous single or stratified layer of cells connected to one another through highly adhesive junctions distributed along their lateral membrane. Cell-cell junctions provide rigidity to the structure of an epithelium. Moreover, the polarized organization of cytoskeletal constituents along the apical-basal axis and interactions of the basal membrane with a specialized extracellular matrix, the basement membrane, are required. Similarly, if mesenchymal cells do not exhibit migratory behavior, they could exist in a partial epithelioid mesenchymal state (for review see (Nakaya and Sheng, 2013)). Prior to their emergence on the surface of the embryo, node and axial mesoderm cells exhibit anisotropic localization of apical markers such as E-cadherin (E-cad, also known as CDH1) on their plasma membrane (Lee et al., 2010). They also bear a primary cilium, which has been assembled prior to their emergence on the embryo’s ventral surface (Lee and Anderson, 2008). Indeed, approximately 30% of epiblast cells possess primary cilia before the onset of gastrulation, and it would be important to investigate whether these cells are the ones ingressing at the primitive streak (Bangs et al., 2015). Thus, for a better understanding of axial mesoderm cell specification, the timeline of appearance of markers that define cellular states during the transition needs to be documented at single-cell resolution.
Following the acquisition of epithelial features, notochordal plate and node cells need to emerge at the surface of the embryo. However, the guidance cues required by these cells to join the existing surface epithelium, the VE, remain unknown. Signals could emerge from the surface of the embryo and thus the arrangement of cells within the VE epithelium might predict where cells forming the midline will insert. Having reached the surface of the embryo, cells of the notochordal plate and node are distinctly identified by their small apical area and are more regularly packed compared to the endoderm, which is positioned laterally to both the left and right sides of the midline. This raises questions of why these cells are so distinct while lying within the same epithelium. Apical constriction and active migration through an epithelium that has loosened their cell-cell adhesion junctions with adjacent basement membrane represent well-described mechanisms. In such cases, actin and myosin cables are visible and enriched in the apical area or protrusions of cells in order to allow for modifications of apical membrane shape by a pulling mechanism (Figure 2) (Martin and Goldstein, 2014; Munjal and Lecuit, 2014). The emergence of the notochordal plate along the midline could also provide a physical barrier and initiate compartmentalization of the prospective right and left sides of the embryo. Another open question concerns the ability of axial mesodermal cells to actively migrate towards the anterior end of the embryo. Morphogenetic forces could also be involved in the egression of axial mesodermal cells. Perhaps the forces generated by the two wings of mesodermal cells moving anteriorly could push the notochordal plate and node cells out of the mesodermal layer and help their emergence at the embryo surface.
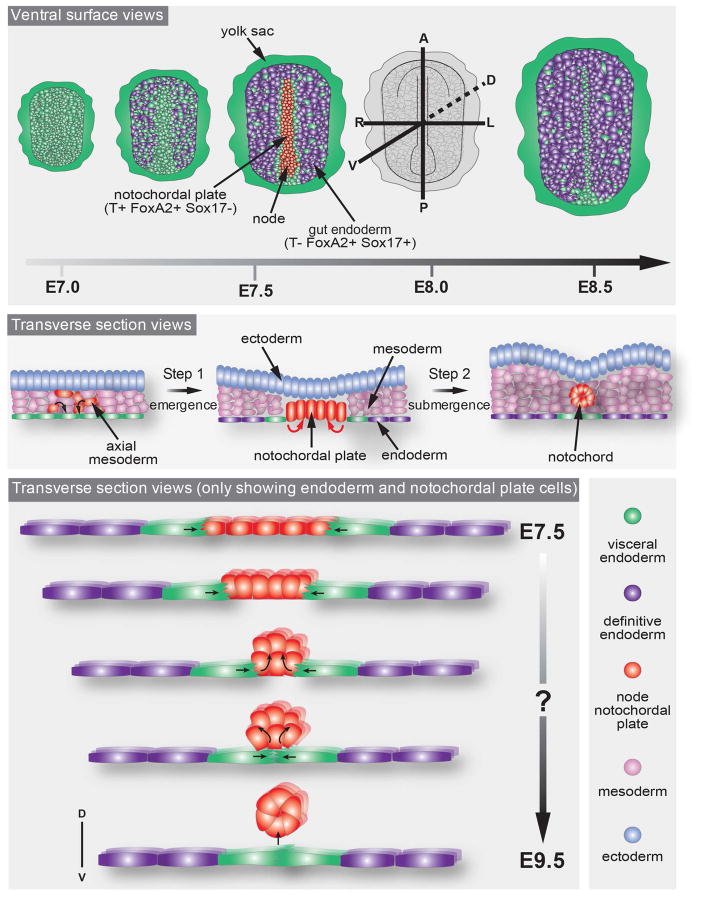
We now know that gut endoderm morphogenesis is driven by the dispersal of the VE and intercalation of streak-derived definitive endoderm (DE) progenitors (Kwon et al., 2008) which arise from within the wings of mesoderm during gastrulation (Viotti et al., 2014a). Cells of the prechordal plate emerge on the embryo’s surface at the midline soon after VE dispersal is underway. Analysis of the HMG-Sry-related transcription factor Sox17 mutants, which exhibit normal formation of a node and notochordal plate, but a failure to specify DE cells and consequently a subsequent failure to disperse the VE, demonstrated the uncoupling of gut endoderm specification from the morphogenesis of midline structures (Viotti et al., 2012). VE cells partially downregulate their epithelial character to allow for definitive endoderm cells to invade the gut endoderm layer (Viotti et al., 2014a). Similar mechanisms could be responsible for the emergence of notochordal plate and node at the surface of the embryo. In fact, the distribution of VE cells on the embryo’s surface as revealed by a panVE reporter such as the Afp-GFP transgene (Kwon et al., 2006) is stereotypical during notochordal plate and node emergence. Within the gut endoderm which is positioned laterally to the midline, VE cells disperse into single cells surrounded by streak-derived DE cells. By contrast, VE cells remain clustered at the midline, and then align on either side of the notochordal plate and the node as they emerge (Figure 3 and 4). This arrangement of VE cells in close proximity to the notochordal plate, might hint at crosstalk between these two cell types. Elucidating these details would be of interest and might also help explain how notochordal plate cells reorganize themselves once they have reached the surface of the embryo to form an invariant 5–10 cell wide structure outlined by a row of VE cells. Perhaps the stereotypical distribution of the VE outlining the narrow furrow comprising the notochordal plate suggests that this population might help define the limits of this population.
Figure 3. Visceral Endoderm (VE) cell dynamics during notochord morphogenesis.
VE cells align along the midline throughout the entire process of its appearance at the embryo surface until its submergence. Cartoons depict the position of VE cells relative to the midline in ventral surface view (upper panel) and transverse section view (middle and lower panels) throughout notochord morphogenesis. Cartoon in the lower panel shows the hypothetical forces (black arrows) emanating from the VE to ensure midline ingression to form the notochord through acto-myosin purse strings.
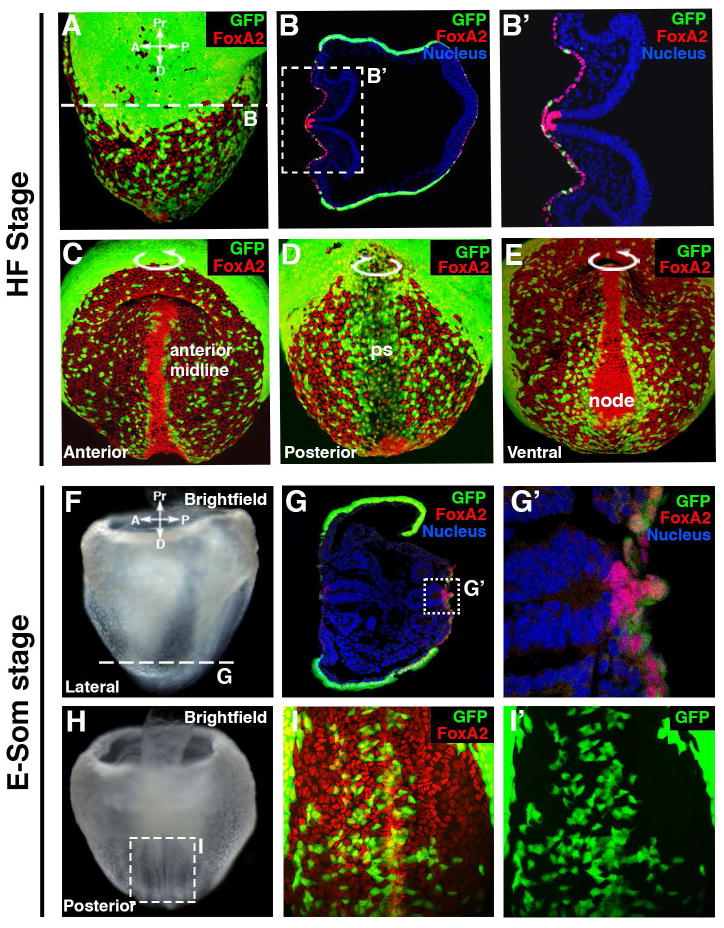
Figure 4. Foxa2 and VE cells distribution during notochord submergence.
A–E: FoxA2 (red) is expressed in the midline, node and gut endoderm of a headfold stage Afp-GFPTG/+ embryo labeling VE cells with GFP. Note the increased cell density and higher levels of FoxA2 expression in the midline and node.
F–I: At early somite (E-Som) stage, FoxA2 (in red) is still expressed in the midline, node and gut endoderm. VE cells are labeled with GFP. Note the FoxA2+ notochordal cells have submerged and VE cells from both side of the midline are now contiguous and recovering the notochordal plate.
Transcription factors associated with the morphogenesis of midline structures
The evolutionarily conserved Forkhead box (FOX) family of transcription factors, also known as the Hepatocyte Nuclear Factor 3 (HNF3) family, were first identified by their regulation of liver-specific genes (Lai et al., 1990). FOX proteins are key transcriptional regulators of endoderm development as well as the specification and maintenance of most, if not all, endoderm derivatives (Ang et al., 1993; Friedman and Kaestner, 2006). FoxA2 (Forkhead box protein A2, previously known as HNF3β Figure 4) is the first member of the family to be expressed at E6.5 in the primitive streak and the node. Its expression is then maintained in the notochord and the definitive endoderm at E7.5. Later on, FoxA2 is expressed in all endoderm-derived structures, as well as parts of the nervous system, such as the ventral neural plate and the floor plate region of the neural tube. In mice, other members of the FoxA family of proteins, for example FoxA1/HNF3α and FoxA3/HNF3γ, are also expressed in endoderm derivatives but are activated later, at E7.0 and E8.5, respectively (Ruiz i Altaba et al., 1993). Among its numerous functions, FoxA2 is necessary for node and notochord formation (Ang et al., 1993; Weinstein et al., 1994). FoxA2 homozygous mutants exhibit a complete absence of node and notochord and die around E11 with abnormalities in their neural tube and somites, as well as defects in gut tube closure. FoxA2 is one of the earliest markers that distinctly identifies cells at the midline on the surface of the embryo before they submerge and form the notochord. FoxA2 has been shown to regulate a molecular program to establish an epithelial cellular phenotype (Burtscher and Lickert, 2009). FoxA2 has also been shown to inhibit EMT in several contexts (Song et al., 2010; Tang et al., 2010; Zhang et al., 2015). Additionally, a microarray-based gene expression screen of wild type versus FoxA2 mutants identified ten new genes expressed in the node/notochord (Tamplin et al., 2008). Of those that have been analyzed functionally, novel components of cilia-based formation or signaling networks have been identified, but none have yet been demonstrated to exhibit key roles in the morphogenesis of midline tissues.
Brachyury/T, the founding member of the T-box family of transcription factors, is expressed in the early primitive streak at the posterior end of the embryo, then in nascent mesoderm, as well as the node and notochord, from late streak stage onwards. T is a dose-sensitive gene in the mouse and has been shown to act both cell-autonomously, in the specification and survival of notochord cell precursors, and non-cell-autonomously, in the development of posterior mesoderm. T mutants display a phenotype of graded severity depending on the allele carried, such as tail shortening in heterozygote or disruption of trunk development and embryonic lethality in homozygous null mutants (Herrmann, 1991; Conlon et al., 1995). Null mutations result in aberrant trunk and mesoderm development whereby the anterior head process and the notochord are specified but fail to be maintained (Gluecksohn-Schoenheimer, 1938; Yanagisawa et al., 1981).
FoxA2 and T expressing axial mesoderm, as well as FoxA2 definitive endoderm cells, are specified early on in the epiblast. Both cell types express FoxA2 before they exit into the primitive streak undergoing (partial) EMT by downregulating polarity markers and adhesion junction proteins (Burtscher and Lickert, 2009; Viotti et al., 2014b). Cells fated to become axial mesoderm upregulate the expression of T and integrate into the overlying epithelium. At the midline. The signals driving the specification of FoxA2+T+ axial mesoderm cells are currently unknown. As presumptive midline cells emanate from the primitive streak, it would be crucial to determine exactly when they initiate expression of FoxA2. Recently, the FoxA2 and T loci have been targeted to generate knock-ins that express Venus (Yamanaka et al., 2007; Burtscher et al., 2013; Imuta et al., 2013), as well GFP and tamoxifen-inducible CreER fusion (Imuta et al., 2013). Reporters such as these are likely to be useful for following the early emergence of axial mesoderm cells and their egression into the endodermal layer by following GFP expression throughout time, as well as subsequent events leading to the formation of the notochord. Additionally, the Cre recombinase present in both alleles should allow perturbation of gene function specifically in midline tissues. For example, as FoxA2 is also expressed in the endoderm or the floorplate, the disruption of FoxA2 using Brachyury/T-driven Cre would allow the identification of specific functions of FoxA2 in notochord development. Similar experiments could be performed for Brachyury and allow more understanding of these events.
Once FoxA2+T+ axial mesoderm cells have been specified, they start expressing markers, which distinctly identify notochord and node cells, for example the homeobox transcription factor Noto. A mouse line containing an eGFP knock-in into the Noto locus facilitated the first notochord live-imaging study in the mouse (Yamanaka et al., 2007). Although noto mutants display severe notochord defects in zebrafish, mouse Noto mutants show only moderate defects in node and notochord patterning suggesting that a yet-to-be identified factor could be responsible for the normal development of the anterior notochord in the Noto mouse mutant. Nonetheless, Noto is required for the specification of cilia (Beckers et al., 2007). Noto-GFP has also been used as a read-out to generate node/notochord-like cells from mouse ES cells via inducing a FoxA2-Brachyury progenitor population by addition of Activin (as a surrogate for the Transforming Growth Factor β signaling molecule Nodal) and inhibition of Bone Morphogenetic Proteins, Wnt and Retinoic Acid signaling pathways. ES cell derived Noto-GFP+ cells expressed markers of the node and notochord such as FoxA2, T, Sonic Hedgehog (Winzi et al., 2011). Additionally, the generation of a Noto-Cre mouse line coupled with a LacZ reporter allowed fate mapping studies and confirmed that notochord progenitors are necessary for intervertebral disc development (McCann et al., 2012).
Lhx1 is a Lim homeobox transcription factor expressed early in the visceral endoderm (VE) and during gastrulation in the primitive streak and the anterior mesendoderm and transiently in nascent mesoderm (Barnes et al. 1994; Shawlot and Behringer 1995; Perea-Gomez et al. 1999). In the VE it has been shown to be a downstream target of Eomesodermin, a T-box transcription factor, expressed in the VE at early postimplantation stages and in mesoderm cells during gastrulation and necessary for Distal VE/Anterior VE formation (Nowotschin et al 2013). Null mutant embryos of Lhx1 exhibit a truncation of the head (Shawlot and Behringer 1995). Conditional deletion of Lhx1 in the epiblast also results in head truncation due to a failure to form the anterior mesendoderm (Shawlot et al. 1999; Fossat et al. 2015). Lhx1 epiblast-deletion also induces the disruption of the node and axial mesoderm morphogenesis (Costello et al., 2015).
From notochordal plate cells on the embryo’s ventral surface to the submerged notochord
Between early headfold and early somite stages (E7.5 and E8.5), the mouse embryo grows dramatically and elongates along the anterior-posterior axis. This elongation is coupled with movements of convergent extension (CE) of notochordal plate cells that both help elongate the anterior-posterior axis as well as promote the addition of cells emerging from the node (Ybot-Gonzalez et al., 2007; Mahaffey et al., 2013). CE is a morphogenetic process whereby tissue is remodeled through narrowing one axis by cellular intercalation in order to elongate in the perpendicular axis (Keller et al., 2000). Yet how the midline tissues elongate and what happens to the notochordal plate and node cells during these events need to be clarified. These movements have been well described in Xenopus and zebrafish regarding the movement necessary for notochord (and body axis) elongation. Recently, similar movements have also been described during mouse notochord elongation (Yamanaka et al., 2007; Imuta et al., 2014). It was shown that the amniotic cavity provides forces for CE movement necessary for notochord formation, highlighting the importance of extra-embryonic tissues as a source of forces to control embryo morphogenesis (Imuta et al., 2014). Additionally, Yamanaka and colleagues used time-lapse imaging to visualize cellular behaviors during notochord formation. Their study defines several distinct morphogenetic processes acting along the axis of the notochord and specific gene regulatory networks necessary to pattern the three different segments of the notochord. The first, composed of cells of the anterior head process emerges independently of the node and is formed by condensation of axial mesoderm dispersed cells anteriorly to the emerging node. Indeed, cells of the head process had been shown to derive from the two opposing ends of the mesodermal wings as they meet and merge at the midline (Tam et al., 1997). Second, trunk notochordal cells derive from the node and use CE movements to elongate the midline. Third, posterior notochord cells, which also derive from node progenitors, actively migrate towards the posterior part of the embryo (Yamanaka et al., 2007). It has been proposed that the node structure regresses as midline tissues elongate. However, node regression could imply that cells actively migrate or translocate towards the posterior end of the embryo, leaving cells alongside the midline to extend it. On the other hand, as the embryo grows, the distance between the node and the posterior end of the embryo might not vary as much as the anterior end and the apparent regression of the node could be perceived due only to its displacement because of the growth and lengthening of the anterior. To affirm these observations, one would need to measure the distance between the node and a fixed point such as the allantois to distinguish between the two possibilities.
To understand how the notochord forms, transverse sections of mouse embryos have been used to construct a sequence of events: from the initial position of cells at the surface of the embryo to their final conglomeration as a rod-shaped internal structure. A 6-stage mechanism for this process has been proposed based on the distribution of basement membrane adjacent to the basal side of midline tissues. Initially, (1) the basement membrane is interposed between notochordal plate cells and the adjacent endoderm and engulfs notochordal plate cells. Thereafter, (2) the basement membrane adopts an angular shape, and (3) comes to encapsulate notochordal cells, which concomitantly downregulate E-cad and have almost left the epithelium of the embryo’s ventral surface. (4) The notochord then completely separates from the endoderm via the basement membrane, and moves towards the floor plate leaving behind a trail of basement membrane (5). By the end of the process (6) the notochord has become internalized and localizes in a position adjacent to the floorplate of the neural tube while the basement membrane trail has disappeared either by degradation or remodeling (Figure 5). Additionally, this study shows by quantitative analyses of the levels of the apical marker E-cad that the downregulation of epithelial characteristics is required for proper ingression of notochordal plate cells. Noggin, an antagonist of BMP signaling is expressed in the notochord and required for proper downregulation of such apical markers in notochordal plate cells. This step is necessary to allow their detachment from the dorsal foregut endoderm and their submergence to form the notochord (Fausett et al., 2014). Another study also showed that Fibronectin and Integrin regulate the position of the node and notochordal plate. Since mutants in Fibronectin and Integrin also display defects in the shape of the notochord and node, they likely are also required for establishing or maintaining the proper morphology of these structures (Pulina et al., 2014).
Figure 5. Notochord resolution model.
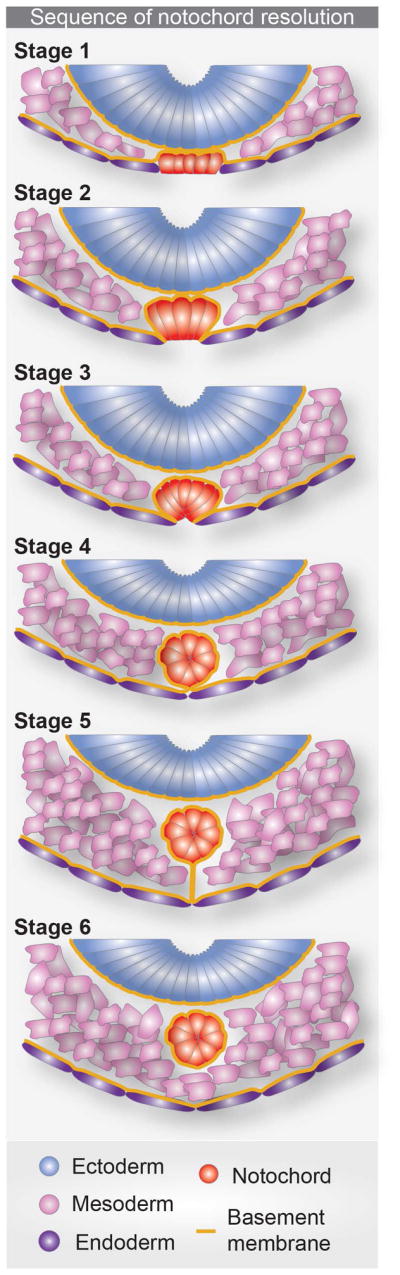
Cartoon representing the analysis of subsequent sections of 6–21 somite embryo from rostral to caudal showing 6 different stages of basement membrane dynamics necessary for the formation of the notochord, as established by (Fausett et al., 2014).
Despite a rudimentary description of this sequence of events, not much is known about the process during which notochordal cells exit the epithelium on the embryo’s ventral surface. Forces coming from the midline could direct their basal extrusion from the epithelium thus leading to their submergence. This may be achieved by apical constriction, epithelial bending, or ingression of single (or groups of) cells (Figure 6) (Martin and Goldstein, 2014). Conversely, forces coming from the adjacent VE cells may act to “push” notochordal cells inside by forming an actomyosin purse-string in a mechanism reminiscent of dorsal closure in Drosophila. During the processes of wound healing or dorsal closure, the two leading-edge epithelia assemble a contractile network of actin and myosin-II and extend projections that contribute to the generation of forces necessary to reach each other and seal the two layers into one congruent epithelium (Kiehart, 1999; Kiehart et al., 2000; Rodriguez-Diaz et al., 2008; Martin and Goldstein, 2014). The distribution of VE cells at this stage is intriguing (Figure 3 and 4). Their alignment along the midline suggests that they could function to send signals to notochordal cells to direct their behavior. Alternatively, VE cells might exhibit an intrinsic movement towards the midline that might serve to push node and notochordal plate cells out of the epithelium on the embryo’s ventral surface driving them inside the embryo. Laser ablation of cell-cell contacts between the VE and notochordal plate cells would reveal contacts that are important for their ingression. If these cells act as purse-string, this set of experiment would allow direct visualization of disruption of this mechanism as such experiments have been successfully applied to study dorsal closure in Drosophila (Rodriguez-Diaz et al., 2008; Harris et al., 2009; Belacortu and Paricio, 2011). However to visualize the dynamics, one would need to analyze the distribution of actin/myosin cables for which a bright live-imaging fluorescent reporter exists but which has not been tested at this developmental stage (Jana et al., 2009). Alternatively, a membrane bound marker in another spectral color would allow the imaging of both cell dynamics and mark a specific population of cells. Moreover, if VE cells cannot align, might notochordal plate cells be unable to ingress? Interestingly, embryos in which Rac1 has been specifically removed from the VE lose this stereotypical distribution of VE cells without disrupting the formation of midline structures (Migeotte et al., 2011). Proteins of the Rho-like GTPase family including Rac1 are important regulators of cytoskeletal properties involved in mechanisms such as cell-cell contacts or lamellipodium formation (Sander and Collard, 1999). It will be interesting to determine if in mutants for genes encoding these proteins, notochordal plate cells positioned at the axial midline on the embryo’s ventral surface can still ingress to form a notochord.
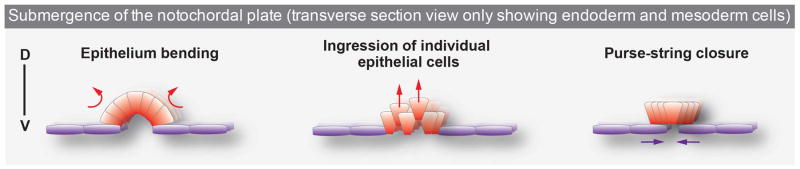
Figure 6. Hypothetical mechanisms driving notochord submergence.
Depicted here are three non-mutually exclusive mechanistic events that could lead to the submergence of midline cells (red) from the endoderm (purple) on the embryo’s surface. The first two mechanisms (epithelium bending and ingression of individual cells) might exert forces emanating directly from the midline (red arrows): apical constriction of midline cells could lead to epithelial bending or ingression of individual cells. In the third (purse-string closure), adjacent endodermal cells could exert forces (purple arrows) to promote internalization of midline cells via a purse-string mechanism, as seen in Drosophila dorsal closure or wound healing events. D = dorsal, V = ventral
Concluding remarks
While we possess a detailed knowledge of the functional and structural roles of the notochord of vertebrate embryo, we still have a very rudimentary understanding of how it arises. Studies of morphogenetic events carried out in mice have up until recently relied heavily on the analysis of sequentially staged fixed tissue preparations. The growing number of mouse reporter lines, improvement of methods for live imaging and quantitative image analyses, are opening the door to a deeper single-cell level mechanistic understanding the formation of key embryonic structures such as the notochord. Some of key open questions include, how presumptive notochordal plate cells emerge onto the surface of the embryo, whether axial mesoderm cells actively migrate or are displaced onto the embryo’s surface. The mechanisms constraining the midline to a 5–10 cell wide domain are also unexplored. Mapping the intrinsic morphogenetic forces, identifying the cellular populations generating them, and investigating the dynamics of the underlying cytoskeletal networks will be critical to further understanding the mechanics of notochord morphogenesis. And finally, it is still unclear why the VE is stereotypically arranged along the midline; is this organization a cause or a consequence of the morphogenesis of the notochordal plate?
Acknowledgments
GRANT SPONSOR
NIH R01-DK084391 & P30-CA008748
We thank Kathryn Anderson and Laina Freyer for comments on this review. Our work on the morphogenesis of structures at the ventral midline of the mouse embryo is supported by NIH grants R01-DK084391 and P30-CA008748.
References
- Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol. 2015;17:113–122. doi: 10.1038/ncb3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Seminars in Cell and Developmental Biology. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Beckers A, Alten L, Viebahn C, Andre P, Gossler A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc Natl Acad Sci U S A. 2007;104:15765–15770. doi: 10.1073/pnas.0704344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddington RS. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. doi: 10.1242/dev.120.3.613. [DOI] [PubMed] [Google Scholar]
- Beddington SP. An autoradiographic analysis of the potency of embryonic ectoderm in the 8th day postimplantation mouse embryo. J Embryol Exp Morphol. 1981;64:87–104. [PubMed] [Google Scholar]
- Belacortu Y, Paricio N. Drosophila as a model of wound healing and tissue regeneration in vertebrates. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:2379–2404. doi: 10.1002/dvdy.22753. [DOI] [PubMed] [Google Scholar]
- Blum M, Andre P, Muders K, Schweickert A, Fischer A, Bitzer E, Bogusch S, Beyer T, van Straaten HW, Viebahn C. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation. 2007;75:133–146. doi: 10.1111/j.1432-0436.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- Blum M, Schweickert A, Vick P, Wright CV, Danilchik MV. Symmetry breakage in the vertebrate embryo: when does it happen and how does it work? Dev Biol. 2014;393:109–123. doi: 10.1016/j.ydbio.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher I, Barkey W, Lickert H. Foxa2-venus fusion reporter mouse line allows live-cell analysis of endoderm-derived organ formation. genesis. 2013;51:596–604. doi: 10.1002/dvg.22404. [DOI] [PubMed] [Google Scholar]
- Burtscher I, Lickert H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development. 2009;136:1029–1038. doi: 10.1242/dev.028415. [DOI] [PubMed] [Google Scholar]
- Camus A, Davidson BP, Billiards S, Khoo P, Rivera-Pérez JA, Wakamiya M, Behringer RR, Tam PP. The morphogenetic role of midline mesendoderm and ectoderm in the development of the forebrain and the midbrain of the mouse embryo. Development. 2000;127:1799–1813. doi: 10.1242/dev.127.9.1799. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Krieg PA. Notochord patterning of the endoderm. Dev Biol. 2001;234:1–12. doi: 10.1006/dbio.2001.0214. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Wright CV, Robertson EJ. Effects of the TWis mutation on notochord formation and mesodermal patterning. Mechanisms of Development. 1995;49:201–209. doi: 10.1016/0925-4773(94)00318-h. [DOI] [PubMed] [Google Scholar]
- Corallo D, Trapani V, Bonaldo P. The notochord: structure and functions. Cell Mol Life Sci. 2015 doi: 10.1007/s00018-015-1897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello I, Nowotschin S, Sun X, Mould AW, Hadjantonakis AK, Bikoff EK, Robertson EJ. Lhx1 functions together with Otx2, Foxa2, and Ldb1 to govern anterior mesendoderm, node, and midline development. Genes Dev. 2015;29:2108–2122. doi: 10.1101/gad.268979.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahia CL, Mahoney EJ, Durrani AA, Wylie C. Intercellular signaling pathways active during intervertebral disc growth, differentiation, and aging. Spine (Phila Pa 1976) 2009;34:456–462. doi: 10.1097/BRS.0b013e3181913e98. [DOI] [PubMed] [Google Scholar]
- Davidson BP, Kinder SJ, Steiner K, Schoenwolf GC, Tam PP. Impact of node ablation on the morphogenesis of the body axis and the lateral asymmetry of the mouse embryo during early organogenesis. Developmental Biology. 1999;211:11–26. doi: 10.1006/dbio.1999.9276. [DOI] [PubMed] [Google Scholar]
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Fausett SR, Brunet LJ, Klingensmith J. BMP antagonism by Noggin is required in presumptive notochord cells for mammalian foregut morphogenesis. Developmental Biology. 2014;391:111–124. doi: 10.1016/j.ydbio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Viotti M, Hadjantonakis A-K. Transitions between epithelial and mesenchymal states and the morphogenesis of the early mouse embryo. Cell adhesion & migration. 2010;4:447–457. doi: 10.4161/cam.4.3.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cellular and molecular life sciences : CMLS. 2006;63:2317–2328. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluecksohn-Schoenheimer S. The Development of Two Tailless Mutants in the House Mouse. Genetics. 1938;23:573–584. doi: 10.1093/genetics/23.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annual review of cell and developmental biology. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Harris TJC, Sawyer JK, Peifer M. How the cytoskeleton helps build the embryonic body plan: models of morphogenesis from Drosophila. Current topics in developmental biology. 2009;89:55–85. doi: 10.1016/S0070-2153(09)89003-0. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Dunwoodie SL, Arkell RM, Lehrach H, Beddington RS. Isolation of novel tissue-specific genes from cDNA libraries representing the individual tissue constituents of the gastrulating mouse embryo. Development. 1995;121:2479–2489. doi: 10.1242/dev.121.8.2479. [DOI] [PubMed] [Google Scholar]
- Herrmann BG. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development. 1991;113:913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- Imuta Y, Kiyonari H, Jang C-W, Behringer RR, Sasaki H. Generation of knock-in mice that express nuclear enhanced green fluorescent protein and tamoxifen-inducible Cre recombinase in the notochord from Foxa2and Tloci. genesis. 2013;51:210–218. doi: 10.1002/dvg.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imuta Y, Koyama H, Shi D, Eiraku M, Fujimori T, Sasaki H. Mechanical control of notochord morphogenesis by extra-embryonic tissues in mouse embryos. Mechanisms of Development. 2014;132:44–58. doi: 10.1016/j.mod.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Jana SS, Kim KY, Mao J, Kawamoto S, Sellers JR, Adelstein RS. An alternatively spliced isoform of non-muscle myosin II-C is not regulated by myosin light chain phosphorylation. J Biol Chem. 2009;284:11563–11571. doi: 10.1074/jbc.M806574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurand A. Some aspects of the development of the notochord in mouse embryos. Journal of embryology and experimental morphology. 1974;32:1–33. [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP. Wound healing: The power of the purse string. Current biology : CB. 1999;9:R602–605. doi: 10.1016/s0960-9822(99)80384-4. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. Journal of Cell Biology. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D, Griffin KJ. Vertebrate mesendoderm induction and patterning. Current Opinion in Genetics & Development. 2000;10:350–356. doi: 10.1016/s0959-437x(00)00095-2. [DOI] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Wakamiya M, Sasaki H, Behringer RR, Nagy A, Tam PP. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development. 2001;128:3623–3634. doi: 10.1242/dev.128.18.3623. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Tam OH, Tam PPL. Timing of developmental events in the early mouse embryo. Seminars in Cell and Developmental Biology. 2014;34:65–75. doi: 10.1016/j.semcdb.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Fraser ST, Eakin GS, Mangano M, Isern J, Sahr KE, Hadjantonakis A-K, Baron MH. Tg(Afp-GFP) expression marks primitive and definitive endoderm lineages during mouse development. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:2549–2558. doi: 10.1002/dvdy.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon GS, Viotti M, Hadjantonakis A-K. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Developmental Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E, Prezioso VR, Smith E, Litvin O, Costa RH, Darnell JE. HNF-3A, a hepatocyte-enriched transcription factor of novel structure is regulated transcriptionally. Genes & development. 1990;4:1427–1436. doi: 10.1101/gad.4.8.1427. [DOI] [PubMed] [Google Scholar]
- Lee JD, Anderson KV. Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:3464–3476. doi: 10.1002/dvdy.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Migeotte I, Anderson KV. Left-right patterning in the mouse requires Epb4.1l5-dependent morphogenesis of the node and midline. Developmental Biology. 2010;346:237–246. doi: 10.1016/j.ydbio.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey JP, Grego-Bessa J, Liem KF, Jr, Anderson KV. Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development. 2013;140:1262–1271. doi: 10.1242/dev.085316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends in neurosciences. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141:1987–1998. doi: 10.1242/dev.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MR, Tamplin OJ, Rossant J, Seguin CA. Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. Dis Model Mech. 2012;5:73–82. doi: 10.1242/dmm.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H. Models for organizer and notochord formation. Comptes Rendus de l’Academie des Sciences Series III Sciences de la Vie. 2011;323:23–30. doi: 10.1016/s0764-4469(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Migeotte I, Grego-Bessa J, Anderson KV. Rac1 mediates morphogenetic responses to intercellular signals in the gastrulating mouse embryo. Development. 2011;138:3011–3020. doi: 10.1242/dev.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjal A, Lecuit T. Actomyosin networks and tissue morphogenesis. Development. 2014;141:1789–1793. doi: 10.1242/dev.091645. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Sheng G. EMT in developmental morphogenesis. Cancer letters. 2013;341:9–15. doi: 10.1016/j.canlet.2013.02.037. [DOI] [PubMed] [Google Scholar]
- Nowotschin S, Ferrer-Vaquer A, Hadjantonakis A-K. Imaging mouse development with confocal time-lapse microscopy. Methods in enzymology. 2010;476:351–377. doi: 10.1016/S0076-6879(10)76020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek M. The role of the notochord and floor plate in inductive interactions. Curr Opin Genet Dev. 1995;5:499–506. doi: 10.1016/0959-437x(95)90055-l. [DOI] [PubMed] [Google Scholar]
- Posfai E, Tam OH, Rossant J. Mechanisms of pluripotency in vivo and in vitro. Current topics in developmental biology. 2014;107:1–37. doi: 10.1016/B978-0-12-416022-4.00001-9. [DOI] [PubMed] [Google Scholar]
- Pulina M, Liang D, Astrof S. Shape and position of the node and notochord along the bilateral plane of symmetry are regulated by cell-extracellular matrix interactions. Biology Open. 2014;3:583–590. doi: 10.1242/bio.20148243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes V, Briscoe J. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb Perspect Biol. 2009;1:a002014. doi: 10.1101/cshperspect.a002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz A, Toyama Y, Abravanel DL, Wiemann JM, Wells AR, Tulu US, Edwards GS, Kiehart DP. Actomyosin purse strings: renewable resources that make morphogenesis robust and resilient. HFSP journal. 2008;2:220–237. doi: 10.2976/1.2955565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Prezioso VR, Darnell JE, Jessell TM. Sequential expression of HNF-3 beta and HNF-3 alpha by embryonic organizing centers: the dorsal lip/node, notochord and floor plate. Mechanisms of Development. 1993;44:91–108. doi: 10.1016/0925-4773(93)90060-b. [DOI] [PubMed] [Google Scholar]
- Sander EE, Collard JG. Rho-like GTPases: their role in epithelial cell-cell adhesion and invasion. Eur J Cancer. 1999;35:1905–1911. doi: 10.1016/s0959-8049(99)00293-2. [DOI] [PubMed] [Google Scholar]
- Sivakamasundari V, Lufkin T. Bridging the Gap: Understanding Embryonic Intervertebral Disc Development. Cell Dev Biol. 2012;1 [PMC free article] [PubMed] [Google Scholar]
- Song Y, Washington MK, Crawford HC. Loss of FOXA1/2 Is Essential for the Epithelial-to-Mesenchymal Transition in Pancreatic Cancer. Cancer research. 2010;70:2115–2125. doi: 10.1158/0008-5472.CAN-09-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Sulik K, Dehart DB, Iangaki T, Carson JL, Vrablic T, Gesteland K, Schoenwolf GC. Morphogenesis of the murine node and notochordal plate. Developmental dynamics : an official publication of the American Association of Anatomists. 1994;201:260–278. doi: 10.1002/aja.1002010309. [DOI] [PubMed] [Google Scholar]
- Tam PP, Beddington RS. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987;99:109–126. doi: 10.1242/dev.99.1.109. [DOI] [PubMed] [Google Scholar]
- Tam PP, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mechanisms of Development. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Tam PP, Steiner KA, Zhou SX, Quinlan GA. Lineage and functional analyses of the mouse organizer. Cold Spring Harb Symp Quant Biol. 1997;62:135–144. [PubMed] [Google Scholar]
- Tamplin OJ, Kinzel D, Cox BJ, Bell CE, Rossant J, Lickert H. Microarray analysis of Foxa2 mutant mouse embryos reveals novel gene expression and inductive roles for the gastrula organizer and its derivatives. BMC Genomics. 2008;9:511. doi: 10.1186/1471-2164-9-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Shu G, Yuan X, Jing N, Song J. FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell research. 2010;21:316–326. doi: 10.1038/cr.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti M, Niu L, Shi SH, Hadjantonakis AK. Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. 2012;10:e1001276. doi: 10.1371/journal.pbio.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti M, Nowotschin S, Hadjantonakis A-K. SOX17 links gut endoderm morphogenesis and germ layer segregation. Nature Cell Biology. 2014a;16:1146–1156. doi: 10.1038/ncb3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti M, Nowotschin S, Hadjantonakis AK. SOX17 links gut endoderm morphogenesis and germ layer segregation. Nat Cell Biol. 2014b;16:1146–1156. doi: 10.1038/ncb3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Developmental Biology. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Winzi MK, Hyttel P, Dale JK, Serup P. Isolation and characterization of node/notochord-like cells from mouse embryonic stem cells. Stem Cells Dev. 2011;20:1817–1827. doi: 10.1089/scd.2011.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Tamplin OJ, Beckers A, Gossler A, Rossant J. Live Imaging and Genetic Analysis of Mouse Notochord Formation Reveals Regional Morphogenetic Mechanisms. Developmental Cell. 2007;13:884–896. doi: 10.1016/j.devcel.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Yanagisawa KO, Fujimoto H, Urushihara H. Effects of the brachyury (T) mutation on morphogenetic movement in the mouse embryo. Dev Biol. 1981;87:242–248. doi: 10.1016/0012-1606(81)90147-0. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene NDE, Copp AJ. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yang C, Gao W, Chen T, Qian T, Hu J, Tan Y. FOXA2 attenuates the epithelial to mesenchymal transition by regulating the transcription of E-cadherin and ZEB2 in human breast cancer. Cancer letters. 2015;361:240–250. doi: 10.1016/j.canlet.2015.03.008. [DOI] [PubMed] [Google Scholar]