Abstract
Objectives
Although it has been previously shown that changes in temporal coding produce changes in pitch in all cochlear regions, research has suggested that temporal coding might be best encoded in relatively apical locations. We hypothesized that although temporal coding may provide useable information at any cochlear location, low rates of stimulation might provide better sound quality in apical regions that are more likely to encode temporal information in the normal ear. In the present study, sound qualities of single electrode pulse trains were scaled to provide insight into the combined effects of cochlear location and stimulation rate on sound quality.
Design
Ten long term users of MED-EL cochlear implants with 31 mm electrode arrays (Standard or FLEXSOFT) were asked to scale the sound quality of single electrode pulse trains in terms of how “Clean”, “Noisy”, “High”, and “Annoying” they sounded. Pulse trains were presented on most electrodes between 1 and 12 representing the entire range of the long electrode array at stimulation rates of 100, 150, 200, 400, or 1500 pulses per second.
Results
While high rates of stimulation are scaled as having a “Clean” sound quality across the entire array, only the most apical electrodes (typically 1 through 3) were considered “Clean” at low rates. Low rates on electrodes 6 through 12 were not rated as “Clean” while the low rate quality of electrodes 4 and 5 were typically in between. Scaling of “Noisy” responses provided an approximately inverse pattern as “Clean” responses. “High” responses show the trade-off between rate and place of stimulation on pitch. Because “High” responses did not correlate with “Clean” responses, subjects were not rating sound quality based on pitch.
Conclusions
If explicit temporal coding is to be provided in a cochlear implant, it is likely to sound better when provided apically. Additionally, the finding that low rates sound clean only at apical places of stimulation is consistent with previous findings that a change in rate of stimulation corresponds to an equivalent change in perceived pitch at apical locations. Collectively, the data strongly suggests that temporal coding with a cochlear implant is optimally provided by electrodes placed well into the second cochlear turn.
Keywords: temporal coding, spectral coding, sound quality, apical stimulation
1. Introduction
Each place within the cochlea responds to a specific frequency due to the tonotopic organization of the cochlea. Similarly, at each cochlear location, the basilar membrane vibrates with a corresponding frequency. Therefore, the pitch of an acoustic tone could theoretically be encoded by either the place of stimulation on the cochlea (e.g. von Helmholtz, 1912) or the vibrations at the corresponding frequency (Wundt, 1904). More recent models have been based on temporal coding (Licklider, 1959; Meddis et al., 1997), spectral coding (Goldstein, 1973; Terhardt, 1979), or a combination of the two (Wever et al., 1930; Wever, 1940). Because the temporal and spectral attributes of an acoustic signal are naturally correlated, it is difficult to disentangle the relative contributions of the two. However, using clever stimulus design such as providing sinusoidal amplitude modulations on a sinusoidal carrier of a different frequency (e.g. Oxenham et al., 2004), it is possible to manipulate the temporal coding separately from the place of stimulation in an acoustic hearing ear. With electrical stimulation, the rate and place of stimulation are easily independently manipulated and can be used to independently study the perceptual attributes of temporal and spectral coding.
It has been well documented that at a given stimulation rate, listeners report that changing the place of stimulation (by changing the electrode used to provide stimulation) provides a change in pitch (e.g. Eddington et al., 1978; Shannon et al., 1983; Donaldson et al., 2005). Similarly, on a fixed electrode, listeners also report that a change in rate of stimulation provides a change in pitch (e.g. Eddington et al., 1978; Tong et al., 1983; Shannon et al., 1983; Landsberger and McKay, 2005). When both rate and place coding are changed in complementary or contradictory directions, the amount of perceived change in pitch is either increased or decreased accordingly (e.g. Zeng et al., 2002; Stohl et al., 2008; Luo et al., 2012). Nevertheless, although both changes in rate and place of stimulation affect pitch, the two are not interchangeable, but are instead perceptually orthogonal (e.g. Tong et al., 1983; McKay et al., 2000).
Cochlear implant users with significant residual hearing (ipsilaterally or contralaterally), including those with normal to near-normal hearing in the contralateral ear (e.g. single-sided deafened (SSD)) have allowed experiments to separately manipulate the temporal and/or spectral components of an electrical signal and compare the percept with that produced acoustically. Several experiments have pitch matched high-rate single electrode pulse trains to acoustic tones (e.g. Blamey et al., 1996; Boex et al., 2006; Baumann and Nobbe, 2006; Dorman et al., 2007; Vermeire et al., 2008; Carlyon et al., 2010; Schatzer et al., 2014; Prentiss et al., 2014; Zeng et al., 2014; Vermeire et al., 2015) and found that a change in place of stimulation (i.e. a change in electrode) does indeed correspond to a change in acoustic frequency pitch match such that more basal stimulation matches a higher pitch. Similarly, using a multi-dimensional scaling technique, Vermeire et al. (2013) demonstrated that a change in acoustic frequency can be represented by the same perceptual dimension as a change in electrode. However, it is worth noting that while a change in place of stimulation produces a change in place pitch for all electrode locations on even long (31 mm) electrode arrays (Landsberger et al., 2014), the changes in pitch corresponding to the most apical electrodes on these arrays are relatively small (e.g. Baumann and Nobbe, 2004; 2006; Dorman et al., 2007; Landsberger et al. 2014). The corresponding pitch from high rate stimulation on the most apical electrodes of long electrode arrays typically asymptotes down to approximately 300 Hz (e.g. Vermeire et al., 2008; 2015; Schatzer et al., 2014; Prentiss et al., 2014).
In addition to studying place-pitch matches, Schatzer et al. (2014) used 8 SSD subjects with a contralateral MED-EL implant (6 with 31 mm FLEXSOFT arrays, 2 with 24 mm M arrays) to explore rate pitch matches. Based on the results of Oxenham et al. (2004), they hypothesized that reliable electrically evoked low-frequency pitch percepts required a matching temporal and place code. In this experiment, Schatzer et al. (2014) played a pure tone (at 100, 150, 200, 300, or 450 Hz) to the acoustic hearing ear. The subject compared the acoustic tone to a single electrode pulse train and adjusted the rate of stimulation of that pulse train using an adaptive pitch ranking task until a match to the pitch of the acoustic tone could be estimated. Initial rates of stimulation were selected in pairs such that one initial rate would be above the pitch match and one initial rate would be below the pitch match. The process was repeated for each subject for each of the 5 acoustic pure tones and on each electrode between 1 and 6. Subjects were not able to make successful matches to all pure tones on all electrodes, regardless of rate of stimulation. Schatzer et al. (2014) kept track of not only the rates that produced successful matches, but also how frequently a match could be successfully made for a given acoustic frequency at a given cochlear location. A successful match was defined as any match such that adaptive tracks from the different initial rates converged according to a ‘sanity check’ rule defined by Carlyon et al. (2010). Considering a match successful did not imply any particular accuracy or sound quality for the match, other than a reasonable (i.e. converging) value could be obtained. The left panel of Figure 1 shows the percentage of successful matches as a function of the acoustic frequency for electrodes at different insertion depths. Electrodes inserted deeper than 430° (plotted in red) are usually able to provide successful matches to low acoustic frequencies (approximately 300 Hz and below). Electrodes placed more shallowly than 362° (plotted in blue) are better at providing pitch matches for higher acoustic frequencies (approximately above 200 Hz) than lower acoustic frequencies. However, electrodes placed between 362° and 430° (plotted in green) usually provided a successful pitch match at all tested acoustic frequencies. From these results, one might conclude that a successful pitch match does not require the corresponding correct place of stimulation. Instead pitch can be presented through temporal coding (at least for frequencies between 100 and 450 Hz) exclusively, on an electrode inserted between 362° and 430° into the cochlea.
Figure 1.
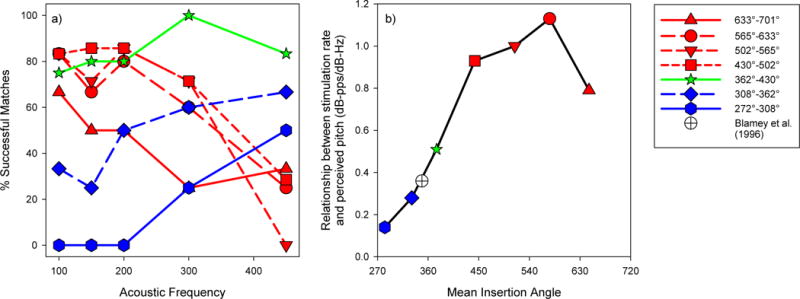
Summary plots adapted from Schatzer et al. (2014). The left panel (a) is adapted from Figure 4 of Schatzer et al. (2014). It shows the percent of successful matches subjects were able to make by varying the rate of stimulation on a single electrode pulse train to an acoustic pure-tone as a function of cochlear region. Regions more apical than 430° are plotted in red, between 362° and 430° are plotted in green, and the regions more basal than 362° are plotted in blue. The right panel (b) is a plot of data from Table 3 of Schatzer et al. (2014). The ratio of the change in perceived acoustic frequency (in dB) to the change in stimulation rate (in dB) is plotted as a function of the the mean insertion angle for each of the cochlear regions. Note that for a rate of stimulation to properly encode pitch, the relationship between rate of stimulation and the frequency corresponding to the perceived pitch must equal 1. The figure legend applies to both panels a and b.
If a pitch is to be conveyed using temporal coding to a cochlear implant user, it is important that not only can a pitch be successfully delivered, but also that relative pitch relationships can be maintained (i.e. that a doubling of frequency is perceived as a shift up by an octave). For the successful rate-pitch matches within a given cochlear region, Schatzer et al. (2014) calculated the ratio of the change in acoustic frequency (in dB) and the corresponding change in rate required to make pitch match (also in dB). When the ratio = 1, a change in stimulation rate maintains the correct pitch relationship. In the right panel of Figure 1, this relationship for the Schatzer et al. (2014) data is plotted as a function of cochlear region. For cochlear locations deeper than 430°, the ratio is not significantly different from 1, while it is significantly different from 1 at shallower cochlear locations. Therefore, although electrodes placed between 362° and 430° can usually provide a successful pitch match to low-frequency acoustic stimuli, using a stimulation rate at a frequency to be perceptually encoded will not maintain the proper perceptual relationship. It is worth noting that multichannel temporal coding strategies, including FSP and FS4 (Riss et al., 2014), F0Mod (Laneau et al., 2006; Milczynski et al., 2009), F0Sync, MEM and PDT (Vandali et al., 2005), MPeak (Pijl, 1994), SAS (Kessler, 1999; Zimmerman-Phillips and Murad, 1999), and single channel analog strategies (Hochmair-Desoyer et al., 1981; Fretz and Fravel, 1985) present the frequencies to be encoded by the corresponding rate of stimulation.
One potential reason that subjects are able to make pitch matches to acoustic pure tones between 100 and 450 Hz on electrodes inserted between 362° and 430° using only a relatively narrow range of stimulation rates might be that the sound quality at low rates for this region might be different than the sound quality at more apical locations where the ratio between pitch and stimulation rate was closer to 1. We hypothesized that more apical regions would be more sensitive to temporal coding and therefore have a better sound quality at low rates as temporal coding is more effective at low rates for CI users (e.g. Tong et al., 1983; Shannon et al., 1983). Conversely, at basal locations, sound quality at low rates would be worse. Furthermore, we expect that there would be a transitional region in the cochlea (probably represented by the region between 362° and 430° in the data collected by Schatzer et al. (2014)) in which the sound quality gradually decreases as low-rate stimulation moves from the apical to basal edge of this region. To test this hypothesis, ten Dutch-speaking bilaterally deafened subjects with 31 mm electrode arrays were asked to scale how “Clean” (using the term “Zuiver” in Dutch) a single electrode pulse train was when presented on electrodes representing all regions of the electrode array at one of five rates of stimulation. Similarly, these subjects were asked to scale how “Noisy” (“Ruis-achtig” in Dutch), “High” (“Hoog” in Dutch), and “Annoying” (“Vervelend” in Dutch). We hoped “Noisy” would provide an approximately inverse response to “Clean” and provide a second measure of sound quality. “High” data was collected to verify that the subjects were not actually pitch scaling for “Clean” and “Noisy” responses. “Annoying” was suggested by the second subject to be tested (UZA-M3) so it was added to the protocol to see if it provided any more useful information. The first subject tested (UZA-M11) was brought in for a second visit to collect the data for “Annoying.”
2. Methods
2.1 Subjects
Ten adult subjects (eleven ears) with fully inserted 31 mm MED-EL electrode arrays (either Standard or FLEXSOFT) were tested at the Antwerp University Hospital (UZA) in Belgium. All subjects were native Flemish speakers. All electrodes were inserted with a cochleostomy approach except UZA-M13. UZA-M13 is a bilateral user whose insertion was via a cochleostomy in the right ear and a round window approach in the left ear. Data was collected for both ears for UZA-M13. However, because responses for the left and right ear cannot be considered statistically independent, all analyses collapsing across subjects only use data from UZA-M13R (the right ear of UZA-M13). Additionally, this assures that all data in across subject analyses comes from ears with full insertions via a cochleostomy approach. Specific subject demographics are presented in Table 1. Impedances for each electrode are presented in Table 2. All subjects provided informed consent in accordance with the IRB regulations of the Antwerp University Hospital.
Table 1.
Subject demographics and performance scores. The speech recognition in quiet scores refer to monosyllables at 65 dB(SPL) using the NVA test material. Scores were based on correct phonemes. Speech in noise scores were based on the LIST sentences (van Weiringen and Wouters, 2008) with a 65 dB (SPL) noise level using a keyword score.
| Subject | Tested Ear | Age at surgery [yrs;mo] |
Duration of CI use at time of testing [mo] |
Implant & Electrode type |
Surgical Approach + Notes |
Clinical Strategy & # of Fine Structure Channels |
Speech recognition in quiet [%] |
Speech recognition in noise [SRT at 65dB noise] |
Clinically Deactiviated Electrodes |
Basal Cochlear Diameter (mm) ("A") |
Etiology | Onset |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UZA-M11 | Left | 43;4 | 61 | SONATA Standard | Cochleostomy, Full Insertion | FS4 ; 4 | 76 | +4 dB | 12 | 9 | CX26 mutation | Congenital (progressive) |
| UZA-M3 | Right | 34;5 | 39 | CONCERTO Standard | Cochleostomy, Full Insertion | FS4 ; 4 | 85 | + 1dB | 11, 12 | 9.4 | Bacterial meningitis | 7/3/1905 |
| UZA-M1 | Left | 55;7 | 178 | COMBI 40+ Standard | Cochleostomy, Full Insertion | FS4 ; 4 | 45 | – | Unknown | Unknown | ||
| UZA-M10 | Right | 44;5 | 98 | PULSAR FLEXSOFT | Cochleostomy, Full Insertion | FS4 ; 4 | 91 | 0 dB | – | 8.1 | Unknown | Congenital (progressive) |
| UZA-M4 | Right | 49;9 | 124 | COMBI 40+ Standard | Cochleostomy, Full Insertion | FS4 ; 4 | 94 | 0 dB | – | Meniere’s disease | 1974 (progressive) | |
| UZA-M12 | Right | 44;11 | 61 | SONATA FLEXSOFT | Cochleostomy, Full Insertion | FSP ; 2 | 85 | +1 dB | – | 9 | Unknown (rhesusincompatibility?) | Congenital |
| UZA-M13L | Left | 27;5 | 41 | CONCERTO FLEXSOFT | Round Window, Full Insertion | FS4 ; 4 | 85 | +3.33 dB | – | 8.8 | Unknown | 1998 (progressive) |
| UZA-M13R | Right | 25;8 | 62 | SONATA FLEXSOFT | Cochleostomy, Full Insertion | FS4 ; 4 | 85 | +3.33 dB | – | 8.9 | Unknown | 1998 (progressive) |
| UZA-M5 | Left | 41;9 | 83 | PULSAR FLEXSOFT | Cochleostomy, Full Insertion | FS4 ; 4 | 73 | +7.67 dB | 12 | Unknown | Unknown | |
| UZA-M14 | Right | 40;8 | 91 | PULSAR FLEXSOFT | Cochleostomy, Full Insertion | FS4 ; 4 | 100 | 0.67 dB | – | 7.8 | Unknown (R) + neurinoma (L) | Congenital (progressive) (R+L) + resection neurinoma (L) |
| UZA-M15 | Left | 50;11 | 87 | PULSAR FLEXSOFT | Cochleostomy, Full Insertion | FS4 ; 4 | 83 | – | Unknown | 1998 (progressive) | ||
|
| ||||||||||||
| MEAN | 43;4 | 86.3 | ||||||||||
Table 2.
Impedances (kΩ) for each electrode and each subject. Values in red represent electrodes which are not included in the subject’s clinical map.
| Subject | EL 1 | EL 2 | EL 3 | EL 4 | EL 5 | EL 6 | EL 7 | EL 8 | EL 9 | EL 10 | EL 11 | EL 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UZA-M11 | 4.6 | 5.97 | 5.32 | 5.97 | 3.87 | 4.19 | 4.27 | 4.44 | 4.44 | 4.52 | 3.63 | 4.03 |
| UZA-M3 | 7.94 | 4.24 | 7.94 | 4.72 | 5 | 8.31 | 6.99 | 9.07 | 9.07 | 15.51 | 15.23 | 19.87 |
| UZA-M1 | 8.33 | 8.26 | 9.19 | 8.2 | 8.26 | 8.82 | 9.19 | 8.94 | 9.62 | 9.93 | 11.1 | >12.3 |
| UZA-M10 | 6.75 | 8.15 | 8.42 | 7.54 | 7.8 | 2.99 | 3.95 | 3.6 | 2.9 | 3.07 | 2.81 | 5.53 |
| UZA-M4 | 7.85 | 4.95 | 5.11 | 5.32 | 7.85 | 6.89 | 5.92 | 4.35 | 4.62 | 5.86 | 6.51 | 5.92 |
| UZA-M12 | 3.73 | 2.69 | 2.24 | 2.46 | 2.54 | 3.36 | 3.58 | 2.84 | 2.54 | 2.61 | 2.76 | 6.27 |
| UZA-M13L | 7.9 | 5.07 | 5.72 | 7.61 | 6.01 | 4.2 | 4.71 | 4.13 | 5 | 6.01 | 6.88 | 6.67 |
| UZA-M13R | 2.5 | 3.23 | 4.19 | 5.24 | 4.6 | 4.6 | 5.16 | 4.19 | 4.03 | 4.19 | 4.84 | 4.76 |
| UZA-M5 | 4.46 | 5.36 | 6.52 | 7.68 | 6.16 | 6.07 | 6.16 | 6.43 | 8.66 | 9.38 | 12.85 | 10.09 |
| UZA-M14 | 5.16 | 5.4 | 4.76 | 4.52 | 4.92 | 4.68 | 5.32 | 4.19 | 4.11 | 6.37 | 4.68 | 5.97 |
| UZA-M15 | 3.88 | 5.32 | 5.4 | 5.08 | 3.96 | 3.56 | 4.52 | 4.12 | 4.52 | 4.36 | 3.8 | 5.72 |
2.2 Stimuli
Stimuli consisted of single electrode, cathodic-first bi-phasic pulse-trains. All stimuli were presented without an interphase gap in monopolar (MP) mode. All pulses in a pulse train were presented at an equal amplitude. By default, stimulation used a 32 μs phase duration. However for some subjects, the phase duration had to be increased to 65 μs in order to obtain a comfortably loud level for all stimuli. For a given subject, all stimuli had the same phase duration. By default, stimuli were presented on electrodes 1, 2, 3, 4, 5, 6, 8, 10, or 12 (where electrode 1 is the most apical contact) at stimulation rates of 100, 150, 200, 400, or 1500 pulses per second (pps). Electrodes that were not present in the subject’s clinical maps were excluded from testing. The duration of the stimuli were either 500, 750, or 1000 ms depending on a subject’s preference for ease of scaling the sound quality. For a given subject, all stimuli were presented for the same duration. Stimuli were presented directly to subjects using the MAX programming interface box using custom written software. Communication with the implant used a pre-release of the RIB2 DLL modified for use with the MAX programming interface box instead of the RIB2 hardware (University of Innsbruck) typically used for psychophysical experiments with the MED-EL system. All stimuli were presented at an equally loud level described as “most comfortable.”
2.3 Procedure
The loudness growth for stimuli at all rates and all electrodes was coarsely estimated. On a single electrode, a stimulus was played initially below threshold, and gradually increased in 10 μa steps until subjects indicated that the sound was at the maximum comfort level. An 11-point loudness scale from Advanced Bionics was used by the subjects to indicate the loudness of each stimulus as the dynamic range was estimated. The experimenter recorded the amplitudes of the pulse trains that corresponded to barely audible, soft, most comfortable, and maximum acceptable loudness.
The amplitudes of each stimulus were adjusted to ensure that all stimuli were equally loud at a most comfortably loud level using a method similar to Landsberger et al. (2014). Four stimuli were presented with a 300 ms inter-stimulus-interval (ISI) at amplitudes corresponding to what had been previously described as the most comfortable loudness. The stimuli differed in either electrode, rate of stimulation, or both. The subject was asked if all of the sounds were the same loudness. If not, the amplitudes were tweaked until all four sounds were the same loudness. The procedure was then repeated with a new set of stimuli until the amplitudes required to produce equal loudness on all stimuli were recorded.
After loudness balancing, all stimuli were presented in series to the subject to familiarize the subject with the range of stimuli in the experiment. Before the familiarization, subjects were told that they would have to rate qualities of the sounds they heard and that all of the possible sounds would be presented to them during the familiarization process. However, the specific terms used to scale the sounds were not told to the subjects in advance. After familiarization, the main perceptual experiment began. In a single trial, subjects were presented one randomly selected single electrode pulse train (on any tested electrode at 100, 150, 200, 400, or 1500 pps) using a method similar to Landsberger et al. (2012). Subjects were asked to scale how well one of four terms described the sound by clicking on a line whose range represented least to most agreeing with the term. The line was approximately 19 cm long and presented on a 15 inch CRT with a screen resolution of 1024 × 768 pixels as can be seen in Figure 2. The location of the line varied between trials to ensure that the subject had to move the mouse to make a new selection for every trial. The four descriptors were “Clean” (Zuiver), “Noisy” (Ruis-Achtig), “High-Pitched” (Hoog), and “Annoying” (Vervelend). Within a block of trials, all stimuli were presented once and subjects were only asked to scale one term. The procedure was repeated until 10 measurements were collected for each stimulus with each of the four descriptors for all subjects.
Figure 2.
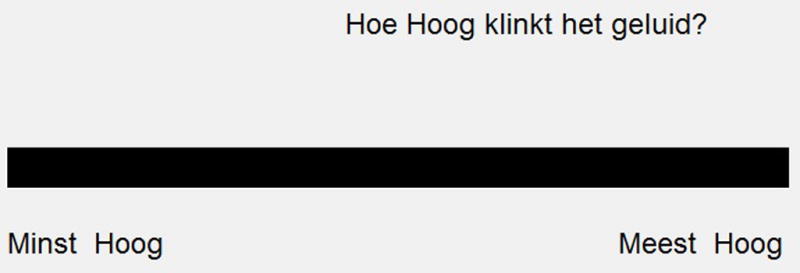
Response interface for the scaling task. The question asked in English is “How high is the sound?” The scale ranges from “least high” to “most high.”
Results
Each response was encoded by a value between 0 and 100 where 100 indicated a complete agreement with the descriptor and 0 indicated complete disagreement with the descriptor. For each subject, electrode, and stimulation rate, the 20% trimmed mean was calculated. A trimmed mean is a cross between a mean and a median. To calculate a 20% trimmed mean, all of the data is rank ordered and then the mean is calculated for the central 60% of the data (Wilcox et al., 1998; Aronoff et al., 2011). Trimmed means were used to reduce the effects of asymmetric tails in the distribution that are likely to occur as a result of floor and ceiling effects created by a restricted response range.
The summary of responses for “Clean” is presented in Figure 3. The small contour plots on the right represent 20% trimmed mean responses of individual subjects while the large contour plot on the left indicates the 20% trimmed mean responses across subjects (i.e. the data in the small contour plots). Responses are color coded such that yellow and red colors indicate a clean sound quality, blue and purple colors indicate not clean sounds, and green colors indicate sounds in between. The plot representing all subjects indicates that the sound quality for the most apical three electrodes is “Clean” for all rates of stimulation. Similarly, the sound quality is “Clean” for 1500 pps stimuli at all cochlear locations. However, between electrodes 3 and 5, the sound quality drops at low rates. For locations more basal than electrode 5, low rates of stimulation do not sound clean. Although the majority of individual subjects demonstrate similar patterns, there are some notable exceptions. For example, UZA-M1 shows no consistent pattern across rate and place. It is worth noting that UZA-M1’s speech scores were much lower than the other subjects tested in this experiment (see Table 1). UZA-M4 scales low rates as not “Clean” regardless of cochlear position. Although the reason why low rates are not as “Clean” for UZA-M4 is unknown, it is consistent with the observation that this subject had a significant drop in performance when switched from HDCIS to FSP. Additionally, UZA-M3 finds high rates and basal electrodes to sound not “Clean”, and UZA-M5 scales all rates and places similarly. UZA-M13R described almost all stimuli as “Clean” as is indicated by her all stimuli as above 65 for “Clean”. Nevertheless, despite using a limited range of responses (65–100) for “Clean”, the pattern of UZA-M13R’s remain consistent with the overall pattern across subjects as can be seen in the Normalized UZA-M13R plot.
Figure 3.
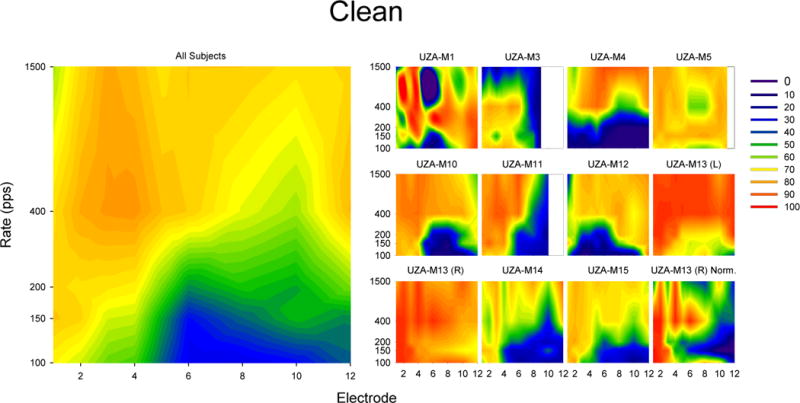
Results of scaling the term “Clean” in response to single electrode pulse trains. The smaller plots on the left show 20% trimmed means results for individual subjects while the larger plot on the left show 20% trimmed means of all subjects (including UZA-M13(R) but not UZA-M13(L)). For each plot, the x-axis indicates the electrode number (where 1 is the most apical and 12 is the most basal) and the y-axis indicates the stimulation rate. The colors indicate the degree to which subjects agree that the sound is “Clean” ranging from blue (indicating the subject reported that the sound was not very clean) to red (indicating that the subject reported that the sound was very clean). Note that the bottom right plot (UZA-M13 (R) Norm.) is a normalized plot of UZA-M13 (R) only used to help visualize the data in UZA-M13 (R). The normalized data is not used for any calculations in the manuscript.
Means of “Clean” scaling weighted by the corresponding rate of stimulation were calculated across all rates of stimulation for each subject and each electrode. A one-way repeated-measures ANOVA on the “Clean” weighted means revealed a statistical difference in “Clean” ratings across electrode locations (F(8,56)=4.155, p = 0.001). The initial hypothesis suggested that the quality of low-rate stimulation would deteriorate as a function of cochlear position in the apical half of the electrode array while the sound quality of low-rate stimulation would remain similar in the cochlear region represented by the basal half of the array. Therefore, planned pairwise comparisons were made between electrodes 1 and 6 (representing change over the apical half of the array), electrodes 6 and 12 (representing change over the basal portion of the electrode array) and electrodes 1 and 12 (representing the two ends of the array). A significant difference was detected between the “Clean” scaling of electrodes 1 and 6 (t(9) = −4.773, p < 0.001) and electrodes 1 and 12 (t(7) = −2.932, p =0.022), but no significant difference was detected between electrodes 6 and 12 (t(7) = 1.484, p = 0.181). The differences between electrodes 1 and 6 and electrodes 1 and 12 remain significant after Type I error correction using Rom’s method (Rom, 1990).
The summary of responses for “Noisy”, “High”, and “Annoying” are presented in Figures 4, 5, and 6. Responses for “Noisy” look like an inverse of responses for “Clean” and were highly negatively correlated with “Clean” (r (43) = −0.878, p < 0.0005). Responses for “High” show the trade-off between rate and place of stimulation of pitch. Responses for “High” were not significantly correlated with “Clean” (r (43) = 0.238, p = 0.115). Responses for “Annoying” seem to be dependent on electrode location and not rate of stimulation. A negative correlation was observed between responses for “Clean” and “Annoying” (r (43) = −0.440, p < 0.003). The significant correlations remain significant after Type I error correction using Rom’s method (Rom, 1990).
Figure 4.
Results of scaling the term “Noisy” in response to single electrode pulse trains. The smaller plots on the left show 20% trimmed means results for individual subjects while the larger plot on the left show 20% trimmed means of all subjects (including UZA-M13(R) but not UZA-M13(L)). For each plot, the x-axis indicates the electrode number (where 1 is the most apical and 12 is the most basal) and the y-axis indicates the stimulation rate. The colors indicate the degree to which subjects agree that the sound is “Noisy” ranging from blue (indicating the subject reported that the sound was not very noisy) to red (indicating that the subject reported that the sound was very noisy).
Figure 5.
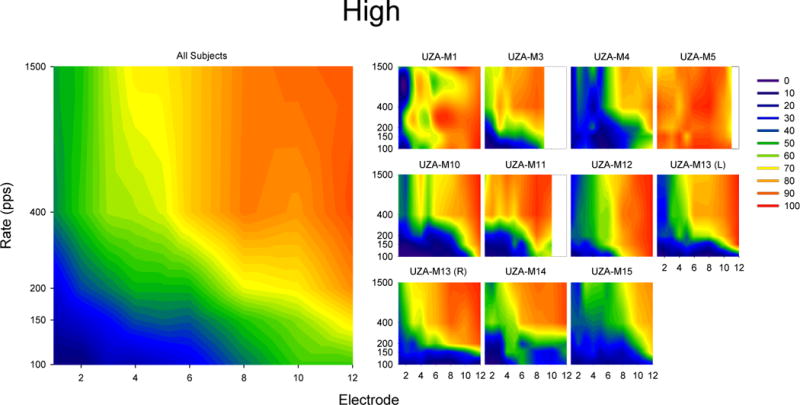
Results of scaling the term “High” in response to single electrode pulse trains. The smaller plots on the left show 20% trimmed means results for individual subjects while the larger plot on the left show 20% trimmed means of all subjects (including UZA-M13(R) but not UZA-M13(L)). For each plot, the x-axis indicates the electrode number (where 1 is the most apical and 12 is the most basal) and the y-axis indicates the stimulation rate. The colors indicate the degree to which subjects agree that the sound is “High” ranging from blue (indicating the subject reported that the sound was not very high) to red (indicating that the subject reported that the sound was very high).
Figure 6.
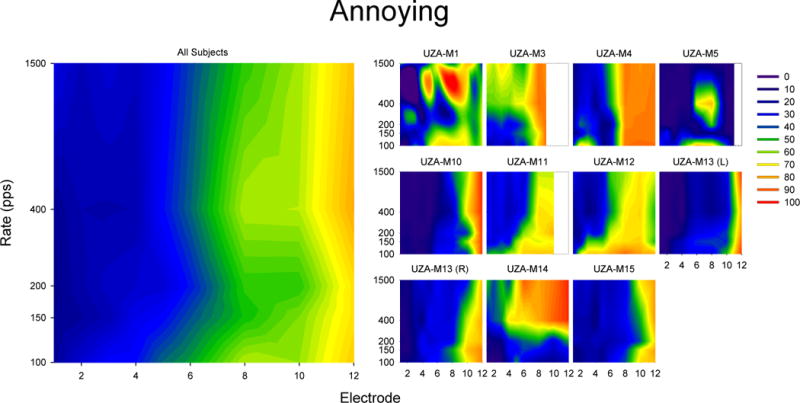
Results of scaling the term “Annoying” in response to single electrode pulse trains. The smaller plots on the left show 20% trimmed means results for individual subjects while the larger plot on the left show 20% trimmed means of all subjects (including UZA-M13(R) but not UZA-M13(L)). For each plot, the x-axis indicates the electrode number (where 1 is the most apical and 12 is the most basal) and the y-axis indicates the stimulation rate. The colors indicate the degree to which subjects agree that the sound is “Annoying” ranging from blue (indicating the subject reported that the sound was not very annoying) to red (indicating that the subject reported that the sound was very annoying).
Discussion
The results from the scaling of “Clean” suggest that although high rates of stimulation sound “Clean” at all cochlear locations, the sound quality of low-rate stimuli are good in the apex and poorer in the middle and basal regions of the cochlea. When changing place of stimulation from apex to base using low-rate pulse trains, the “cleanness” of the sound deteriorates between electrodes 2 and 6 with the most dramatic changes between electrodes 4 and 5. Although it is impossible to know exactly where the electrodes lie without radiology, in Schatzer et al. (2014) the region between 362° and 430° is represented by approximately electrodes 4 (mean electrode position = 4.25, median and mode electrode position = 4; see Figure 7). As all of the subjects with FLEXSOFT arrays in Schatzer et al. (2014) were implanted by the same surgeon in the same hospital and with the same surgical approach as all of the subjects in the present experiment, it is likely that electrodes 4 and 5 are also typically placed between 362° and 430°. If so, this would suggest that the region in which low rates of stimulation begin to sound less “Clean” is also the region in which there is no longer a 1 to 1 relationship between a change in stimulation rate and the corresponding change in pitch. Results from the descriptor “Noisy” provide a similar story as results from “Clean” in that the region in which low rates of stimulation begin to sound more “Noisy” occurs between electrodes 4 and 5 and therefore likely in the region in which there is no longer a 1 to 1 relationship between a change in stimulation rate and the corresponding pitch change.
Figure 7.
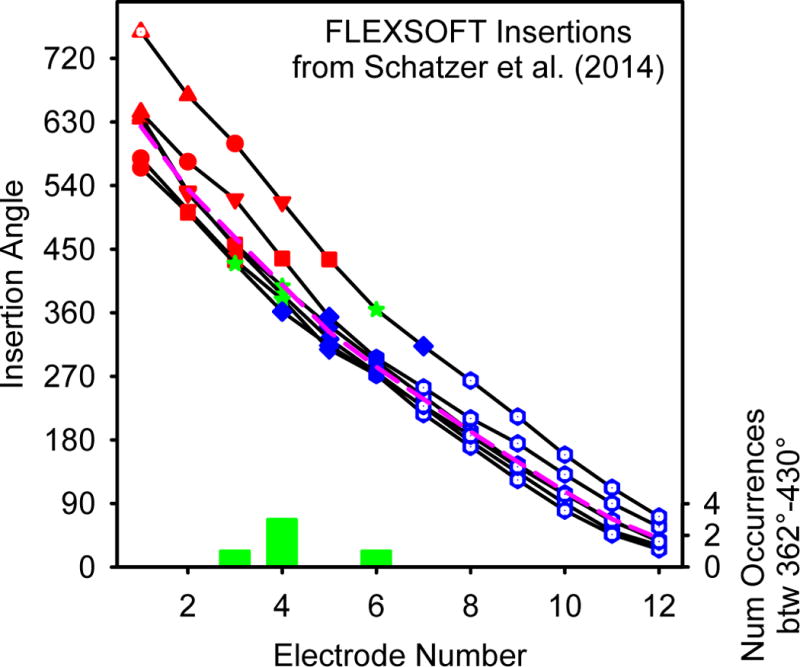
The insertion angle for each electrode of the six FLEXSOFT users evaluated in Schatzer et al. (2014). The pink dashed line indicates the 20% trimmed mean insertion angle for each electrode position. The 20% trimmed mean of the most apical electrode is 624°. The symbols and colors are selected to correspond to the symbols and colors used in Figure 1. Points with white centers represent electrodes which were not evaluated in Schatzer et al. (2014). The histogram at the bottom (in green) represents the frequency that a given electrode number was between 362° and 430°.
The results from our “High” scaling are consistent with previous findings in that they show both the independent and combined effects of a change in rate and place on pitch perception. However the present data represent a more detailed map of the relationships across multiple rates and the entire electrode array than has been previously reported (e.g. Nobbe, 2004; Zeng et al., 2002; Stohl et al., 2008). As each color in the “High” scaling pitch plot represents an equal pitch, the trade-off between rate and place on pitch are documented. For example, electrode 1 at 1500 pps has a similar pitch as electrode 8 at 100 pps. While changes in rate and place both influence pitch, using multi-dimensional scaling, it has been shown that the percepts corresponding to rate and place are independent of each other (e.g. Tong et al., 1983) and that although two different electrodes at two different rates might provide a similar pitch, they will have a different sound quality. The lack of correlation between the “Clean” scaling data and the “High” scaling data also suggests that although different rate and place combinations may have the same pitch, they have different sound qualities. For example, while electrode 1 at 1500 pps and electrode 8 at 100 pps may have the same pitch, the stimulus on electrode 1 is described as much cleaner than the stimulus on electrode 8. Therefore, it seems that while a range of pitches can be provided through a combination of rate and place, low pitches tend to sound cleaner when provided on more apical electrodes despite having the same pitch as stimulus at a lower rate on a less apical electrode.
While the manuscript focuses on the overall scaling results across subjects, it is worth highlighting that there is variability across subjects in each of the scaling tasks as can be seen in the individual subject plots of Figures 3, 4, 5, and 6. One potential source of variability in responses is variability in the spread of excitation for each stimulus. Landsberger et al. (2012) demonstrated that narrower spreads of excitation were consistently described as “cleaner”, “more pure”, as well as “higher in pitch” while broader spreads of excitation were consistently described as “dirtier”, “noisier”, and “lower in pitch”. Eisen and Franck (2005) and Stickney et al. (2006) found a smaller spread of excitation with basal electrodes on arrays (Nucleus Contour and Advanced Bionics HiFocus) designed to be inserted at a maximal angle of 430°. Modeling data of Kalkman et al. (2014) suggests that spread of excitation should be broader at cochlear locations beyond 540°. Combining these results, one could predict that apical electrodes would sound less “clean” and basal electrodes would sound more “clean”. This prediction is contrary to what was observed in the present experiment. Because an increase of amplitude yields an increase in current spread and presumably spread of excitation, the amplitudes at which stimuli were delivered in this experiment are likely to correlate with spread of excitation. Nevertheless, for all subjects but UZA-M1, the correlation between amplitude and “clean” scaling was negative although after Type I error correction (Rom, 1990), none of the correlations were statistically significant. Similarly, for all subjects but UZA-M13 (left and right ears), a positive correlation was found between amplitude and “noisy” scaling. After Type I error correction, only one subject’s correlation (UZA-M10; r (43) = 0.72, p = 0.000266) remained significant. Because higher rates of stimulation require lower amplitudes to maintain a fixed loudness, one would expect a negative correlation between amplitude and pitch. Eight of eleven ears have a negative correlation between amplitude and “high” scaling. Even after Type I error correction (Rom, 1990), the negative correlation is significant for 3 subjects (UZA-M4: r (43) = −0.8118, p = 0. 000000858; UZA-M10: r (43) = −0.694, p = 0.000825; UZA-M13R: r (43) = 0.7405, p = 0. 0000962). Interestingly, one of the two subjects with a positive correlation between amplitude and “high” was also significant (UZA-M1: r (43) = 0.6526, p = 0.002544) after Type I correction. In summary, the data collected in the present experiment does not strongly support the hypothesis that spread of excitation effects the sound quality of single electrode stimuli. To make a stronger statement about the relationship, a direct measure of spread of excitation (as was measured in Landsberger et al. (2012)) is needed.
Similar results were found for unpublished experiments presented in a Ph.D. thesis by Nobbe (2004). Nobbe asked patients to scale the pitch and sound quality of single electrode pulse trains at various stimulation rates on electrodes 1, 3, 7 and 10 of MED-EL Standard (31 mm) array users. Similar to our results, Nobbe found that high rates tend to sound better than low rates, and apical electrodes tend to sound better than basal electrodes. However, the most apical electrode was reported to sound worse at low rates in the Nobbe data set while the sound quality at all rates was similar for electrode 1 in the present data set. Nobbe’s (2004) pitch scaling results were also similar to ours (and Schatzer et al., 2014) in that a change in rate provided a greater change in pitch for the basal locations. The range of pitch changes was larger for the present experiment than found in Nobbe (2004) which is likely to be the result of slightly different methodologies. Nobbe (2004) played every stimulus paired with a reference that was “middle pitch” and subjects were told to avoid the extreme points of the scale. This protocol would inherently compress the range of responses. Conversely, in the present experiment, subjects were pre-familiarized with the stimuli and as a result had a concept of the highest and lowest potential sounds. Therefore, subjects were not discouraged from using the extreme points of the scale. This protocol would predictably result in an expanded response range relative to the methodology of Nobbe (2004).
One limitation of the data collected for this manuscript is that postoperative temporal bone CT-scans were not available. Therefore, we are unable to determine the precise locations of the electrodes on each array for each individual subject. Therefore, we collapsed our data across subjects by electrode number and not cochlear location, effectively assuming that each subject has the same exact insertion depth and cochlear geometry. However, it should be noted that there is likely to be great deal of variation in the insertion angles of the arrays used in this study as there is a great deal of variation in cochlear anatomy (e.g. Erixon et al., 2009). The users evaluated in Schatzer et al. (2014) were implanted by the same surgeon at the same hospital as the subjects in this study. Therefore, the mean placements of the electrode arrays for Schatzer et al. (2014) should be similar to the mean placements of the arrays in the present study despite the across subject variations. The placements for each electrode from Schatzer et al. (2014) are presented in Figure 7. While it is impossible to know the specific location of the array for any given subject in the present study, it is assumed that the population in our study is represented by the 20% trimmed mean (624° ± 62° Standard Deviation) of the Schatzer et al. (2014) positions (plotted in a pinked dashed line in Figure 7). It is worth noting that the Schatzer et al. (2014) insertion is similar to other insertions in the field. The 20% trimmed mean insertion of 31 mm MED-EL electrodes from the 8 additional studies (Baumann and Nobbe, 2004; Kos et al., 2005; Hamzavi and Arnoldner, 2006; Gani et al., 2007; Radeloff et al., 2008; Vermeire et al., 2008; Trieger et al., 2011; Landsberger et al., 2015) reported in Table 1 of Landsberger et al. (2015) is 627°. We therefore anticipate that collapsing across electrode numbers instead of electrode angles will produce a similar but noisier result than had we collapsed across electrode position. Pre-operative CT scans were available for seven of the ears and therefore we were able to calculate the basal diameter of cochlea (known as “A” in the literature). “A” can be used to estimate the size of the cochlea (e.g. Éscude et al., 2006; Alexiades et al., 2014; Würfel et al., 2014). As a larger “A” value predicts a larger cochlear duct length, it can be assumed that subjects with larger “A” values have shallower insertions for an equivalent surgery with the same electrode array model. The “A” values we have for the seven ears are presented in Table 1.
All of the subjects in the present experiment are MED-EL users with 31 mm electrode arrays (i.e. either the FLEXSOFT or Standard) who have been clinically mapped with a fine structure strategy (see Table 1). The specific results in this experiment could be due to adaptation to low-rate temporal cues on the apical electrodes as well as adaptation to the frequency to electrode allocation that corresponds to FSP/FS4. While the effect of adaptation to stimulation from the specific electrode array configuration and processing strategy cannot be estimated from this dataset, it is worth noting that the related results from both Nobbe (2004) and Schatzer et al. (2014) were obtained with users of the CIS+ strategy (an envelope extracting strategy without fine structure channels.) It is also worth noting that Blamey et al. (1996) conducted a rate pitch matching experiment with bimodal Nucleus 22 users. On the most apical electrode with an average insertion of 349°, the ratio of a change in stimulation rate to a change in pitch-matched frequency was 0.36. This point (plotted on Figure 1) is consistent with the data collected with the MED-EL users by Schatzer et al. (2014). Although unspecified, it is likely that the Nucleus users all used the SPEAK speech processing strategy which is an envelope extracting strategy with only a 250 pps stimulation rate. Despite these findings with different strategies and/or electrode arrays, follow-up replications of the current experiment with users of different electrode arrays or strategies are required to understand the potential effect of adaptation on sound quality as a function of rate and place.
The combined results from this experiment and Schatzer et al. (2014) suggest that temporal coding beyond 430° into the cochlea provide both a better sound quality but also a better relationship between the temporal code provided by the implant and the corresponding perceived pitch. These results might support the decision of the cochlear implant field which primarily uses electrodes inserted more shallowly than 430° to use strategies which emphasize spectral coding over temporal coding by encoding place of stimulation but discarding temporal fine structure information. Similarly, this might suggest that a sound coding strategy that provides fine structure via temporal coding (like FSP or FS4) might be desirable for longer electrode arrays like the MED-EL FLEXSOFT which typically provide electrical stimulation well beyond 430° into the cochlea, but may be less desirable for electrode arrays that are typically inserted less than 430° such as the MED-EL FLEX24, Advanced Bionics HiFocus 1J, or Cochlear Contour Advance (Landsberger et al., 2015). Nevertheless, follow-up experiments need to be conducted to verify that the results observed in the present experiment are not dependent on adaptation to the specific properties of the electrode array and speech-processing system used in the current study.
Acknowledgments
We are very grateful to Otto Peter at the University of Innsbruck for providing support and a newly developed software revision for the RIB2. Furthermore, we are grateful to Ellen Cochet and Anouk Hofkens for helping with a number of logistical issues including recruiting subjects. Reinhold Schatzer was generous enough to provide his own raw data for reanalysis as well as provide his own perspectives on the data. Further appreciation is given to Ann Todd, Monica Padilla, and Natalia Stupak for their helpful comments on an earlier version of the manuscript. Thanks are given to two anonymous reviewers for their helpful comments and for informing us about the relevant unpublished experiments in Andrea Nobbe’s Ph.D. thesis. Support for this research was provided by the NIH/NIDCD (R01 DC012152) and a MED-EL Hearing Solutions grant.
References
- Alexiades G, Dhanasingh A, Jolly C. Method to Estimate the Complete and Two-Turn Cochlear Duct Length. Otology & Neurotology. 2015;36 doi: 10.1097/MAO.0000000000000620. [DOI] [PubMed] [Google Scholar]
- Aronoff JM, Freed DJ, Fisher LM, et al. The effect of different cochlear implant microphones on acoustic hearing individuals’ binaural benefits for speech perception in noise. Ear Hear. 2011;32:468–484. doi: 10.1097/AUD.0b013e31820dd3f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann U, Nobbe A. Pitch ranking with deeply inserted electrode arrays. Ear Hear. 2004;25:275–283. doi: 10.1097/00003446-200406000-00008. [DOI] [PubMed] [Google Scholar]
- Baumann U, Nobbe A. The cochlear implant electrode-pitch function. Hear Res. 2006;213:34–42. doi: 10.1016/j.heares.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Blamey PJ, Dooley GJ, Parisi ES, et al. Pitch comparisons of acoustically and electrically evoked auditory sensations. Hear Res. 1996;99:139–150. doi: 10.1016/s0378-5955(96)00095-0. [DOI] [PubMed] [Google Scholar]
- Boex C, Baud L, Cosendai G, et al. Acoustic to electric pitch comparisons in cochlear implant subjects with residual hearing. J Assoc Res Otolaryngol. 2006;7:110–124. doi: 10.1007/s10162-005-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon RP, Macherey O, Frijns JH, et al. Pitch Comparisons between Electrical Stimulation of a Cochlear Implant and Acoustic Stimuli Presented to a Normal-hearing Contralateral Ear. J Assoc Res Otolaryngol. 2010;11:625–640. doi: 10.1007/s10162-010-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GS, Kreft HA, Litvak L. Place-pitch discrimination of single- versus dual-electrode stimuli by cochlear implant users (L) J Acoust Soc Am. 2005;118:623–626. doi: 10.1121/1.1937362. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Spahr T, Gifford R, et al. An electric frequency-to-place map for a cochlear implant patient with hearing in the nonimplanted ear. J Assoc Res Otolaryngol. 2007;8:234–240. doi: 10.1007/s10162-007-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington DK, Dobelle WH, Brackmann DE, et al. Place and periodicity pitch by stimulation of multiple scala tympani electrodes in deaf volunteers. Trans Am Soc Artif Intern Organs. 1978;24:1–5. [PubMed] [Google Scholar]
- Eisen MD, Franck KH. Electrode interaction in pediatric cochlear implant subjects. J Assoc Res Otolaryngol. 2005;6:160–170. doi: 10.1007/s10162-005-5057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erixon E, Hogstorp H, Wadin K, et al. Variational anatomy of the human cochlea: implications for cochlear implantation. Otol Neurotol. 2009;30:14–22. doi: 10.1097/MAO.0b013e31818a08e8. [DOI] [PubMed] [Google Scholar]
- Escude B, James C, Deguine O, et al. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurootol. 2006;11(Suppl 1):27–33. doi: 10.1159/000095611. [DOI] [PubMed] [Google Scholar]
- Fretz RJ, Fravel RP. Design and function: a physical and electrical description of the 3M House cochlear implant system. Ear Hear. 1985;6:14S–19S. [PubMed] [Google Scholar]
- Gani M, Valentini G, Sigrist A, et al. Implications of deep electrode insertion on cochlear implant fitting. J Assoc Res Otolaryngol. 2007;8:69–83. doi: 10.1007/s10162-006-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL. An optimum processor theory for the central formation of the pitch of complex tones. J Acoust Soc Am. 1973;54:1496–1516. doi: 10.1121/1.1914448. [DOI] [PubMed] [Google Scholar]
- Hamzavi J, Arnoldner C. Effect of deep insertion of the cochlear implant electrode array on pitch estimation and speech perception. Acta Otolaryngol. 2006;126:1182–1187. doi: 10.1080/00016480600672683. [DOI] [PubMed] [Google Scholar]
- Hochmair-Desoyer IJ, Hochmair ES, Burian K, et al. Four years of experience with cochlear prostheses. Med Prog Technol. 1981;8:107–119. [PubMed] [Google Scholar]
- Kalkman RK, Briaire JJ, Dekker DM, et al. Place pitch versus electrode location in a realistic computational model of the implanted human cochlea. Hear Res. 2014;315:10–24. doi: 10.1016/j.heares.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Kessler DK. The CLARION Multi-Strategy Cochlear Implant. Ann Otol Rhinol Laryngol Suppl. 1999;177:8–16. [PubMed] [Google Scholar]
- Kos MI, Boex C, Sigrist A, et al. Measurements of electrode position inside the cochlea for different cochlear implant systems. Acta Otolaryngol. 2005;125:474–480. doi: 10.1080/00016480510039995. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, McKay CM. Perceptual differences between low and high rates of stimulation on single electrodes for cochlear implantees. J Acoust Soc Am. 2005;117:319–327. doi: 10.1121/1.1830672. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Mertens G, Kleine Punte A, et al. Perceptual changes in place of stimulation with long cochlear implant electrode arrays. J Acoust Soc Am. 2014;135:EL75–81. doi: 10.1121/1.4862875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger DM, Padilla M, Srinivasan AG. Reducing current spread using current focusing in cochlear implant users. Hear Res. 2012;284:16–24. doi: 10.1016/j.heares.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger DM, Svrakic M, Roland JT, Jr, et al. The Relationship between Insertion Angles, Default Frequency Allocations, and Spiral Ganglion Place Pitch in Cochlear Implants. Ear and Hearing. 2015 doi: 10.1097/AUD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneau J, Wouters J, Moonen M. Improved music perception with explicit pitch coding in cochlear implants. Audiol Neurootol. 2006;11:38–52. doi: 10.1159/000088853. [DOI] [PubMed] [Google Scholar]
- Licklider JC. Three auditory theories. In: Koch S, editor. Psychology: a Study of a Science. New York: McGraw-Hill; 1959. pp. 41–144. [Google Scholar]
- Luo X, Padilla M, Landsberger DM. Pitch contour identification with combined place and temporal cues using cochlear implants. J Acoust Soc Am. 2012;131:1325–1336. doi: 10.1121/1.3672708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ, Carlyon RP. Place and temporal cues in pitch perception: are they truly independent? Acoustics Research Letters Online. 2000;1:25–30. [Google Scholar]
- Meddis R, O’Mard L. A unitary model of pitch perception. J Acoust Soc Am. 1997;102:1811–1820. doi: 10.1121/1.420088. [DOI] [PubMed] [Google Scholar]
- Milczynski M, Wouters J, van Wieringen A. Improved fundamental frequency coding in cochlear implant signal processing. J Acoust Soc Am. 2009;125:2260–2271. doi: 10.1121/1.3085642. [DOI] [PubMed] [Google Scholar]
- Nobbe A. Pitch Perception and Signal Processing in Electric Hearing. Faculty of Medicine: LMU Munich 2004 [Google Scholar]
- Oxenham AJ, Bernstein JG, Penagos H. Correct tonotopic representation is necessary for complex pitch perception. Proc Natl Acad Sci U S A. 2004;101:1421–1425. doi: 10.1073/pnas.0306958101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijl S. Audiologic results with the MSP/MPEAK and WSP/F0F1F2 processors and coding strategies for the nucleus cochlear implant. J Otolaryngol. 1994;23:286–291. [PubMed] [Google Scholar]
- Prentiss S, Staecker H, Wolford B. Ipsilateral acoustic electric pitch matching: A case study of cochlear implantation in an up-sloping hearing loss with preserved hearing across multiple frequencies. Cochlear Implants Int. 2014 doi: 10.1179/1754762814Y.0000000066. [DOI] [PubMed] [Google Scholar]
- Radeloff A, Mack M, Baghi M, et al. Variance of angular insertion depths in free-fitting and perimodiolar cochlear implant electrodes. Otol Neurotol. 2008;29:131–136. doi: 10.1097/MAO.0b013e318157f0ea. [DOI] [PubMed] [Google Scholar]
- Riss D, Hamzavi JS, Blineder M, et al. FS4, FS4-p, and FSP: a 4-month crossover study of 3 fine structure sound-coding strategies. Ear Hear. 2014;35:e272–281. doi: 10.1097/AUD.0000000000000063. [DOI] [PubMed] [Google Scholar]
- Rom DM. A sequentially rejective test procedure based on a modified Bonferroni inequality. Biometrika. 1990;77:663–665. [Google Scholar]
- Schatzer R, Vermeire K, Visser D, et al. Electric-acoustic pitch comparisons in single-sided-deaf cochlear implant users: Frequency-place functions and rate pitch. Hear Res. 2014;309C:26–35. doi: 10.1016/j.heares.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Multichannel electrical stimulation of the auditory nerve in man. I. Basic psychophysics. Hear Res. 1983;11:157–189. doi: 10.1016/0378-5955(83)90077-1. [DOI] [PubMed] [Google Scholar]
- Stickney GS, Loizou PC, Mishra LN, et al. Effects of electrode design and configuration on channel interactions. Hear Res. 2006;211:33–45. doi: 10.1016/j.heares.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Stohl JS, Throckmorton CS, Collins LM. Assessing the pitch structure associated with multiple rates and places for cochlear implant users. J Acoust Soc Am. 2008;123:1043–1053. doi: 10.1121/1.2821980. [DOI] [PubMed] [Google Scholar]
- Terhardt E. Calculating virtual pitch. Hear Res. 1979;1:155–182. doi: 10.1016/0378-5955(79)90025-x. [DOI] [PubMed] [Google Scholar]
- Tong YC, Blamey PJ, Dowell RC, et al. Psychophysical studies evaluating the feasibility of a speech processing strategy for a multiple-channel cochlear implant. J Acoust Soc Am. 1983;74:73–80. doi: 10.1121/1.389620. [DOI] [PubMed] [Google Scholar]
- Trieger A, Schulze A, Schneider M, et al. In vivo measurements of the insertion depth of cochlear implant arrays using flat-panel volume computed tomography. Otol Neurotol. 2011;32:152–157. doi: 10.1097/MAO.0b013e3181fcf04d. [DOI] [PubMed] [Google Scholar]
- van Wieringen A, Wouters J. LIST and LINT: sentences and numbers for quantifying speech understanding in severely impaired listeners for Flanders and the Netherlands. Int J Audiol. 2008;47:348–355. doi: 10.1080/14992020801895144. [DOI] [PubMed] [Google Scholar]
- Vandali AE, Sucher C, Tsang DJ, et al. Pitch ranking ability of cochlear implant recipients: a comparison of sound-processing strategies. J Acoust Soc Am. 2005;117:3126–3138. doi: 10.1121/1.1874632. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Landsberger DM, Schleich P, et al. Multidimensional scaling between acoustic and electric stimuli in cochlear implant users with contralateral hearing. Hear Res. 2013;306:29–36. doi: 10.1016/j.heares.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire K, Landsberger DM, Van de Heyning PH, et al. Frequency-place map for electrical stimulation in cochlear implants: Change over time. Hear Res. 2015;326:8–14. doi: 10.1016/j.heares.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire K, Nobbe A, Schleich P, et al. Neural tonotopy in cochlear implants: an evaluation in unilateral cochlear implant patients with unilateral deafness and tinnitus. Hear Res. 2008;245:98–106. doi: 10.1016/j.heares.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Von Helmholtz H. On the Sensations of tone as a physiological basis for the theory of music. 4th. Braunschweig: Friedrich Vieweg; 1912. [Google Scholar]
- Wever EG. Theory of Hearing. New York: John Wiley & Sons; 1940. [Google Scholar]
- Wever EG, Bray CW. Present possibilities for auditory theory. Psychological Review. 1930;37:365–380. [Google Scholar]
- Wilcox RR, Keselman HJ, Kowalchuk RK. Can tests for treatment group equality be improved?: The bootstrap and trimmed means conjecture. British Journal of Mathematical and Statistical Psychology. 1998;51:123–134. [Google Scholar]
- Wundt W. Principles of physiological psychology. 5th. Leipzig; Germany: 1904. [Google Scholar]
- Wurfel W, Lanfermann H, Lenarz T, et al. Cochlear length determination using Cone Beam Computed Tomography in a clinical setting. Hear Res. 2014;316:65–72. doi: 10.1016/j.heares.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Zeng FG. Temporal pitch in electric hearing. Hear Res. 2002;174:101–106. doi: 10.1016/s0378-5955(02)00644-5. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Tang Q, Lu T. Abnormal pitch perception produced by cochlear implant stimulation. PLoS One. 2014;9:e88662. doi: 10.1371/journal.pone.0088662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman-Phillips S, Murad C. Programming features of the CLARION Multi-Strategy Cochlear Implant. Ann Otol Rhinol Laryngol Suppl. 1999;177:17–21. [PubMed] [Google Scholar]


