Abstract
Objective
Most midlife women report vasomotor symptoms, yet their physiology remains poorly understood. This study tested whether acute decreases in cardiac vagal control would occur with vasomotor symptoms in a large sample of women monitored during wake and sleep.
Methods
215 nonsmoking women ages 40–60 with evidence of vasomotor symptoms were included. Women were free of a history of clinical cardiovascular disease or arrhythmia; or use of insulin, beta blockers, calcium channel blockers, or medications impacting vasomotor symptoms. Women underwent 24 hours of ambulatory monitoring for physiological (sternal skin conductance) and self-report (electronic diary) measurement of vasomotor symptoms; heart rate variability (electrocardiogram); and respiratory rate. Changes in cardiac vagal control as assessed by respiratory sinus arrhythmia during vasomotor symptoms relative to periods preceding and following vasomotor symptoms were tested in linear mixed models.
Results
Significant decreases in respiratory sinus arrhythmia were observed during physiologically-measured vasomotor symptoms relative to periods preceding (b(SE)=.13(.004), p<.0001) and following the VMS (b(SE)=.13(.004), p<.0001), adjusted for age, race, body mass index, sleep/wake. Decreases were observed for women not aware of their vasomotor symptoms, and persisted controlling for respiration rate. Interactions indicated that respiratory sinus arrhythmia decreases were most pronounced during sleep and for younger women.
Conclusions
Physiologically-measured vasomotor symptoms were accompanied by an inhibition of cardiac vagal control in a large sample of women. Changes were observed irrespective of whether the vasomotor symptoms were reported, were most pronounced during sleep, and were greatest among younger women. These findings contribute to the understanding of vasomotor symptom physiology.
Keywords: vasomotor symptoms, hot flashes, heart rate variability, autonomic nervous system, cardiac vagal control
Introduction
Vasomotor symptoms (VMS), or hot flashes and night sweats, are common during the menopause transition, experienced by over 70% of women.1 Frequent or severe VMS last much longer than previously thought; an average of 7–10 years.2, 3 For many women, VMS are associated with impairments in quality of life, mood, and sleep.4–6 New data also has indicated that VMS may be linked to physical health, including indicators of cardiovascular disease (CVD) risk.7, 8 In the wake of findings of potential risk associated with the most effective treatment for VMS, hormone therapy9, many women have discontinued hormone therapy,10 and no other comparatively effective treatment options have emerged. Thus, there has been great interest in clarifying the underlying physiology of VMS to better understand this common midlife experience, to inform new treatments for VMS, and to elucidate the implications of VMS for the health of midlife women.
Despite their ubiquity, the physiology of VMS is not well established. Some work has implicated the autonomic nervous system and its innervation of cardiac and vascular tissue in the etiology of VMS. Alpha sympathetic control of the skin vasculature is important for thermoregulation, and vagal and beta sympathetic control directly influence cardiac function (heart rate (HR), contractility).11, 12 Vagal activation often, though not uniformly, acts to counter sympathetic activation. Some early research shows increased plasma levels of epinephrine13 and of a metabolite of brain norepinephrine occurring with VMS.14 Recently investigators have been using electrocardiogram (ECG)-assessed heart rate variability (HRV), an index of autonomic control of heart rate. One HRV index is respiratory sinus arrhythmia (RSA), which provides an index of parasympathetic, or vagal control of the heart.11, 15 Although some limited research16,17,18 suggests acute vagal withdrawal occurring during physiologically-assessed VMS, this work has notable limitations, including being based upon very small sample sizes.
In this study, we examined whether physiologically-monitored VMS are accompanied by acute reductions in cardiac vagal control during wake and sleep among 216 women undergoing 24 hours of ambulatory ECG and physiologic and diary VMS monitoring. We further considered changes in heart rate and respiration during VMS. Finally, we considered changes in cardiac vagal control with VMS as a function of whether or not the VMS was reported.
Methods
Study Sample
The study sample included 215 late perimenopausal (no menstrual period in the prior two-12 months) and postmenopausal (no menstrual period in the prior 12 months)19 women participating in a study about VMS and cardiovascular function. All women were between ages 40 and 60, showed evidence of VMS on skin conductance monitoring, and had ≥17 hours (75%) of valid ECG and sternal skin conductance data. Exclusion criteria included hysterectomy and/or oophorectomy; current smoking; reported heart disease, stroke, or arrhythmia; pregnancy; use of oral or transdermal estrogen or progesterone, gabapentin, insulin, clonidine, beta blockers, calcium channel blockers, SSRI/SNRI antidepressants within the past three months; and currently undergoing chemotherapy for breast cancer. 215 women (152 non-Hispanic White, 55 African American, and 8 women of other ethnicities) were included in primary analytic models.
Design and Procedures
Women were recruited from the community via advertisements, mailings, and postings on message boards. After a telephone and in-person screening, height and weight were measured, questionnaires administered, and participants equipped with an ambulatory monitor (VU-AMS, VU University Amsterdam, www.vu-ams.nl, Amsterdam, the Netherlands)20–22 that they wore for 24 hours as they went about their daily activities. This portable device worn in a pouch around the waist measures sternal skin conductance (for VMS), ECG (for HRV), and thoracic impedance (for respiration) continuously. Women were also provided with an electronic diary to be completed during waking hours when experiencing a VMS. Procedures were approved by the University of Pittsburgh Institutional Review Board. Participants provided written informed consent.
Measures
VMS
Sternal skin conductance was sampled at 1 Hz from the sternum via a 0.5 Volt constant voltage circuit passed between two Ag/AgCl electrodes (UFI) filled with 0.05M KCL Velvachol/glycol paste.23 Participants were instructed to avoid exercising and showering during monitoring. At the end of monitoring, data was downloaded, and VMS were scored using UFI software (DPSv3.7; Morro Bay, CA). This software automatically flags skin conductance rises of 2 µmho in 30 seconds, the standard criterion for VMS.24 All events were also visually reviewed. Given that some women show submaximal VMS failing to reach the 2 µmho criterion,25, 26 all potential VMS events (submaximal VMS that show the characteristic hot flash pattern27) were also visually inspected, and events showing the characteristic pattern yet <2 micro mho/30 sec rise were coded and independently verified by two coders. This coding approach has been shown to be reliable (κ=.86).25, 26 A 20-minute lockout period was implemented after the start of the VMS during which no VMS were coded. Women were also asked to report VMS they subjectively experienced by 1) completing a portable electronic diary (Palm Z22, Palm, Inc.) and 2) pressing event mark buttons on the monitor that provided a date and time-stamped event mark.
HR, HRV and respiration
HR was measured by ECG via three Ag/Ag Cl electrodes (Ultratrace 1690, Conmed; Utica, NY) in a standard 3-lead configuration sampled at 1 KHz via the VU-AMS. Respiration was measured via thoracic impedance, sampled at 1 KHz via 4 Ag/Ag Cl electrodes.20, 28 Thoracic impedance to index respiration rate is preferable in the ambulatory setting for participant comfort and data quality.29
Each heartbeat or R wave markers in the ECG signal were assessed for artifacts by an artifact detection algorithm (VU-AMS.5fs software; VU University Amsterdam, www.vu-ams.nl, Amsterdam, the Netherlands). All data were also reviewed and edited in detail by trained coders for the classification of normal from ectopic and artifactual beats, the latter being removed or interpolated. Minutes with ≥75% ectopic beats were eliminated. Min-by-min estimates of HR and RSA were conducted using VU-AMS software in accordance with guidelines.11, 15 RSA was scored by VU-AMS software based on the peak-valley method. This method uses the inter-beat interval (IBI) time series extracted from the ECG together with the respiration signal obtained from the thorax impedance change signal to obtain heart period variability that is associated with respiration.15, 28, 29 RSA indexes changes in heart rate that occur during each respiratory cycle. Heart rate increases during inspiration and decreases during expiration, variations which are largely vagally mediated.15 RSA is computed for each respiratory cycle from the shortest IBI during an interval starting at the begin of inspiration and ending 1000 msec after the end of inspiration and the longest IBI during an interval starting at the beginning of expiration and ending 1000 msec delay after the end of expiration. RSA is calculated by subtracting the shortest IBI from the longest IBI. For secondary analyses SDNN, the standard deviation of all normal R-R intervals and a time domain approach to calculating HRV was also calculated.11
Covariates
Height and weight were measured via a fixed stadiometer and a calibrated balance beam scale, respectively. Demographics, menstrual history, and health behaviors were assessed by demographic and medical history questionnaires. Sleep/wake times were determined from a sleep diary completed before the woman went to bed at night and upon waking the following morning. Race/ethnicity was determined in response to “How would you describe your primary racial or ethnic group?” Menopause status was obtained from reported bleeding patterns, categorized as perimenopausal (bleeding in previous three months with decrease in cycle predictability in past year or >3–<12 months amenorrhea), or postmenopausal (≥12 months amenorrhea).19 Time since the final menstrual period was calculated from the recalled date of the woman’s last menstrual period.
Statistical analysis
RSA and SDNN values were log (ln) transformed for analysis due to skewness. Consistent with prior work16, 17 pre-VMS, VMS, and post-VMS time periods were identified by comparing RSA during the minute at which the onset of the VMS occurred (i.e., minute zero) to each of the preceding and following intervals within a single mixed effects regression. Three periods were yielded: 1) a pre-VMS period, ranging from 8 to 2 min prior to flash onset; 2) a VMS period, ranging from 1 min prior to 5 min following VMS onset; and 3) a post-VMS period, ranging from 6 min to 12 min following onset. These 6 min pre- and post-VMS periods were next entered into subsequent models as within-woman factors, with the during-flash time segment considered the referent. We also considered longer pre and post-VMS intervals (encompassing the 20 min before and after the VMS)17 and findings were comparable, so analytic models with VMS intervals of equal length (6 min) are presented here.
Relations between VMS intervals, RSA, heart rate, and respiration were estimated with linear mixed effects models, with random intercepts for VMS nested within participants and maximum likelihood estimation. The within-group correlation structure was modeled as a first order continuous autoregressive, nested within VMS and participants. Covariates included age, race/ethnicity, and body mass index (BMI) (note that menopause stage was not related to RSA). Physiologically-detected VMS (regardless of whether they were reported) and onset times were used for all primary models; subjective VMS (regardless of whether they were physiologically detected) and times were considered in secondary models. Additional models considered RSA changes during physiologically-detected VMS that were also reported, physiologically-detected VMS that were not reported, and reported VMS that lacked a corresponding physiologically-detected VMS. Respiratory rate was added to covariate-adjusted models to examine whether it accounted for any relations between (physiologically-detected or self-reported) VMS and RSA. Further, given potential dependence of HRV on HR, in secondary analyses we used the formula of Monfredi to correct for HR level (SDNN/exp(HR/58.8)).30 Interactions between VMS period and sleep-wake status, VMS reporting status, menopause stage, time since the final menstrual period, age, and race/ethnicity in relation to RSA were tested with cross product terms for VMS intervals and the relevant moderator via the likelihood ratio test. Analyses were performed with SAS v9.4 (SAS Institute, Cary, NC). Models were 2-sided, alpha=0.05.
Results
Participants were on average 54 years old, postmenopausal, normotensive and overweight (Table 1). Of the 215 women, 148 (69%) women reported having VMS and 67 (31%) reported that they did not have VMS in the past 3 months. The sample showed a median of 10 physiologically-detected VMS/24 hours (7 wake, 3 sleep) and reported 3 VMS/24 hours (2 wake, 0 sleep). The women who reported having VMS reported a median of 6 VMS/24 hours. The total number of VMS across the sample was 2386 physiologically-detected VMS and 958 self-reported VMS.
Table 1.
Subject characteristics (N=215)
| Age, M (SD) | 53.9 (3.8) |
| Race/ethnicity, N (%) | |
| White | 152 (70.7) |
| Black | 55 (25.6) |
| Asian/Hispanic/mixed | 8 (3.7) |
| Menopause stage, N (%) | |
| Perimenopausal | 34 (15.8) |
| Postmenopausal | 181 (84.2) |
| Education, N (%) | |
| High school/some college/vocational | 100 (46.5) |
| College graduate | 57 (26.5) |
| > College | 58 (27.0) |
| BMI, M (SD) | 28.8 (6.6) |
| SBP, M (SD) | 120.1 (13.9) |
| DBP, M (SD) | 71.1 (8.9) |
| CESD, M (SD) | 8.0 (7.8) |
| Self-rated health, N (%) | |
| Good/fair/poor | 68 (31.6) |
| Very good | 93 (43.3) |
| Excellent | 54 (25.1) |
| RSA, Median (IQR) | |
| Total | 36.9 (26.5, 48.1) |
| Wake | 32.4 (24.6, 42.5) |
| Sleep | 44.0 (34.2, 60.9) |
| Heart rate, beats/min, M (SD) | |
| Total | 77.4 (10.1) |
| Wake | 81.9 (10.2) |
| Sleep | 67.6 (9.4) |
| Respiration rate, breaths/min, M (SD) | |
| Total | 16.3 (1.7) |
| Wake | 17.0 (1.6) |
| Sleep | 14.8 (2.2) |
| Physiologic hot flashes, number/woman Median (IQR) | |
| Total | 10 (4–16) |
| Wake† | 7 (3–12) |
| Sleep§ | 3 (1–4) |
| Self-reported hot flashes, number/woman Median (IQR) | |
| Total | 3 (0–7) |
| Wake† | 2 (0–6) |
| Sleep§ | 0 (0–1) |
Number of hot flashes/monitoring time standardized to 17 hour waking period;
Number of hot flashes/monitoring time standardized to 7 hour sleep period
We found a significant reduction in RSA observed during the VMS period relative to the periods preceding and following physiologically-detected VMS (Table 2, Figure 1A). Reductions were somewhat more pronounced during sleep than during wake (interaction p<.0001), differences that were not fully due to the higher RSA during sleep (interaction p<.0001 when controlling for average pre-flash RSA). We also examined subjectively-reported VMS and found significant reductions in RSA during subjectively-reported VMS (Table 2, Figure 1B).
Table 2.
Regression weights for the difference of RSA from pre and post-VMS period relative to RSA during the VMS
| RSA (24 hour) | RSA (wake) | RSA (sleep) | ||||
|---|---|---|---|---|---|---|
| B (SE) | P value | B (SE) | P value | B (SE) | P value | |
| Physiologic VMS | ||||||
| Pre-VMS period | .13 (.004) | <.0001 | .11 (.005) | <.0001 | .16 (.007) | <.0001 |
| Post-VMS period | .13 (.004) | .13 (.005) | .13 (.007) | |||
| Self-report VMS | <.0001 | |||||
| Pre-VMS period | .12 (.007) | .11 (.008) | <.0001 | .18 (.01) | <.0001 | |
| Post-VMS period | .14 (.007) | .13 (.008) | .17 (.01) | |||
Controlling for age, race, BMI, and (in 24-hour RSA models) wake/sleep; RSA log transformed; N=215;
Figure 1.
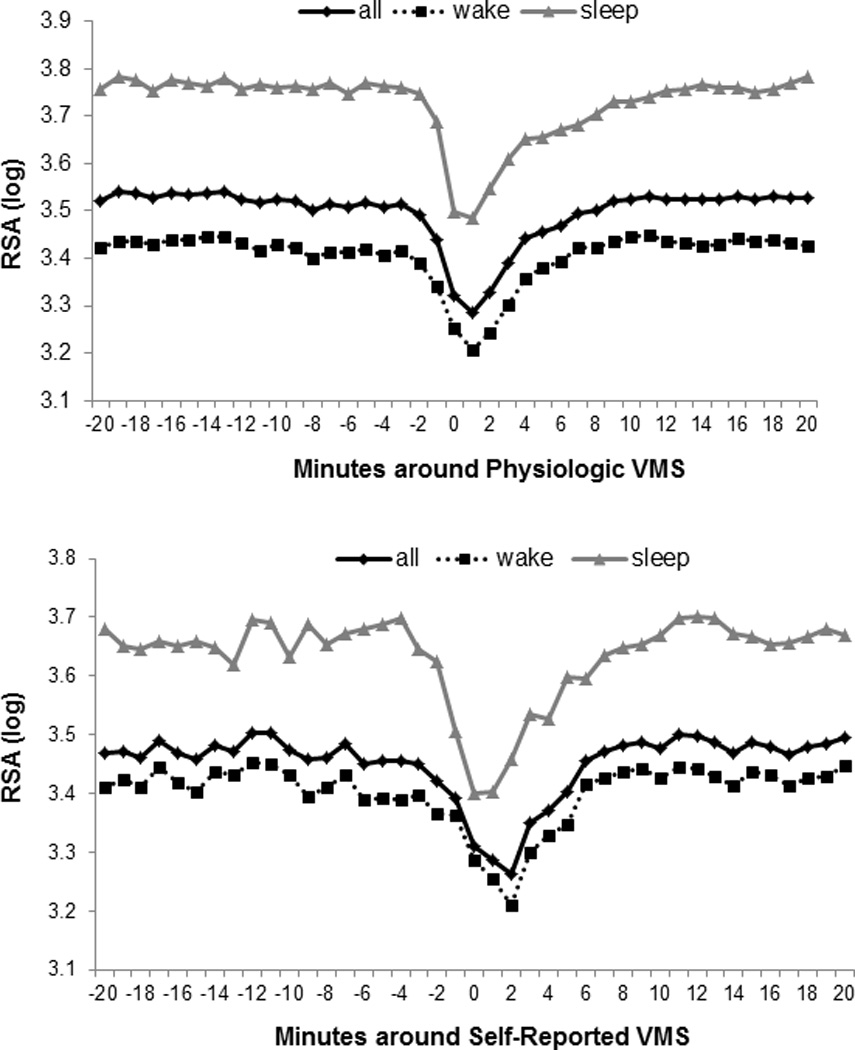
Mean RSA during physiologically-monitored VMS (A), and self-reported VMS (B), stratified by wake/sleep (N=215)
Both HR and respiratory rate significantly increased during physiologically-detected VMS as compared to pre- and post-VMS periods (Figure 2; HR: pre-VMS b(SE)=−3.56 (.08), p<.0001; post-VMS b(SE)= −3.77 (.07), p<.0001; respiratory rate: pre-VMS b(SE)=−.38 (.02), p<.0001; post-VMS b(SE)=−.40 (.02), p<.0001; both models relative to VMS period and adjusted for age, BMI, race/ethnicity, wake/sleep). Changes were similar for self-reported VMS (Supplementary Figure 1). Given the role of respiration in RSA, we examined RSA changes during VMS also controlling for respiration, and findings persisted for physiologically-detected and self-reported VMS (physiologically-detected: pre-VMS b(SE)=.09 (.004), post-VMS b(SE)=.10 (.004), p<.0001; self-reported: pre-VMS b(SE)=.09 (.006), post-VMS b(SE)=.10 (.006), p<.0001; both models relative to VMS period and adjusted for age, BMI, race/ethnicity, wake/sleep, respiration rate). Further, given the potential dependence of HRV on HR, we used a formula that corrects for HR level in examining autonomic changes during VMS (SDNN/exp(HR/58.8))30 and findings persisted (relative to VMS period: pre-VMS b(SE)=−.10 (.005), post-VMS b(SE)= −.07 (.005), p<.0001; adjusted for age, BMI, race/ethnicity, wake/sleep).
Figure 2.
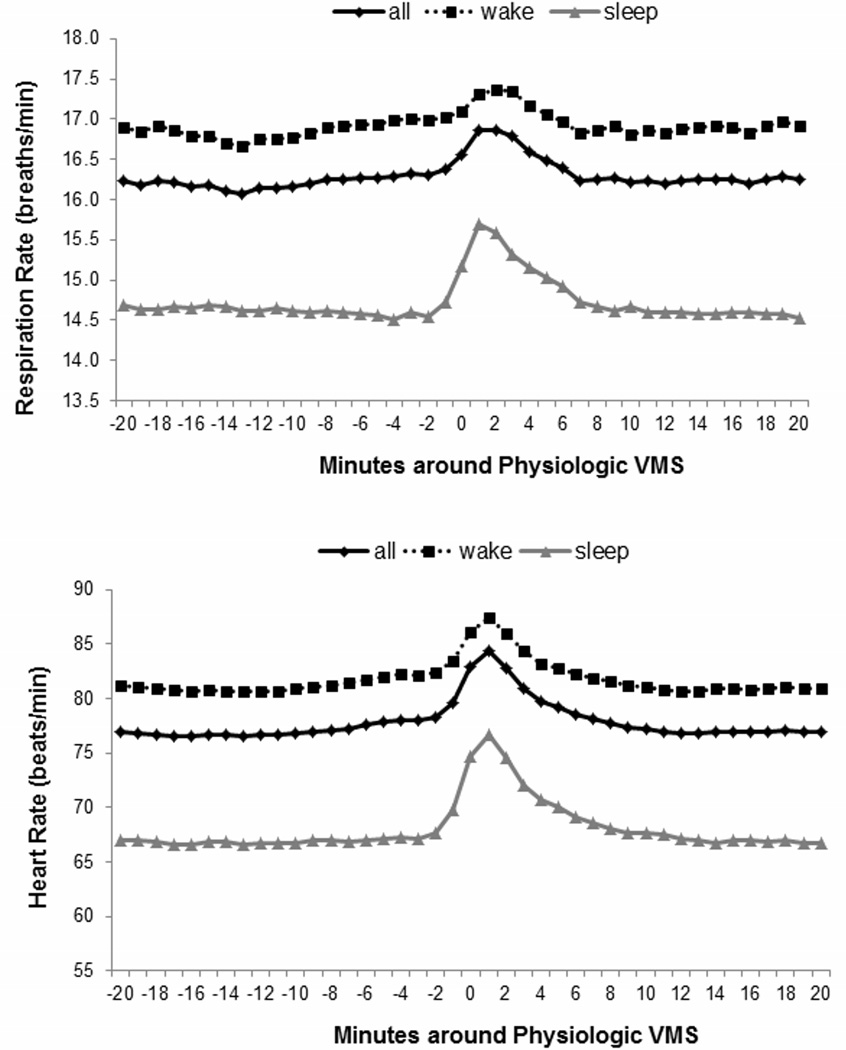
Mean heart rate (A) and respiration rate (B) during physiologically-monitored VMS (N=215)
We next examined RSA changes during physiologically-detected VMS that were also reported (VMS, N=783), self-reported VMS that were not physiologically detected (N=174) and physiologically-detected VMS that were not reported (N=1585; Table 3). Although all types of VMS showed significant reductions in RSA, examination of effect sizes indicated particularly pronounced associations for VMS that were both physiologically detected and reported. Further, as many women who showed VMS here (31%) denied having current VMS, we examined whether the RSA changes during the physiologically-detected VMS of women who denied currently having VMS were comparable to those of the women who reported having VMS. Reductions in RSA during VMS among women who did not report having VMS were slightly smaller (interaction p=.002) but also statistically significant (Supplementary Table 1, Supplementary Figure 2).
Table 3.
Regression weights for the difference of RSA from pre and post-VMS period relative to RSA during the VMS, by physiologic occurrence and self-report of VMS, wake and sleep
| Physiologic VMS that are reported | Physiologic VMS that were not self- reported |
Self-reported VMS not physiologically detected |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wake | Sleep | Wake | Sleep | Wake | Sleep | |||||||
| B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | |
| VMS period | ||||||||||||
| Pre-VMS | .13 (.009) | <.0001 | .23 (.02) | <.0001 | .10 (.007) | <.0001 | .14 (.008) | <.0001 | .07 (.02) | .001 | .13 (.03) | <.0001 |
| Post-VMS | .15 (.009) | .18 (.02) | .12 (.007) | .11 (.008) | .07 (.02) | .14 (.03) | ||||||
Controlling for age, race, BMI; RSA log transformed; N=215
We conducted several additional analyses. As 19 women were taking medications that could impact autonomic function (inhaled beta agonists, anticholinergics, epinephrine), we conducted analyses without these women and findings were largely unchanged (relative to VMS period: pre-VMS b(SE)=.12 (.004), post-VMS b(SE)=.13 (.004), p<.0001; adjusted for age, BMI, race/ethnicity, wake/sleep). Notably, these medications were not related to RSA here (p’s>.50). Further, we examined any differences in RSA during VMS by age, menopause stage, time since the last menstrual period, and race/ethnicity. Significant interactions by age were observed (p<.0001), with changes in RSA during VMS somewhat more pronounced among younger women (Supplementary Table 2, Supplementary Figure 3). These differences were not driven by average RSA differences by age (interaction p=.0004, controlling for average RSA level). Further, there was a statistically significant interaction between race/ethnicity and VMS period (p=.002), but examination of RSA changes with VMS by race/ethnicity indicated that they were quite similar between racial/ethnic groups (Supplementary Figure 4).
Discussion
In this study we monitored 215 women over 24 hours during wake and sleep. Findings showed acute reductions in cardiac vagal control during VMS. These findings were apparent for both self-reported and physiologically-assessed VMS, with particularly pronounced RSA changes during VMS that were both reported and physiologically detected. Vagal withdrawal with physiologically-detected VMS was even apparent among women who were not aware of having VMS. HR and respiration rate also increased during VMS, yet the autonomic changes observed during VMS were apparent when controlling for HR level as well as controlling for respiration rate. Further, RSA changes with VMS were observed during both wake and sleep, with slightly larger RSA declines with VMS during sleep.
This work extends prior work to a much larger sample of women. Three prior small studies showed acute reductions in cardiac vagal control during VMS, including a study of 30 women performing various tasks in the laboratory,16 11 women measured in the laboratory during sleep,18 and 21 women monitored in the ambulatory setting.17 An additional small study of 20 women indicated potential increases in sympathetic activation with physiologically-detected VMS during sleep,31 yet interpretation of this study is limited by the mixture of sympathetic and parasympathetic contributions to the lower frequency spectral bands examined.11 These initial studies suggest vagal withdrawal and possibly sympathetic activation during VMS. However, the small sample sizes and restriction of many of these studies to sleep limited conclusions. Our study includes a larger and more ethnically diverse sample that includes women, considers wake and sleep, and indexes respiration rate. We more definitively demonstrate increases in HR and reductions in cardiac vagal control with VMS.
Despite the prevalence of VMS, a complete understanding of their physiology has remained elusive. Notably, leading models indicate that VMS reflect altered thermoregulatory function, potentially driven by reproductive hormonal changes, yet also with potential autonomic inputs.32 HRV reflects autonomic control of the heart, and autonomic control of other organs cannot be inferred from our data. However, other evidence more broadly supports a role of the autonomic nervous system in VMS. Prior work has indicated that alterations in sympathetic nerve activity to the skin may play a role in the VMS-associated alterations in skin blood flow.33 Freedman showed clonidine, a centrally acting alpha-2 adrenergic agonist to reduce VMS and yohimbine, an alpha-2 adrenergic antagonist, to increase VMS.34 Other studies have suggested alterations in peripheral catecholamines13 or central norepinephrine14 with VMS. Our results suggest that vagal inhibition may be important to the occurrence of VMS.
These findings may contribute to the emerging literature on VMS and cardiovascular risk. In some (but not all35) prior studies, VMS have been linked to higher subclinical CVD7, 8, 36 and a more adverse CVD risk factor profile.37–39 Notably, reduced cardiac vagal control has been implicated in CVD development.40 The physiologic significance of the transient reductions in cardiac vagal control with VMS observed here is not entirely clear. However, vagal withdrawal has been linked to disinhibition of inflammatory control of tissue macrophages.41 Notably, women in this sample were having many VMS per day. The cardiovascular implications of these repeated reductions in RSA should be investigated in future work.
Given the large number of women and VMS events included examined here, we were able to test variations in RSA during VMS depending on whether the VMS was reported. Reductions in RSA were observed during both physiologically-detected and self-reported VMS, with the most pronounced reductions in RSA observed during physiologically-detected VMS that were also reported. It is widely observed that many VMS that are detected on physiologic monitoring are not reported,42 similar to observations here. Interestingly, 30% of women in the present investigation denied currently having VMS, yet showed evidence of VMS on monitoring. Whether these VMS reflect actual VMS or poor specificity of sternal skin conductance monitoring remains a question. However, these VMS showed a similar autonomic signature as women who reported having VMS, supporting the events as VMS. Thus, these data indicate that RSA reductions are observed during both physiologically-detected and self-reported VMS.
There were significant interactions by age, with greater reductions in RSA observed among the younger midlife women in the sample relative to the older women. These differences were not driven by baseline differences by age. There is some data indicating that the cardiovascular implications of VMS may vary by the timing of VMS in a woman’s lifespan;43 these findings suggest attention to younger midlife women. Notably, we did not observe interactions by menopause stage or time since the last final menstrual period, suggesting that this age moderation is more attributable to chronological than ovarian aging (although it is notable that date of birth is likely reported with more precision than the date of the last menstrual period). We also found significant interactions by race/ethnicity, yet the magnitude of these race-related differences were quite small.
Several limitations deserve mention. First, physiologic measures of VMS have limitations.44 While our methods of quantifying VMS addressed certain of these limitations, questions of the validity of these measures remain. However, it is notable that findings were apparent for VMS physiologically-detected but not reported, supporting the significance of these events. Further, while the sample was large and included both White and non-White (principally African American) women, the ethnic diversity of the sample was somewhat limited, therefore findings may not generalize to other ethnic groups, particularly Hispanic and Asian women.
This study had several strengths. This study included a large sample of women studied over day and night in their home environments. Thus, it had greater power and generalizability than other studies on the topic. The women were free of major cardiovascular co-morbidities. None of the women were taking medications impacting VMS nor key medications impacting cardiovascular function. While some of the women were taking medications that would have some impact on the autonomic nervous system, removal of these women did not alter findings. VMS, ECG, and respiratory rate were measured prospectively and rigorously throughout the monitoring period. Physiologic VMS measures, while subject to the caveats mentioned above, provide a more precise estimation of VMS timing and do not reply upon participant reporting. Further, we carefully considered the role of respiration, as well as potential dependence of HRV on HR. We considered VMS during wake and sleep, allowing comparisons of changes in HRV during both intervals.
Conclusions
This is the first study to examine acute changes in cardiac vagal control during physiologically-assessed and self-reported VMS in a large sample of women monitored over 24 hours. This study provides further evidence that VMS are accompanied by acute increases in heart rate and reductions in cardiac vagal control. These changes were observed for VMS occurring during wake and sleep, for both self-reported and physiologically-detected VMS, and even among women who show VMS that they do not perceive. Together with the larger literature, they indicate vagal withdrawal during VMS and further add to the understanding of this highly prevalent experience.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health, National Heart Lung and Blood Institute (R01HL105647 and K24123565 to Thurston).
Footnotes
N Santoro received a research grant from Bayer Healthcare and owns stock options in Menogenix. No other authors have conflicts of interests or disclosures.
References
- 1.Gold E, Colvin A, Avis N, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Am J Public Health. 2006;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104. doi: 10.1097/AOG.0b013e318214f0de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avis N, Crawford S, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–539. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103(1–3):267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 6.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women's Health Across the Nation. Menopause. 2009;16(5):860–869. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women's Health Across the Nation Heart Study. Circulation. 2008;118(12):1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18(4):352–358. doi: 10.1097/gme.0b013e3181fa27fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause. 2011;18(4):385–392. doi: 10.1097/gme.0b013e3181f43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berntson GG, Bigger JT, Jr, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 12.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clinic proceedings. 2003;78(5):603–612. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg F, Cote LJ, Linkie DM, Dyrenfurth I, Downey JA. Menopausal hot flashes: thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas. 1984;6(1):31–43. doi: 10.1016/0378-5122(84)90063-x. [DOI] [PubMed] [Google Scholar]
- 14.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70(2):332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 15.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 16.Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control: a link to cardiovascular risk? Menopause. 2010;17(3):456–461. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurston R, Christie I, Matthews K. Hot flashes and cardiac vagal control during women’s daily lives. Menopause. 2012;19(4):406–412. doi: 10.1097/gme.0b013e3182337166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Zambotti M, Colrain IM, Sassoon SA, Nicholas CL, Trinder J, Baker FC. Vagal withdrawal during hot flashes occurring in undisturbed sleep. Menopause. 2013 doi: 10.1097/GME.0b013e31828aa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlow SD, Gass M, Hall JE, et al. Executive Summary of the Stages of Reproductive Aging Workshop + 10: Addressing the Unfinished Agenda of Staging Reproductive Aging. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Geus EJ, Willemsen GH, Klaver CH, van Doornen LJ. Ambulatory measurement of respiratory sinus arrhythmia and respiration rate. Biol Psychol. 1995;41(3):205–227. doi: 10.1016/0301-0511(95)05137-6. [DOI] [PubMed] [Google Scholar]
- 21.van Dijk AE, van Lien R, van Eijsden M, Gemke RJ, Vrijkotte TG, de Geus EJ. Measuring cardiac autonomic nervous system (ANS) activity in children. J Vis Exp. 2013;(74):e50073. doi: 10.3791/50073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willemsen GH, De Geus EJ, Klaver CH, Van Doornen LJ, Carroll D. Ambulatory monitoring of the impedance cardiogram. Psychophysiology. 1996;33(2):184–193. doi: 10.1111/j.1469-8986.1996.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 23.Dormire SL, Carpenter JS. An alternative to Unibase/glycol as an effective nonhydrating electrolyte medium for the measurement of electrodermal activity. Psychophysiology. 2002;39(4):423–426. doi: 10.1017.S0048577201393149. [DOI] [PubMed] [Google Scholar]
- 24.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26(5):573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 25.Thurston R, Matthews K, Hernandez J, De La Torre F. Improving the performance of physiologic hot flash measures with support vector machines. Psychophysiology. 2009;46(2):285–292. doi: 10.1111/j.1469-8986.2008.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurston R, Hernandez J, Del Rio J, De la Torre F. Support vector machines to improve physiologic hot flash measures: Application to the ambulatory setting. Psychophysiology. 2011;48(7):1015–1021. doi: 10.1111/j.1469-8986.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6(3):209–215. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 28.Houtveen JH, Groot PF, de Geus EJ. Validation of the thoracic impedance derived respiratory signal using multilevel analysis. Int J Psychophysiol. 2006;59(2):97–106. doi: 10.1016/j.ijpsycho.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Ernst JM, Litvack DA, Lozano DL, Cacioppo JT, Berntson GG. Impedance pneumography: noise as signal in impedance cardiography. Psychophysiology. 1999;36(3):333–338. doi: 10.1017/s0048577299981003. [DOI] [PubMed] [Google Scholar]
- 30.Monfredi O, Lyashkov AE, Johnsen AB, et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64(6):1334–1343. doi: 10.1161/HYPERTENSIONAHA.114.03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman RR, Kruger ML, Wasson SL. Heart rate variability in menopausal hot flashes during sleep. Menopause. 2011;18(8):897–900. doi: 10.1097/gme.0b013e31820ac941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. The Journal of steroid biochemistry and molecular biology. 2014;142:115–120. doi: 10.1016/j.jsbmb.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause. 2008;15(2):290–295. doi: 10.1097/gme.0b013e3180ca7cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman RR, Woodward S, Sabharwal SC. Alpha 2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol. 1990;76(4):573–578. [PubMed] [Google Scholar]
- 35.Wolff EF, He Y, Black DM, et al. Self-reported menopausal symptoms, coronary artery calcification, and carotid intima-media thickness in recently menopausal women screened for the Kronos early estrogen prevention study (KEEPS) Fertility and Sterility. 2013;99(5):1385–1391. doi: 10.1016/j.fertnstert.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bechlioulis A, Kalantaridou SN, Naka KK, et al. Endothelial function, but not carotid intima-media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab. 2010;95(3):1199–1206. doi: 10.1210/jc.2009-2262. [DOI] [PubMed] [Google Scholar]
- 37.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet gynecol. 2012;119(4):753–761. doi: 10.1097/AOG.0b013e31824a09ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and insulin resistance in the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gast GC, Grobbee DE, Pop VJ, et al. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51(6):1492–1498. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- 40.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Thayer JF. Vagal tone and the inflammatory reflex. Cleveland Clinic journal of medicine. 2009;76(Suppl 2):S23–S26. doi: 10.3949/ccjm.76.s2.05. [DOI] [PubMed] [Google Scholar]
- 42.Mann E, Hunter MS. Concordance between self-reported and sternal skin conductance measures of hot flushes in symptomatic perimenopausal and postmenopausal women: a systematic review. Menopause. 2011;18(6):709–722. doi: 10.1097/gme.0b013e318204a1fb. [DOI] [PubMed] [Google Scholar]
- 43.Szmuilowicz ED, Manson JE, Rossouw JE, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011;18(6):603–610. doi: 10.1097/gme.0b013e3182014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79(6):777–781. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


