Abstract
Objective
Neuroendocrine carcinomas of the cervix (NECC) are rare and thought to be aggressive. We performed a population-based analysis to examine the natural history, treatment patterns and outcomes of women with NECC compared to squamous cell carcinoma (SCCC) and adenocarcinoma (AC) of the cervix.
Methods
The National Cancer Database (NCDB) was utilized to identify women with NECC, SCCC, and AC treated from 1998–2011. Clinical, demographic, and treatment characteristics were compared between the groups. The association between tumor histology and survival was examined using Kaplan-Meier analyses and multivariable Cox proportional hazards regression models.
Results
We identified 127,332 patients, including 1,896 (1.5%) with NECC and 101,240 (79.5%) with SCCC and 24,196 (19.0%) with AC. Patients with NECC were younger, more often white, commercially insured, and diagnosed with metastatic disease at presentation compared to women with SCCC. Patients with early-stage NECC were more likely to receive adjuvant chemotherapy and radiation after surgery (P<0.05 for both). In multivariable models stratified by stage and adjusted for clinical and demographic characteristics, the risk of death was higher for patients with NECC compared to SCCC for all stages of disease: stage IB–IIA (HR=2.96; 95% CI, 2.48–3.52), stage IIB–IVA (HR=1.70; 95% CI, 1.45–1.99) and stage IVB (HR=1.14; 95% CI, 0.91–1.43).
Conclusion
NECC are aggressive tumors associated with an increased risk of death. Survival is inferior for NECC compared to squamous cell tumors for women with both early and advanced stage disease.
Introduction
Neuroendocrine carcinomas of the cervix (NECC) are rare tumors that comprise 2–5% of cervical malignancies.1–4 This category of tumors is comprised of small cell, large cell, carcinoid and atypical carcinoid histologic types. Small cell and large cell tumors are poorly differentiated and are characterized by a high mitotic rate, necrosis, frequent lymphovascular space involvement and a more aggressive clinical course. Carcinoid and atypical carcinoid tumors are well-differentiated, are thought to be derived from neural crest cells, and are extremely rare.1 Atypical carcinoid tumors display significant nuclear atypia and are poorly studied.
In general, NECCs are characterized by early spread beyond the cervix and a poor prognosis. Five-year survival rates for NECC are reported at 0–30%.5 Given that NECC is uncommon, data to guide clinical decision-making is limited and treatment regimens are often extrapolated from neuroendocrine tumors from other sites. Therapy for early stage disease involves radical surgery for tumors <4cm, often in combination with adjuvant chemotherapy. Advanced stage disease is treated with combination chemotherapy and radiation. The chemotherapy regimens most commonly utilized include cisplatin and etoposide (EP) or vincristine, doxorubicin, and cyclophosphamide (VAC). Prior work has shown that among women with NECC chemotherapy improves survival, particularly for locally advanced stage disease.6
Institutional reviews of NECC typically include small numbers of women treated over the course of many years or decades.7–11 To date, large studies examining the patterns of care and outcomes of women with NECC are limited.12 We performed a population-based analysis of NECC to examine the natural history, treatment patterns and outcomes of women with these tumors.
Methods
Data Source and Patient Selection
The National Cancer Data Base (NCDB) was analyzed. The NCDB is a registry developed and sponsored by the American College of Surgeons and American Cancer Society that includes hospitals from across the United States.13,14 The NCDB captures all patients with newly diagnosed invasive cancers from over 1500 Commission on Cancer (CoC) affiliated hospitals from across the nation. NCDB collects data on patient demographics, tumor characteristics, including stage, primary and adjuvant therapy, and survival.13,14 Incident tumor cases are abstracted by trained registrars and the data is audited regularly to ensure accuracy. It is estimated that nearly 80% of women with invasive cervical cancer in the U.S. are recorded in NCDB.15 The Columbia University Institutional Review Board deemed the study exempt.
We identified all women with stage I–IV neuroendocrine carcinomas of the cervix, squamous cell carcinoma of the cervix (SCCC), and adenocarcinomas (AC) diagnosed from 1998–2011. As NCDB only reports survival for patients with five or more years of follow-up, survival was analyzed in the subset of patients diagnosed from 1998–2006.
Clinical and Demographic Characteristics
Demographic data analyzed included age at diagnosis (<30, 30–39, 40–49, 50–59, 60–69, ≥70 years), race (white, black, Hispanic, other or unknown), and insurance status (commercial, Medicare, Medicaid, uninsured and unknown). Tumor stage (stages IA–IVB or unknown) was recorded for each patient. Hospital characteristics included region (Northeast, Midwest, South, or West) and location (metropolitan, urban, rural or unknown). Based on the ACS CoC criteria, hospitals were also classified as academic centers, community centers or comprehensive community cancer centers.14
Primary treatment was classified as surgery, radiation (including chemoradiation), chemotherapy, or none. Patients were stratified by stage (IA2–IIA, IIB–IVA, IVB) for analysis, and primary and adjuvant therapies were recorded for each stage group.
Statistical Analysis
Frequency distributions between categorical variables were compared using χ2 tests. Overall survival was estimated as the number of months from diagnosis until death from any cause. Cox proportional hazards regression models were developed to evaluate the association between type of histology and survival while adjusting for other clinical and demographic characteristics. Separate models stratified by stage were developed for early-stage (stage IB–IIA), locally advanced (stage IIB–IVA), and metastatic (stage IVB) tumors. Given that NECC is uncommon and potentially subject to pathologic misclassification, we performed a sensitivity analysis limited to academic centers and comprehensive community cancer centers to mitigate any histologic misclassification at hospitals that treat NECC infrequently. Kaplan-Meier survival curves were developed to contrast survival between women with NECC and SCCC. The log-rank test was used to compare the curves. All hypothesis tests were two-sided. A P-value of <0.05 was considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
We identified a total of 127,332 patients including 1,896 (1.5%) with NECC,101,240 (79.5%) with SCCC, and 24,196 (19.0%) with AC (Table 1). Patients with NECC were more likely to be diagnosed before age 30 (11.2% NECC vs 5.3% SCCC vs. 5.3% AC, P<0.001), present with stage IVB disease (23.6% NECC vs 5.9% SCCC vs. 5.9% AC, P<0.001) and not receive either surgery or radiation therapy (18.3% NECC vs 6.5% SCCC vs. 6.2% AC, P<0.001). Patients with NECC, patients were less likely to be black (15.5% NECC vs.18.2% SCCC vs 8.8% AC, P<0.001), Hispanic (10.3% NECC vs 13.1% SCCC vs. 10.8% AC,, P<0.001) and have Medicaid insurance coverage (14.2% NECC vs 19.6% SCCC vs. 10.7% AC, P<0.001).
Table 1.
Descriptive characteristics of the cohort stratified by histology.
| Squamous cell | Adenocarcinoma | Neuroendocrine | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |||
| All | 101,240 | (79.5) | 24,196 | (19.0) | 1,896 | (1.5) | ||
| Year of diagnosis | <0.001 | |||||||
| 1998 | 8,567 | (8.5) | 1,752 | (7.2) | 130 | (6.9) | ||
| 1999 | 8,211 | (8.1) | 1,653 | (6.8) | 112 | (5.9) | ||
| 2000 | 8,138 | (8.0) | 1,695 | (7.0) | 126 | (6.7) | ||
| 2001 | 7,744 | (7.7) | 1,623 | (6.7) | 118 | (6.2) | ||
| 2002 | 7,440 | (7.4) | 1,626 | (6.7) | 138 | (7.3) | ||
| 2003 | 6,982 | (6.9) | 1,529 | (6.3) | 141 | (7.4) | ||
| 2004 | 6,848 | (6.8) | 1,585 | (6.6) | 118 | (6.2) | ||
| 2005 | 6,894 | (6.8) | 1,633 | (6.8) | 133 | (7.0) | ||
| 2006 | 6,864 | (6.8) | 1,732 | (7.2) | 135 | (7.1) | ||
| 2007 | 6,884 | (6.8) | 1,746 | (7.2) | 129 | (6.8) | ||
| 2008 | 6,869 | (6.8) | 1,820 | (7.5) | 162 | (8.5) | ||
| 2009 | 6,898 | (6.8) | 1,985 | (8.2) | 142 | (7.5) | ||
| 2010 | 6,513 | (6.4) | 1,922 | (7.9) | 144 | (7.6) | ||
| 2011 | 6,388 | (6.3) | 1,895 | (7.8) | 168 | (8.9) | ||
| Age | <0.001 | |||||||
| <30 | 5,410 | (5.3) | 1,291 | (5.3) | 213 | (11.2) | ||
| 30–39 | 20,677 | (20.4) | 6,055 | (25.0) | 377 | (19.9) | ||
| 40–49 | 27,069 | (26.7) | 6,926 | (28.6) | 409 | (21.6) | ||
| 50–59 | 20,850 | (20.6) | 4,343 | (18.0) | 377 | (19.9) | ||
| 60–69 | 13,961 | (13.8) | 2,754 | (11.4) | 259 | (13.7) | ||
| ≥70 | 13,273 | (13.1) | 2,827 | (11.7) | 261 | (13.8) | ||
| Race | <0.001 | |||||||
| White | 63,615 | (62.8) | 17,992 | (74.4) | 1,296 | (68.4) | ||
| Black | 18,444 | (18.2) | 2,124 | (8.8) | 293 | (15.5) | ||
| Hispanic | 13,237 | (13.1) | 2,601 | (10.8) | 195 | (10.3) | ||
| Other | 4,642 | (4.6) | 1,141 | (4.7) | 102 | (5.4) | ||
| Unknown | 1,302 | (1.3) | 338 | (1.4) | 10 | (0.5) | ||
| Insurance status | <0.001 | |||||||
| Commercial | 45,845 | (45.3) | 15,057 | (62.2) | 1,006 | (53.1) | ||
| Medicare | 18,452 | (18.2) | 3,746 | (15.5) | 366 | (19.3) | ||
| Medicaid | 19,791 | (19.6) | 2,598 | (10.7) | 270 | (14.2) | ||
| Uninsured | 10,978 | (10.8) | 1,542 | (6.4) | 152 | (8.0) | ||
| Other | 1,001 | (1.0) | 241 | (1.0) | 11 | (0.6) | ||
| Unknown | 5,173 | (5.1) | 1,012 | (4.2) | 91 | (4.8) | ||
| Region | <0.001 | |||||||
| Northeast | 18,606 | (18.4) | 4,624 | (19.1) | 361 | (19.0) | ||
| Midwest | 22,946 | (22.7) | 5,644 | (23.3) | 391 | (20.6) | ||
| South | 42,071 | (41.6) | 9,105 | (37.6) | 758 | (40.0) | ||
| West | 17,617 | (17.4) | 4,823 | (19.9) | 386 | (20.4) | ||
| Location | <0.001 | |||||||
| Metropolitan | 77,896 | (76.9) | 18,882 | (78.0) | 1,512 | (79.8) | ||
| Urban | 15,965 | (15.8) | 3,439 | (14.2) | 265 | (14.0) | ||
| Rural | 1,924 | (1.9) | 384 | (1.6) | 35 | (1.9) | ||
| Unknown | 5,455 | (5.4) | 1,491 | (6.2) | 84 | (4.4) | ||
| Hospital type | <0.001 | |||||||
| Community cancer program | 8,222 | (8.1) | 1,582 | (6.5) | 108 | (5.7) | ||
| Comprehensive community cancer program | 46,489 | (45.9) | 11,890 | (49.1) | 901 | (47.5) | ||
| Academic | 43,997 | (43.5) | 10,170 | (42.0) | 856 | (45.2) | ||
| Other | 2,532 | (2.5) | 554 | (2.3) | 31 | (1.6) | ||
| Stage | <0.001 | |||||||
| IA (IA, IA1, IA2) | 15,408 | (15.2) | 4,185 | (17.3) | 49 | (2.6) | ||
| IB (IB, IB1, IB2, INOS) | 29,461 | (29.1) | 10,592 | (43.8) | 519 | (27.4) | ||
| IIA (IIA, IIA1, IIA2) | 929 | (0.9) | 245 | (1.0) | 14 | (0.7) | ||
| IIB (IIB, IINOS) | 18,146 | (17.9) | 2,500 | (10.3) | 187 | (9.9) | ||
| IIIA | 1,531 | (1.5) | 236 | (1.0) | 24 | (1.3) | ||
| IIIB | 18,938 | (18.7) | 2,248 | (9.3) | 380 | (20.0) | ||
| IVA | 2,924 | (2.9) | 337 | (1.4) | 55 | (2.9) | ||
| IVB | 6,017 | (5.9) | 1,424 | (5.9) | 447 | (23.6) | ||
| IVNOS | 1,042 | (1.0) | 274 | (1.1) | 64 | (3.4) | ||
| Unknown | 6,844 | (6.8) | 2,155 | (8.9) | 157 | (8.3) | ||
| Primary treatment | <0.001 | |||||||
| Surgery | 48,855 | (48.3) | 16,462 | (68.0) | 716 | (37.8) | ||
| Radiation | 36,404 | (36.0) | 4,949 | (20.5) | 710 | (37.5) | ||
| No surgery or radiation | 6,528 | (6.5) | 1,493 | (6.2) | 347 | (18.3) | ||
| Unknown | 9,453 | (9.3) | 1,292 | (5.3) | 123 | (6.5) | ||
Treatment patterns are displayed in Table 2. For women with early-stage disease, surgery was the most common primary treatment for all histologic subtypes (72.5% for SCCC, 82.9% for AC, and 69.1% for NECC), however, more patients with NECC were treated primarily with radiation (22.4% versus 17.6% SCCC and 10.6% AC, P<0.001). Adjuvant chemotherapy was used in 72.9% of those with NECC compared to 18.1% of women with SCC and 12.9% with AC (P<0.001). Similarly, use of external beam radiotherapy was more common for NECC (31.4% versus 16.8% for SCC and 11.7% for AC).
Table 2.
Patterns of treatment stratified by stage and histology.
| Squamous cell | Adenocarcinoma | Neuroendocrine | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | ||
| Stage IA2–IIA | |||||||
| Primary treatment | <0.001 | ||||||
| Surgery | 23,131 | (72.5) | 9,494 | (82.9) | 373 | (69.1) | |
| Radiation | 5,624 | (17.6) | 1,217 | (10.6) | 121 | (22.4) | |
| No surgery or radiation | 738 | (2.3) | 224 | (2.0) | 18 | (3.3) | |
| Unknown | 2,410 | (7.6) | 512 | (4.5) | 28 | (5.2) | |
| Adjuvant therapy1 | |||||||
| Radiation | <0.001 | ||||||
| Combination | 2,390 | (10.3) | 726 | (7.7) | 36 | (9.7) | |
| External beam | 3,879 | (16.8) | 1,110 | (11.7) | 117 | (31.4) | |
| Brachytherapy | 1,051 | (4.5) | 347 | (3.7) | 18 | (4.8) | |
| None/unknown | 15,811 | (68.4) | 7,311 | (77.0) | 202 | (54.2) | |
| Chemotherapy1 | <0.001 | ||||||
| Yes | 4,194 | (18.1) | 1,222 | (12.9) | 272 | (72.9) | |
| No | 18,363 | (79.4) | 8,056 | (84.9) | 87 | (23.3) | |
| Unknown | 574 | (2.5) | 216 | (2.3) | 14 | (3.8) | |
| Stage IIB–IVA | |||||||
| Primary treatment | <0.001 | ||||||
| Surgery | 8,300 | (20.0) | 1,753 | (32.9) | 188 | (29.1) | |
| Radiation | 25,227 | (60.7) | 2,738 | (51.5) | 337 | (52.2) | |
| No treatment | 2,326 | (5.6) | 319 | (6.0) | 73 | (11.3) | |
| Unknown | 5,686 | (13.7) | 511 | (9.6) | 48 | (7.4) | |
| Concurrent chemotherapy2 | <0.001 | ||||||
| Yes | 19,909 | (78.9) | 2,077 | (75.9) | 301 | (89.3) | |
| No | 5,036 | (20.0) | 634 | (23.2) | 33 | (9.8) | |
| Unknown | 282 | (1.1) | 27 | (1.0) | 3 | (0.9) | |
| Stage IVB | |||||||
| Chemotherapy | 0.001 | ||||||
| Yes | 3,933 | (65.4) | 871 | (61.2) | 320 | (71.6) | |
| No | 1,924 | (32.0) | 504 | (35.4) | 116 | (26.0) | |
| Unknown | 160 | (2.7) | 49 | (3.4) | 11 | (2.5) | |
Among patients treated with primary surgery.
Among patients treated with primary radiotherapy.
For patients with advanced stage (IIB–IVA) disease, radiation was the most common treatment modality. Concurrent chemotherapy was administered to 78.9% of women with SCCC, 75.9% of those with AC and 89.3% of those with NECC (P<0.001). Among those with metastatic disease (stage IVB neoplasms), chemotherapy was utilized in 65.4% of women with SCCC, 61.2% of those with AC and 71.6% of those with NECC (P=0.001).
The risk of death was higher for patients with NECC compared to SCCC for all stages (Table 3). Among women with early-stage disease, the hazard ratio for death for patients with NECC was 2.96 (95% CI, 2.48–3.52) times higher than for SCCC. Compared to women with SCCC, the hazard ratio for death for women with stage IIB–IVA NECC was 1.70 (95% CI, 1.45–1.99) and 1.14 (95% CI, 0.91–1.43) for those with stage IVB neoplasms. In models that only included women with NECC, year of diagnosis and race were independent predictors of death for women with locally advanced tumors (Table 4). In a sensitivity analysis limited to women treated at comprehensive community cancer centers and academic hospitals, the findings were similar (Supplemental Table 1).
Table 3.
Multivariable models of risk of death stratified by stage.
| Stage IB–IIA1 | Stage IIB–IVA2 | Stage IVB3 | ||
|---|---|---|---|---|
| Histology | ||||
| Squamous cell | Referent | Referent | Referent | |
| Adenocarcinoma | 0.86 (0.80–0.92)* | 1.19 (1.11–1.27)* | 1.05 (0.89–1.25) | |
| Neuroendocrine | 2.96 (2.48–3.52)* | 1.70 (1.45–1.99)* | 1.14 (0.91–1.43) | |
| Year of diagnosis | ||||
| 1998 | Referent | Referent | Referent | |
| 1999 | 0.99 (0.88–1.10) | 1.29 (1.19–1.41)* | 1.06 (0.78–1.43) | |
| 2000 | 1.12 (1.00–1.24)* | 1.39 (1.27–1.51)* | 1.10 (0.81–1.48) | |
| 2001 | 1.09 (0.97–1.22) | 1.47 (1.35–1.60)* | 1.13 (0.84–1.51) | |
| 2002 | 1.30 (1.16–1.45)* | 1.49 (1.37–1.62)* | 1.03 (0.76–1.39) | |
| 2003 | 0.85 (0.76–0.95)* | 0.81 (0.74–0.87)* | 1.01 (0.76–1.35) | |
| 2004 | 1.03 (0.92–1.15) | 0.83 (0.77–0.90)* | 0.95 (0.72–1.26) | |
| 2005 | 0.95 (0.85–1.06) | 0.81 (0.75–0.88)* | 0.96 (0.72–1.27) | |
| 2006 | 0.98 (0.87–1.10) | 0.79 (0.73–0.86)* | 0.87 (0.66–1.15) | |
| Age | ||||
| <30 | Referent | Referent | Referent | |
| 30–39 | 0.87 (0.75–1.01) | 1.01 (0.87–1.18) | 1.17 (0.64–2.14) | |
| 40–49 | 1.00 (0.87–1.15) | 1.02 (0.88–1.18) | 1.00 (0.56–1.79) | |
| 50–59 | 1.30 (1.12–1.50)* | 1.02 (0.88–1.19) | 1.25 (0.70–2.22) | |
| 60–69 | 1.73 (1.49–2.01)* | 1.07 (0.92–1.25) | 1.44 (0.81–2.58) | |
| ≥70 | 2.83 (2.41–3.32)* | 1.43 (1.22–1.67)* | 1.50 (0.83–2.73) | |
| Race | ||||
| White | Referent | Referent | Referent | |
| Black | 1.16 (1.08–1.25)* | 0.99 (0.94–1.04) | 0.82 (0.69–0.97)* | |
| Hispanic | 0.73 (0.66–0.81)* | 0.71 (0.66–0.76)* | 0.76 (0.58–0.99)* | |
| Other | 0.74 (0.64–0.86)* | 0.72 (0.65–0.80)* | 0.63 (0.42–0.94)* | |
| Unknown | 0.80 (0.61–1.05) | 0.91 (0.72–1.14) | 0.93 (0.57–1.51) | |
| Insurance status | ||||
| Commercial | Referent | Referent | Referent | |
| Medicare | 1.61 (1.46–1.77)* | 1.27 (1.19–1.36)* | 1.14 (0.93–1.40) | |
| Medicaid | 1.61 (1.48–1.76)* | 1.17 (1.10–1.24)* | 1.09 (0.89–1.33) | |
| Uninsured | 1.40 (1.25–1.57)* | 1.01 (0.94–1.08) | 1.21 (0.98–1.49) | |
| Other | 1.38 (1.01–1.88)* | 1.24 (1.01–1.52)* | 1.29 (0.65–2.56) | |
| Unknown | 1.19 (1.05–1.35)* | 1.19 (1.09–1.31)* | 1.02 (0.75–1.40) | |
| Region | ||||
| Northeast | Referent | Referent | Referent | |
| Midwest | 0.98 (0.90–1.07) | 1.02 (0.96–1.08) | 0.98 (0.80–1.19) | |
| South | 1.03 (0.95–1.12) | 1.02 (0.97–1.08) | 0.87 (0.73–1.03) | |
| West | 0.90 (0.82–0.99)* | 1.09 (1.02–1.16)* | 1.03 (0.81–1.30) | |
| Location | ||||
| Metropolitan | Referent | Referent | Referent | |
| Urban | 1.12 (1.04–1.21)* | 1.01 (0.96–1.07) | 1.00 (0.83–1.20) | |
| Rural | 0.99 (0.81–1.20) | 0.93 (0.81–1.08) | 1.38 (0.93–2.03) | |
| Unknown | 1.00 (0.89–1.14) | 1.02 (0.94–1.12) | 1.18 (0.90–1.54) | |
| Hospital type | ||||
| Academic | Referent | Referent | Referent | |
| Community cancer program | 0.98 (0.88–1.10) | 1.04 (0.96–1.11) | 1.11 (0.89–1.39) | |
| Comprehensive community cancer program | 1.07 (1.01–1.14)* | 1.06 (1.01–1.10)* | 1.24 (1.07–1.43)* | |
| Other | 1.18 (0.94–1.49) | 1.01 (0.88–1.16) | 0.95 (0.65–1.40) | |
| Stage | ||||
| IB1 | Referent | — | — | |
| IB2 | 1.41 (1.24–1.61)* | — | — | |
| IB NOS (IB, IB NOS) | 1.95 (1.80–2.11)* | — | — | |
| IIA | 2.36 (2.02–2.75)* | — | — | |
| IIB | — | Referent | — | |
| IIIA | — | 1.61 (1.47–1.77)* | — | |
| IIIB | — | 1.83 (1.75–1.91)* | — | |
| IVA | — | 2.73 (2.55–2.94)* | — | |
| Treatment | ||||
| Surgery | Referent | — | — | |
| Radiation | 2.54 (2.38–2.71)* | — | — | |
| No therapy | — | — | — | |
| Unknown | — | — | — | |
| Chemotherapy | ||||
| Yes | — | Referent | Referent | |
| No | — | 1.47 (1.41–1.55)* | 1.86 (1.61–2.15)* | |
| Unknown | — | 1.17 (0.98–1.40) | 0.88 (0.61–1.27) | |
Adjusted hazard ratio (95% confidence interval).
Limited to patients who received primary treatment with either radiation or surgery (n=23,758)
Limited to patients who received radiation (n=16,535)
Patients in no treatment group (n=1069)
Indicates P value <0.05
Table 4.
Multivariable models of risk of death stratified by stage among neuroendocrine patients.
| Stage IB–IIA1 | Stage IIB–IVA2 | Stage IVB3 | ||
|---|---|---|---|---|
| Year of diagnosis | ||||
| 1998 | Referent | Referent | Referent | |
| 1999 | 0.84 (0.36–1.98) | 0.25 (0.10–0.62)* | 1.68 (0.55–5.19) | |
| 2000 | 0.54 (0.25–1.20) | 0.23 (0.09–0.58)* | 1.85 (0.58–5.86) | |
| 2001 | 0.48 (0.20–1.14) | 0.28 (0.13–0.61)* | 0.82 (0.24–2.82) | |
| 2002 | 1.04 (0.49–2.24) | 0.37 (0.16–0.84)* | 0.81 (0.27–2.42) | |
| 2003 | 1.08 (0.56–2.08) | 0.24 (0.10–0.56)* | 1.87 (0.65–5.42) | |
| 2004 | 1.48 (0.61–3.59) | 0.27 (0.12–0.59)* | 0.81 (0.32–2.01) | |
| 2005 | 0.57 (0.26–1.24) | 0.15 (0.07–0.34)* | 0.34 (0.12–0.97)* | |
| 2006 | 0.82 (0.40–1.68) | 0.25 (0.10–0.58)* | 1.53 (0.47–5.00) | |
| Age | ||||
| <30 | Referent | Referent | Referent | |
| 30–39 | 0.41 (0.23–0.72)* | 1.41 (0.56–3.54) | 1.47 (0.28–7.80) | |
| 40–49 | 0.76 (0.42–1.38) | 1.47 (0.66–3.29) | 1.14 (0.25–5.12) | |
| 50–59 | 0.72 (0.39–1.34) | 1.76 (0.81–3.81) | 1.87 (0.41–8.59) | |
| 60–69 | 0.50 (0.20–1.27) | 1.85 (0.71–4.81) | 1.08 (0.20–5.75) | |
| ≥70 | 0.59 (0.22–1.60) | 1.43 (0.40–5.13) | 1.16 (0.15–8.64) | |
| Race | ||||
| White | Referent | Referent | Referent | |
| Black | 1.30 (0.65–2.60) | 0.77 (0.45–1.33) | 0.79 (0.36–1.75) | |
| Hispanic | 0.61 (0.29–1.29) | 0.33 (0.16–0.65)* | 0.43 (0.14–1.34) | |
| Other | 0.90 (0.39–2.10) | 0.42 (0.20–0.91)* | 0.91 (0.13–6.26) | |
| Unknown | 1.37 (0.16–11.50) | 0.69 (0.08–5.66) | 0.86 (0.16–4.60) | |
| Insurance status | ||||
| Commercial | Referent | Referent | Referent | |
| Medicare | 1.62 (0.72–3.61) | 1.72 (0.77–3.81) | 1.00 (0.41–2.44) | |
| Medicaid | 1.43 (0.74–2.78) | 1.62 (0.94–2.79) | 1.49 (0.56–3.93) | |
| Uninsured | 1.07 (0.48–2.40) | 0.93 (0.46–1.89) | 0.98 (0.39–2.43) | |
| Other | — | 1.30 (0.12–13.70) | 0.46 (0.04–4.95) | |
| Unknown | 1.12 (0.52–2.42) | 1.79 (0.62–5.16) | 0.93 (0.33–2.61) | |
| Region | ||||
| Northeast | Referent | Referent | Referent | |
| Midwest | 1.69 (0.90–3.20) | 0.97 (0.49–1.89) | 1.83 (0.80–4.17) | |
| South | 1.45 (0.83–2.53) | 1.21 (0.65–2.26) | 0.65 (0.30–1.39) | |
| West | 1.06 (0.54–2.08) | 1.21 (0.62–2.36) | 0.51 (0.17–1.53) | |
| Location | ||||
| Metropolitan | Referent | Referent | Referent | |
| Urban | 1.02 (0.62–1.69) | 1.60 (0.93–2.76) | 0.72 (0.30–1.74) | |
| Rural | 0.36 (0.08–1.54) | 0.29 (0.04–2.42) | 1.71 (0.51–5.70) | |
| Unknown | 1.34 (0.60–2.99) | 2.02 (0.80–5.10) | 0.74 (0.09–5.79) | |
| Hospital type | ||||
| Academic | Referent | Referent | Referent | |
| Community cancer program | 1.66 (0.77–3.61) | 1.01 (0.47–2.17) | 0.69 (0.25–1.92) | |
| Comprehensive community cancer program | 1.38 (0.90–2.13) | 0.80 (0.54–1.18) | 1.34 (0.67–2.68) | |
| Other | 1.95 (0.21–17.91) | 0.64 (0.07–5.74) | 0.42 (0.04–4.17) | |
| Stage | ||||
| IB1 | Referent | — | — | |
| IB2 | 1.21 (0.53–2.74) | — | — | |
| IB NOS (IB, IB NOS) | 3.01 (1.69–5.37)* | — | — | |
| IIA | 15.76 (4.97–50.01)* | — | — | |
| IIB | — | Referent | — | |
| IIIA | — | 2.72 (1.22–6.07)* | — | |
| IIIB | — | 1.35 (0.90–2.01) | — | |
| IVA | — | 2.91 (1.11–7.63)* | — | |
| Treatment | ||||
| Surgery | Referent | — | — | |
| Radiation | 1.59 (0.97–2.60) | — | — | |
| No therapy | — | — | — | |
| Unknown | — | — | — | |
| Chemotherapy | ||||
| Yes | — | Referent | Referent | |
| No | — | 1.58 (0.81–3.08) | 3.36 (1.69–6.67)* | |
| Unknown | — | 0.39 (0.05–3.09) | 1.76 (0.40–7.67) | |
Adjusted hazard ratio (95% confidence interval).
Limited to patients who received primary treatment with either radiation or surgery (n=276)
Limited to patients who received radiation (n=202)
Patients in no treatment group (n=103)
Indicates P value <0.05
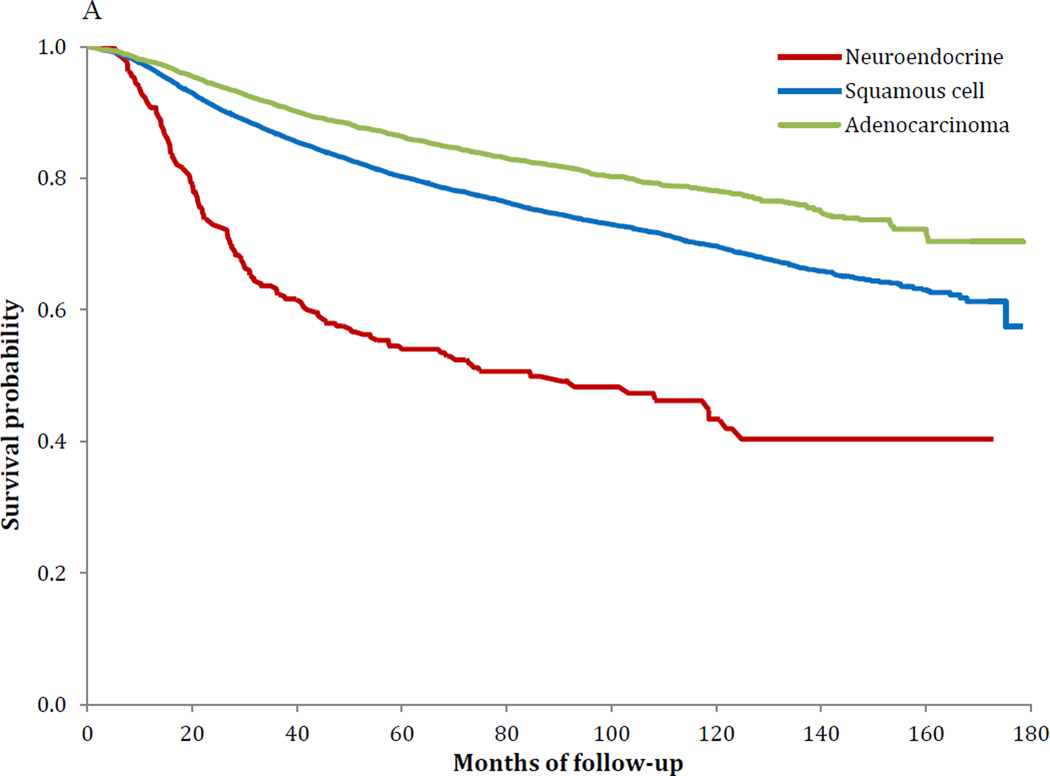
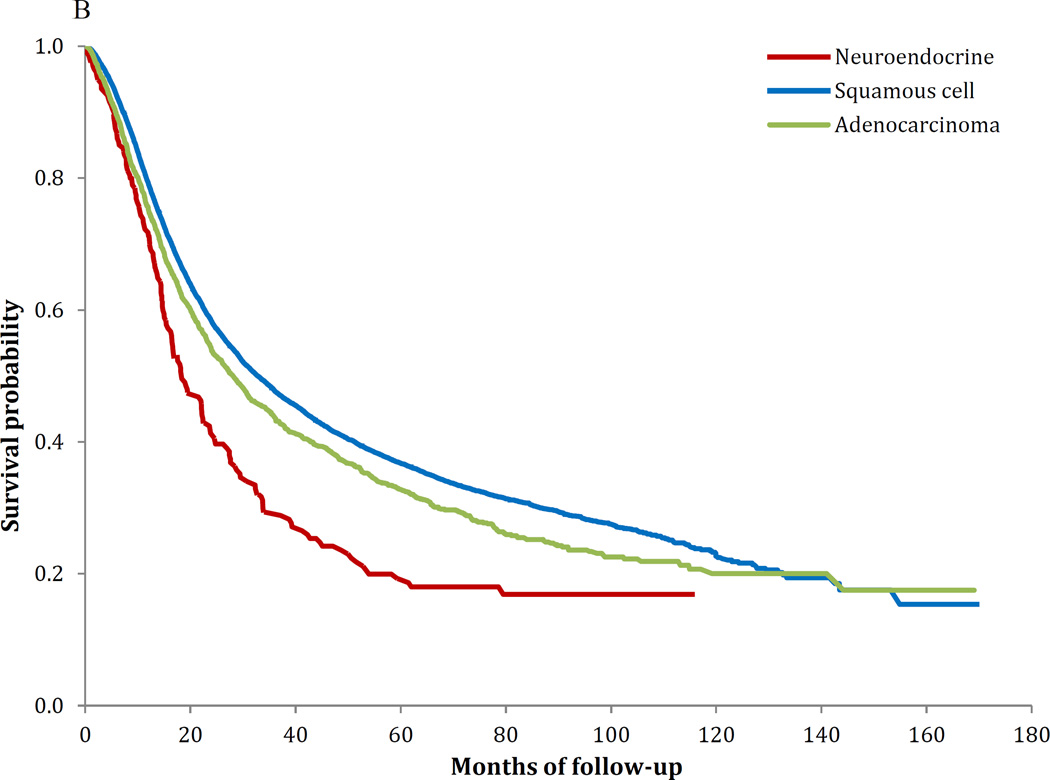
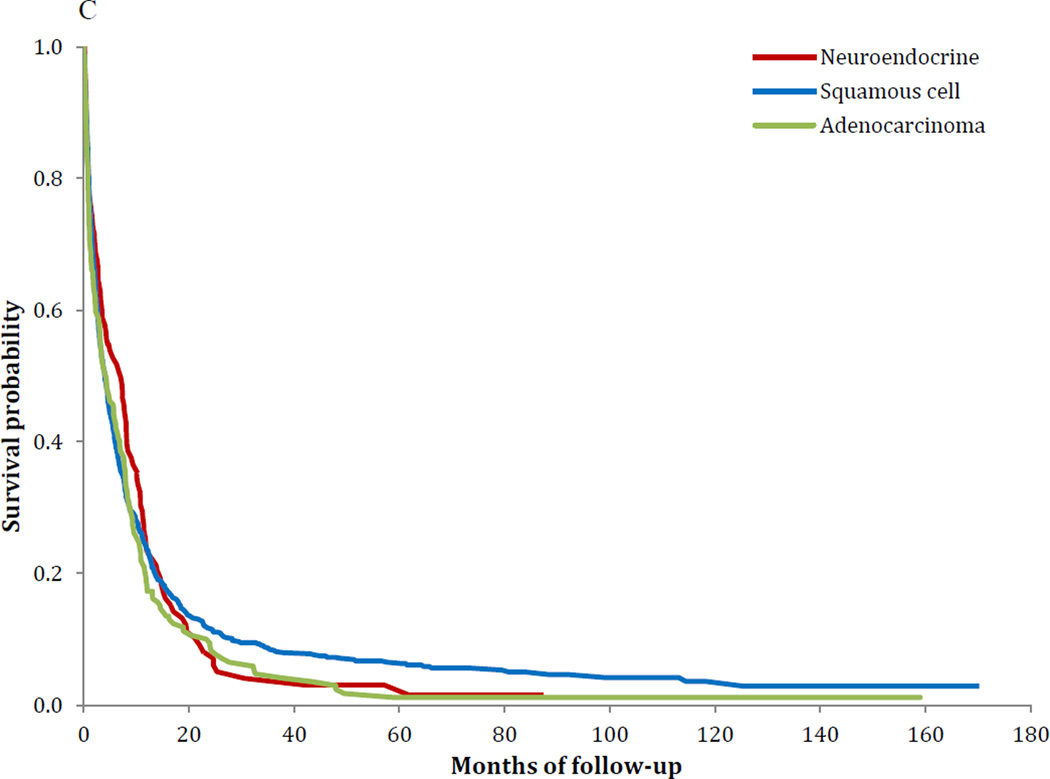
Similar survival findings were noted in the Kaplan-Meier analyses (Figure 1A–C). Survival was worse for NECC compared to SCCC for stages IB–IIA (P<0.001) and stages IIB–IVA (P<0.001), while the findings for stage IVB tumors was not significant (P=0.37). Among women with stage IB tumors, five-year survival was 80.4% (95% CI, 79.7–81.0%) for squamous cell tumors, 85.7% (95% CI, 84.7–86.6%) for AC and 55.4% (95% CI, 49.3–61.2%) for NECC. Similarly, for stage IIIB tumors, five-year survival was 43.3% (95% CI, 42.3–44.2%) for SCCC, 39.4% (95% CI, 36.6–42.1%) for AC and 24.4% (95% CI, 18.8–30.4%) for those with NECC (Table 5).
Figure 1.
A Kaplan-Meier curve of survival stratified by histology: stage IB–IIA (P<0.001)
B Kaplan-Meier curve of survival stratified by histology: stages IIB–IVA (P<0.001)
C Kaplan-Meier curve of survival stratified by histology: stage IVB (P=0.37).
Table 5.
Five-year survival.
| Squamous cell | Adenocarcinoma | Neuroendocrine | P-value 1 | ||||
|---|---|---|---|---|---|---|---|
| 5-year survival | (95% CI) | 5-year survival | (95% CI) | 5-year survival | (95% CI) | ||
| Stage IA (1A1, 1A2) | 96.4% | (95.8%–97.0%) | 98.2% | (97.2%–98.9%) | 81.8% | (44.7%–95.1%) | 0.02 |
| Stage IB (1B1, IB2, IB) | 80.4% | (79.7%–81.0%) | 85.7% | (84.7%–86.6%) | 55.4% | (49.3%–61.2%) | <0.001 |
| IIB | 57.2% | (56.2%–58.2%) | 51.2% | (48.3%–54.1%) | 22.2% | (14.1%–31.4%) | <0.001 |
| IIIB | 43.3% | (42.3%–44.2%) | 39.4% | (36.6%–42.1%) | 24.4% | (18.8%–30.4%) | <0.001 |
| IVA | 20.0% | (18.1%–22.0%) | 13.3% | (9.0%–18.5%) | 4.1% | (0.3%–17.5%) | 0.12 |
| IVB | 13.3% | (12.1%–14.5%) | 12.3% | (10.1%–14.8%) | 7.1% | (4.3%–10.9%) | <0.001 |
P-value was from log-rank test.
Discussion
Our data suggest that neuroendocrine tumors of the cervix have a distinct natural history and follow an aggressive course. Survival for both early and late stage tumors is inferior to that of squamous cell carcinomas. The differential in survival is greatest for women with early-stage tumors. Compared to women with squamous cell tumors, those with NECC are more likely to receive adjuvant chemotherapy and radiotherapy.
Given that NECC are relatively uncommon, few large scale studies of these tumors have been reported.12 Like other cervical cancers, NECC appear to be associated with human papillomavirus (HPV).16 One report from a cohort in Thailand found HPV 16 and/or 18 in 89% of NECCs, however the prevalence varies widely in the literature.16 Despite the association, it is unknown whether HPV vaccination is protective against the development of NECC.
Our data confirm that survival for women with NECC tumors is poor overall. For women with stage IB tumors, we found that five-year survival was only 55% compared to 80% for patients with squamous cell tumors and 86% for adenocarcinomas. For those with stage IIIB tumors, five-year survival rates were 24% and 43%, respectively. A prior institutional series reported median survival rates for women with NECC of 31 months for those with stage IA–IB2 and 6 months for patients with stage IIB–IV neoplasms.9 Women with NECC are at particularly high risk for distant failure.17 Unlike prior studies, we found that early-stage AC was associated with a more favorable prognosis than SCCC.18 These findings warrant further investigation.
Most commonly, patients with NECC are diagnosed with early-stage disease. In our cohort, we noted that over 60% of women with NECC were diagnosed with stages I or II tumors. Despite the early-stage at diagnosis, these patients are at higher risk for the development of systemic disease. Recent consensus guidelines by the Society of Gynecologic Oncology (SGO) recommend multimodal therapy for early stage tumors.19 Initial treatment usually consists of surgery with radical hysterectomy and regional lymphadenectomy followed by adjuvant chemotherapy along with consideration for adjuvant radiation.10,11,17,19 The chemotherapy regimens that have been the most widely studied include etoposide/cisplatin (EP), vincristine/doxorubicin/cyclophosphamide (VAC), and alternating VAC/EP regimens.5 Although the addition of radiation is often recommended, the survival benefit of adjuvant radiotherapy is poorly defined.20 For women with early-stage tumors >4 cm in diameter, treatment with either primary chemoradiation or neoadjuvant chemotherapy prior to surgical resection may be considered.21,22
For women with advanced stage NECC, treatment typically consists of a combination of chemotherapy and radiation. While these recommendations are extrapolated from treatment regimens for other histologic subtypes of cervical cancer, the British Columbia Cancer Agency reported results from patients treated from 1998–2002 with a regimen of etoposide and cisplatin in combination with external beam radiation and intracavitary brachytherapy. Of note, there were only 8 patients in this study with stage III/IV disease, highlighting the need for more data to guide treatment.17 Survival estimates for advanced stage disease range from 38–40% at 3 years.12,17
Given the fact that many of the current treatment recommendations for NECC are extrapolated from data in patients with small cell lung cancer, it is reasonable to look towards emerging SCLC therapies including targeted therapy.23 Multiple targeted therapies are under study including angiogenesis inhibitors, mTOR inhibitors, and PARP inhibitors. Improvements in 3 month PFS have been shown in small cell lung cancer with the addition of aflibercept, a VEGF inhibitor in combination with topotecan for recurrent disease.24 When bevacizumab is incorporated into first line therapy it has been shown to increase PFS and is also under study for recurrent disease.25 While not studied specifically for NECC, bevacizumab is now approved for the treatment of recurrent cervical cancer and likely represents a reasonable option for women with NECC.26 The tyrosine kinase inhibitor sunitinib has also been shown to increase PFS in small cell lung cancer in a phase II randomized trial.27 Given that small cell lung cancers have high expression of PARP1, PARP inhibition is another attractive strategy. PARP inhibitors are currently being explored in clinical trials.28 Given the rarity of NECC, trials of targeted therapy specifically designed for women with neuroendocrine cervical tumors are unlikely.
While our study benefits from the inclusion of a large sample of women with NECC, we recognize a number of important limitations. The NCDB captures approximately 70% of newly diagnosed cancer patients in the US. As such, these findings may not be generalizable to the entire population. Given that neuroendocrine tumors are relatively rare, we cannot exclude the possibility that a small number of women were misclassified, as central pathologic review was not performed. Within the NCDB, data on some important tumor characteristics, including tumor size, are limited. Additionally, while we can identify use of adjuvant chemotherapy, we cannot discern the particular agents utilized and lack data on the treatment of recurrences. Finally, as with any study using administrative data, we cannot capture individual patient and physician preferences that influenced treatment.
We found that NECC is a rare and aggressive type of cervical cancer that is generally associated with poor survival. Survival is inferior for NECC compared to squamous cell tumors for women with both early and advanced stage disease. Although treatment guidelines exist for NECC, they are derived from small cohort studies and from experience with neuroendocrine tumors from other sites. Further studies to prospectively document the natural history, treatment and outcomes of women with NECC are clearly needed.
Supplementary Material
Research Highlights.
-
-
NECC are aggressive tumors associated with an increased risk of death.
-
-
Survival is inferior for NECC compared to squamous cell tumors for women with both early and advanced stage disease.
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1 and Dr. Hershman (NCI R01CA134964) are recipients of grants from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interests.
References
- 1.Albores-Saavedra J, Larraza O, Poucell S, Rodriguez Martinez HA. Carcinoid of the uterine cervix: additional observations on a new tumor entity. Cancer. 1976;38:2328–2342. doi: 10.1002/1097-0142(197612)38:6<2328::aid-cncr2820380620>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Scully RE, Aguirre P, DeLellis RA. Argyrophilia, serotonin, and peptide hormones in the female genital tract and its tumors. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 1984;3:51–70. doi: 10.1097/00004347-198403010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Miller B, Dockter M, el Torky M, Photopulos G. Small cell carcinoma of the cervix: a clinical and flow-cytometric study. Gynecologic oncology. 1991;42:27–33. doi: 10.1016/0090-8258(91)90225-t. [DOI] [PubMed] [Google Scholar]
- 4.van Nagell JR, Jr, Donaldson ES, Parker JC, van Dyke AH, Wood EG. The prognostic significance of pelvic lymph node morphology in carcinoma of the uterine cervix. Cancer. 1977;39:2624–2632. doi: 10.1002/1097-0142(197706)39:6<2624::aid-cncr2820390648>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Chan JK, Loizzi V, Burger RA, Rutgers J, Monk BJ. Prognostic factors in neuroendocrine small cell cervical carcinoma: a multivariate analysis. Cancer. 2003;97:568–574. doi: 10.1002/cncr.11086. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JG, Kapp DS, Shin JY, et al. Small cell carcinoma of the cervix: treatment and survival outcomes of 188 patients. Am J Obstet Gynecol. 2010;203:347 e1–347 e6. doi: 10.1016/j.ajog.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Kasamatsu T, Sasajima Y, Onda T, Sawada M, Kato T, Tanikawa M. Surgical treatment for neuroendocrine carcinoma of the uterine cervix. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2007;99:225–228. doi: 10.1016/j.ijgo.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan AN, Deavers MT, Jhingran A, Ramirez PT, Levenback C, Eifel PJ. Small cell neuroendocrine carcinoma of the cervix: outcome and patterns of recurrence. Gynecologic oncology. 2004;93:27–33. doi: 10.1016/j.ygyno.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Zivanovic O, Leitao MM, Jr, Park KJ, et al. Small cell neuroendocrine carcinoma of the cervix: Analysis of outcome, recurrence pattern and the impact of platinum-based combination chemotherapy. Gynecologic oncology. 2009;112:590–593. doi: 10.1016/j.ygyno.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Boruta DM, 2nd, Schorge JO, Duska LA, Crum CP, Castrillon DH, Sheets EE. Multimodality therapy in early-stage neuroendocrine carcinoma of the uterine cervix. Gynecologic oncology. 2001;81:82–87. doi: 10.1006/gyno.2000.6118. [DOI] [PubMed] [Google Scholar]
- 11.McCann GA, Boutsicaris CE, Preston MM, et al. Neuroendocrine carcinoma of the uterine cervix: the role of multimodality therapy in early-stage disease. Gynecologic oncology. 2013;129:135–139. doi: 10.1016/j.ygyno.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 12.McCusker ME, Cote TR, Clegg LX, Tavassoli FJ. Endocrine tumors of the uterine cervix: incidence, demographics, and survival with comparison to squamous cell carcinoma. Gynecologic oncology. 2003;88:333–339. doi: 10.1016/s0090-8258(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 13.The National Cancer Data Base. at https://www.facs.org/qualityprograms/cancer/ncdb. [Google Scholar]
- 14.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20:1759–1765. doi: 10.1245/s10434-013-2901-1. [DOI] [PubMed] [Google Scholar]
- 16.Siriaunkgul S, Utaipat U, Settakorn J, Sukpan K, Srisomboon J, Khunamornpong S. HPV genotyping in neuroendocrine carcinoma of the uterine cervix in northern Thailand. Int J Gynaecol Obstet. 2011;115:175–179. doi: 10.1016/j.ijgo.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Hoskins PJ, Swenerton KD, Pike JA, et al. Small-cell carcinoma of the cervix: fourteen years of experience at a single institution using a combined-modality regimen of involved-field irradiation and platinum-based combination chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3495–3501. doi: 10.1200/JCO.2003.01.501. [DOI] [PubMed] [Google Scholar]
- 18.Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125:287–291. doi: 10.1016/j.ygyno.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Gardner GJ, Reidy-Lagunes D, Gehrig PA. Neuroendocrine tumors of the gynecologic tract: A Society of Gynecologic Oncology (SGO) clinical document. Gynecologic oncology. 2011;122:190–198. doi: 10.1016/j.ygyno.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Lan-Fang L, Hai-Yan S, Zuo-Ming Y, Jian-Qing Z, Ya-Qing C. Small cell neuroendocrine carcinoma of the cervix: analysis of the prognosis and role of radiation therapy for 43 cases. European journal of gynaecological oncology. 2012;33:68–73. [PubMed] [Google Scholar]
- 21.Lee SW, Nam JH, Kim DY, et al. Unfavorable prognosis of small cell neuroendocrine carcinoma of the uterine cervix: a retrospective matched case-control study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20:411–416. doi: 10.1111/IGC.0b013e3181ce427b. [DOI] [PubMed] [Google Scholar]
- 22.Chang TC, Hsueh S, Lai CH, et al. Phase II trial of neoadjuvant chemotherapy in early-stage small cell cervical cancer. Anti-cancer drugs. 1999;10:641–646. doi: 10.1097/00001813-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes & development. 2015;29:1447–1462. doi: 10.1101/gad.263145.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen JW, Moon J, Redman M, et al. Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2463–2470. doi: 10.1200/JCO.2013.51.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2215–2222. doi: 10.1200/JCO.2010.29.3423. [DOI] [PubMed] [Google Scholar]
- 26.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morabito A, Carillio G, Daniele G, et al. Treatment of small cell lung cancer. Critical reviews in oncology/hematology. 2014;91:257–270. doi: 10.1016/j.critrevonc.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer discovery. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.