Abstract
Objective
High-grade serous carcinoma (HGSC) generally presents at an advanced stage with poor long-term (LT) survival. Here we describe clinical features found in women surviving HGSC for ten or more years.
Methods
A multi-center research consortium was established between five participating academic centers. Patient selection criteria included high-grade serous ovarian, fallopian tube, or peritoneal carcinoma with at least ten years of follow up. Non-serous, borderline tumors and low-grade serous subtypes were excluded.
Results
The 203 identified LT ten-year survivors with HGSC were diagnosed at a median age of 57 years (range 37–84 years). The majority of patients had stage IIIC (72.4%) disease at presentation. Of those who underwent primary cytoreductive surgery, optimal cytoreduction was achieved in 143 (85.6%) patients. After a median follow up of 144 months, 88 (46.8%) patients did not develop recurrent disease after initial treatment. Unexpected findings from this survey of LT survivors includes 14% of patients having had suboptimal cytoreduction, 11% of patients having an initial platinum free interval of less than 12 months, and nearly 53% of patients having recurrent disease, yet still surviving more than ten years after diagnosis.
Conclusions
LT survivors of HGSC of the ovary generally have favorable clinical features including optimal surgical cytoreduction and primary platinum sensitive disease. The majority of patients will develop recurrent disease, however many remained disease free for more than 10 years. Future work will compare the clinical features of this unusual cohort of LT survivors with the characteristics of HGSC patients having less favorable outcomes.
Keywords: Ovarian cancer, long-term survival, neoadjuvant chemotherapy, surgical cytoreduction, outcome, survival
Introduction
Ovarian cancer has extensive heterogeneity within and between histologic subtypes [1]. High-grade serous carcinoma is the most aggressive subtype and accounts for the majority of advanced stage cases [2]. Ten-year survival for all ovarian cancer is approximately 30–40% according to the SEER registry and other studies [3, 4]. Long-term (LT) survival of women with high-grade serous carcinoma (HGSC) is low and often associated with completely resected disease (no gross residual). While many factors have been reported to have prognostic value for HGSC beyond the current FIGO staging system, most have limited value for patients with advanced stage disease [5, 6]. Nonetheless, the use of intraperitoneal therapy in patients with microscopic and small volume residual disease after cytoreductive surgery has been associated with long-term survival [7]. Nomograms have potential superiority over traditional staging systems to predict individual probabilities of survival [8, 9]. Many studies have demonstrated that molecular markers may improve outcome prediction alone or in combination with clinical variables, however, none have been sufficiently robust to incorporate into clinical practice to date [10–13]. Although many recently developed resources are tremendously valuable for studying HGSC, in general, they contain few LT survivors and those that are included often lack detailed clinical and pathologic data [14]. The goal of this pilot report is to describe clinical variables, alone or in combination, that are found among ten year survivors of HGSC with the overall goal of identifying approaches for improving the outcome of patients with shorter survival.
Methods
A multi-center research consortium was established between five participating academic centers: Memorial Sloan Kettering Cancer Center, MD Anderson Cancer Center, Cedars-Sinai Medical Center, Stephenson Oklahoma Cancer Center at the University of Oklahoma, and University of Iowa. IRB approval was obtained locally at each center. Study criteria were established that included patients with a diagnosis of high-grade serous ovarian, fallopian tube, or peritoneal carcinoma with at least ten years of follow up from the date of initial diagnosis to the date of death or last follow-up. All stages of cancer were permitted. Diagnosis dates for patients in this study range from 1979 to 2005. Non-serous, borderline tumors and low-grade serous subtypes were excluded in addition to any patients with insufficient follow up data. Grade 2 tumors were excluded unless they were re-reviewed and met contemporary criteria for high-grade serous carcinoma. Clinical and disease characteristics, including age at diagnosis, stage, residual disease after primary surgery, treatment history, recurrence history, and results of BRCA1 and BRCA2 germline genetic testing were collected from medical records. Major components (primary surgery and/or adjuvant chemotherapy) of initial treatment were performed at one of the five participating consortium centers. Cytoreductive surgery was characterized as optimal if there was ≤1cm of residual disease and suboptimal if >1cm of disease remained at the end of the surgical procedure. Consult cases or other cases referred after the completion of initial therapy were excluded. Data are reported in a descriptive fashion with summary statistics provided, as appropriate. An overall survival analysis using the method of Kaplan and Meier was performed using the time from diagnosis to last follow-up for patients who did not recur and the landmark event of recurrence to define the start of the overall survival interval for patients who had recurred. Statistical analysis and visualization was performed using R (http://www.R-project.org).
Results
Patient characteristics
Across the five centers, 203 patients were identified as LT ten-year survivors and included in this study. Demographic and clinical characteristics of the study group are shown in the Table. All patients were diagnosed with high-grade serous carcinoma of the ovary, fallopian tube, or peritoneum at their respective institution. The median age of patients was 57 years (range 37–84 years). Twenty-eight patients (13.8%) were older than 70 years at the time of diagnosis. The majority of patients were white (88.7%) and diagnosed with stage IIIC (72.4%) disease at presentation.
Table.
Demographic and clinical characteristics of long-term survivors
| N* | % | |
|---|---|---|
| Age at Diagnosis (years) | ||
| Median (Range) | 57 (37–84) | |
| FIGO Stage | ||
| I | 7 | 3.5 |
| II | 9 | 4.4 |
| III | 164 | 80.8 |
| IV | 23 | 11.3 |
| Grade | ||
| High | 203 | 100 |
| Histology | ||
| Serous | 203 | 100 |
| BRCA mutations | ||
| BRCA1 | 22 | 27.8 |
| BRCA2 | 21 | 26.6 |
| None | 36 | 45.6 |
| Residual Disease | ||
| None | 76 | 46.6 |
| ≤1cm | 61 | 37.4 |
| >1cm | 26 | 16.0 |
| Neoadjuvant therapy | ||
| No | 184 | 92.9 |
| Yes | 14 | 7.1 |
| Intraperitoneal therapy | ||
| No | 145 | 79.2 |
| Yes | 38 | 20.8 |
| Number of Recurrences | ||
| 0 | 88 | 46.6 |
| 1 | 21 | 11.2 |
| 2 | 19 | 10.1 |
| >2 | 60 | 31.9 |
| Platinum-free Interval | ||
| <6 months | 5 | 3.6 |
| 6–12 months | 11 | 7.9 |
| >12 months | 124 | 88.6 |
FIGO, International Federation of Gynecology and Obstetrics
Numbers do not sum to the total for some variables due to missing values.
Of the 23 patients with stage IV disease, 12 (52%) had available data that indicated 5 had stage IV disease based on pleural effusion only. Of the seven patients with distant metastases or solid organ involvement, 3 patients had parenchymal liver metastases, two patients had supraclavicular nodal involvement and one patient each had pleural metastasis or abdominal wall involvement. All three patients with liver metastases remained progression free for more than 10 years and have not developed recurrence. Available data for 157 (96%) of 164 stage III patients indicate that 26 (17%) had stage III disease due to lymph node only disease.
Of the 198 patients with available treatment information, 92.9% underwent primary cytoreductive surgery and 7.1% received neoadjuvant chemotherapy prior to interval cytoreductive surgery. Of those who underwent primary cytoreductive surgery, optimal cytoreduction, defined as residual disease of less than or equal to 1cm in maximum diameter, was achieved in 143 (85.6%) patients. A complete gross resection to no macroscopic residual disease was achieved in 70 (47.0%) patients. Ascites was present at the time of surgery in 103 (70.5%) patients. All but one patient (99.5%) received a platinum agent as part of their adjuvant or neoadjuvant treatment regimens. Intraperitoneal chemotherapy was given to 38 (20.8%) patients, with most of these patients receiving IP treatment as part of consolidation therapy.
Treatment outcome
After a median follow up of 144 months, 88 (46.8%) patients did not develop recurrent disease after initial treatment. This group was characterized by a high optimal cytoreduction rate (93%, 78/84) and a low rate of neoadjuvant chemotherapy (6%, 5/88). The majority of patients in the entire cohort were reported to be platinum sensitive (96.4%) with a platinum-free interval of greater than 12 months for 124 (88.6%) patients. The median progression-free survival (PFS) of the entire cohort was 147 months. Twenty-one (11.2%) patients had one recurrence during the follow-up interval, 19 (10.1%) recurred twice, and 60 (31.9%) suffered from more than two recurrences, with 13 patients having more than 10 reported recurrences. In the group of patients who experienced one recurrence, eight (44%) of 18 with available data underwent secondary cytoreduction and 16 (89%) received a platinum agent. The two patients who did not receive a platinum agent for their first recurrence received single-agent paclitaxel or cytoreduction alone. Only 19% (n=4) of the 21 patients with a single recurrence recurred within 24 months of diagnosis. BRCA1 and BRCA2 germline mutation status was known for 79 (38.9%) patients and of those tested 43 (54.4%) were found to carry a deleterious mutation. There were no identifiable survival differences between the subsets of patients with or without BRCA1 and BRCA2 germline mutations.
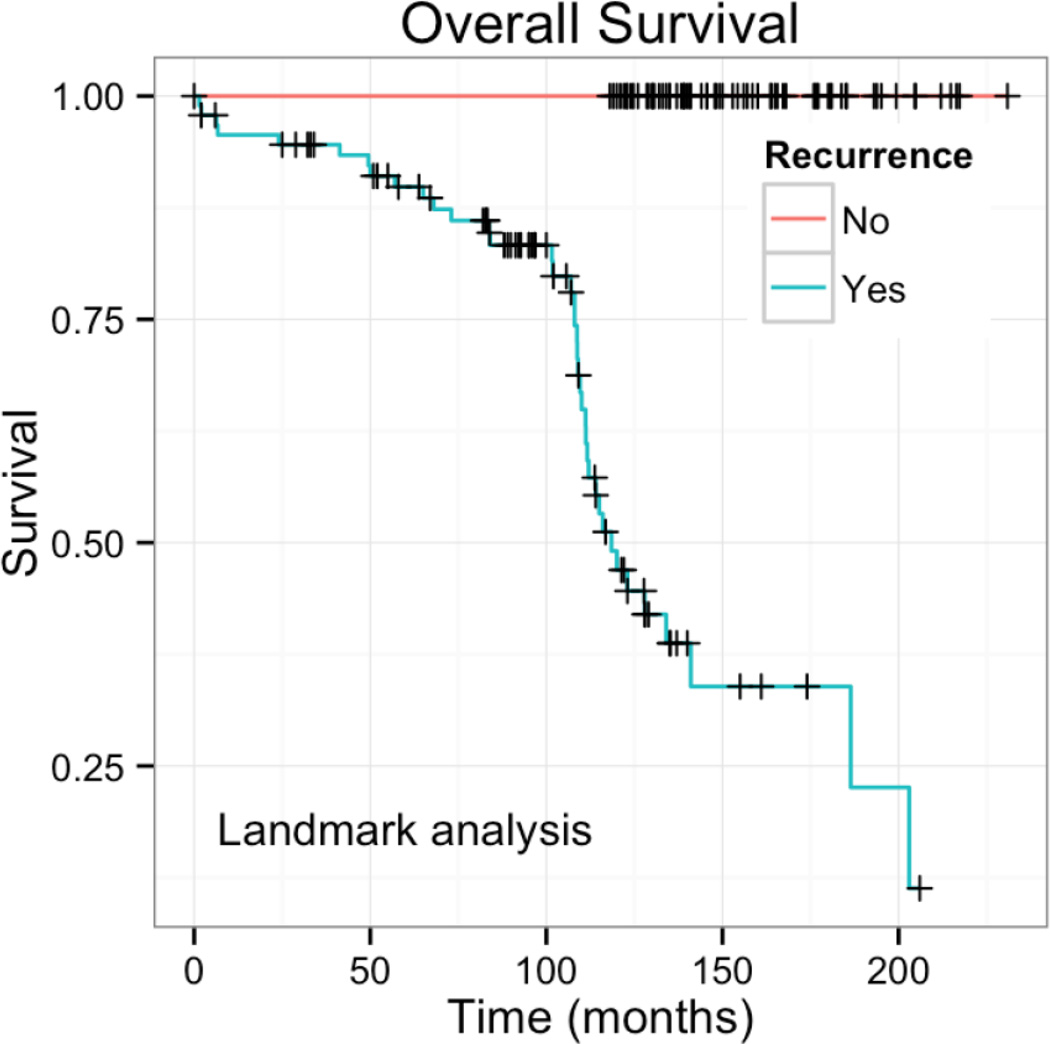
Data regarding disease-free intervals after the first recurrence were not collected as part of this pilot study. Therefore, a landmark analysis was performed to examine the survival pattern after first recurrence. For patients with recurrence, the landmark time of first recurrence was used as the start of the survival interval and date of diagnosis was used as the start of the survival interval for patients without recurrence. This analysis demonstrated that some patients recurred early and continued to have LT survival, whereas other patients recurred late and had shorter survival after recurrence (Figure). The survival curve suggests that both situations are common and equally represented in the cohort.
Figure.
Landmark survival analysis. Patients with recurrence have a survival curve with two component slopes suggesting both early and late events. Since this is a landmark analysis from time of recurrence in a cohort that has 10 or more year survival, the early events are late recurrence and the later events are early recurrence in patients who have a true overall survival of at least 10 years.
Twenty-four patients (13.4%) of 179 patients with available data participated in a clinical trial as part of initial adjuvant therapy. Four patients who received targeted therapy with cetuximab including 3 with stage IIIC disease, and one had stage IV disease. All were BRCA carriers (two with BRCA1 mutations and two with BRCA2 mutations). Three patients developed recurrence once between 17 and 39 months after diagnosis and were treated with secondary cytoreduction followed by additional platinum-based therapy. The one stage IV patient never recurred. All four were without disease at a median overall survival of 122 months (range 121 – 126 months).
Unusual responders
Unexpected findings from this survey of LT survivors includes 15% of patients having suboptimal cytoreduction at the time of primary surgical resection, 11% of patients having a platinum free interval of less than 12 months, and 53% of patients having recurrent disease, yet still surviving more than ten years after diagnosis. Other unusual features of LT survivors include four patients with suboptimal cytoreduction and a platinum-free interval (PFI) of < 12 months. All were diagnosed with stage IIIC disease and none of them participated in a clinical trial as part of their adjuvant therapy. Within the subset of patients who received neoadjuvant chemotherapy, 50% survived 10 or more years without recurrence, a proportion similar to the overall study population.
There were eight patients with stage IV disease who did not experience any recurrence of their disease after initial treatment. The median OS of these survivors was 131 months (range 122–204 months). All eight women had an optimal debulking with less than 1 cm of residual disease, five at primary debulking and three at interval debulking surgery after neoadjuvant chemotherapy. Those that received neoadjuvant chemotherapy all received carboplatin and paclitaxel, ranging from three to six cycles. Two women in this group had BRCA1 mutations; one had tested negative for a BRCA1 or BRCA2 mutation and the remaining five patients were not tested. Thirty patients had a disease-free interval of less than 2 years. The median age at diagnosis was 54. The majority (25, 83%) had stage III disease. Five patients remained without disease following their first recurrence and the others suffered from additional recurrences.
Of the patients undergoing primary surgical resection, 23 (15%) of 151 patients with available data had suboptimal cytoreduction and these patients had a median PFS of 76 months. The patients with suboptimal tumor resection were more likely to receive consolidation therapy after completing primary chemotherapy. Consolidation data was available for 139 (92%) of the 151 patients that underwent primary surgical resection with a known size of residual disease. Of these patients, 72% of the 18 patients who had suboptimal cytoreduction received consolidation therapy compared to only 31% of the 121 patients having optimal cytoreduction (P=0.001, Fisher’s exact test).
Discussion
In this study, we identify demographic, pathologic, surgical, and treatment-related clinical factors associated with LT survival in HGSC. LT survivors exist both with and without multiple recurrences. They appear to have a slightly younger age than the average patient with advanced stage HGSC and are likely to have had optimal surgical cytoreduction, defined at the time of this study as less than 1cm of residual disease. There are intrinsic biologic factors associated with platinum sensitivity that are generally associated with long-term survival, but a surprising yet small fraction of patients who had either suboptimal cytoreduction or primary platinum resistance achieved LT survival. The study is descriptive in nature to highlight the characteristics of patients with HGSC who survive 10 or more years after diagnosis. Future studies will compare this group of patients with others who have HGSC and are shorter-term survivors. We will also examine genetic and reproductive factors in future work, including the role of germline mutations in BRCA1, BRCA2 and other functionally-related genes.
The low use of neoadjuvant chemotherapy may be directly associated with long-term survival or may be a reflection of the time period under study, which is by definition at least 10 years ago. Furthermore, the complete gross resection rate of nearly 50% may be unexpectedly high during a time period when the goals of cytoreductive surgery were generally defined no more than 1cm of residual disease in maximal dimension.
Though studies have shown that survival of advanced stage HGSC patients is poor, there is a dearth of collective data on 10-year survivors of HGSC with sufficient clinical annotations to determine which modifiable and predictive characteristics are reliably associated with outcome. The few studies that have been conducted generally examined multiple histologic types together. For example, in a study of mixed histologic subtypes from Sweden, age, stage, residual disease and post-operative CA-125 were all associated with LT survival [3]. A SEER registry study found that advanced stage patients and those who did not receive primary surgical resection had worse LT outcomes [4]. A recent study reported a 31% 10-year survival of mixed histologic subtypes from a state registry [15]. The authors reported that 16% of patients with advanced stage HGSC survived 10 years, but there was no indication of treatment regimens, patterns of recurrence or other clinical features of their disease provided.
The data from this pilot study indicate that long-term survivors of HGSC from major academic medical centers have high surgical resection to no visible disease and there are many patients without any episodes of disease recurrence among 10-year survivors. We do not know how these and other variables compare to shorter-term survivors and future work will compare these 10-year survivors to shorter-term ovarian cancer survivors.
Highlights.
Ovarian cancer survivors who live for 10 or more years comprise a heterogeneous patient population with and without recurrent disease.
Long-term survivors may have suboptimal cytoreduction or short platinum free intervals.
BRCA1 and BRCA2 germline mutations appear common among long-term survivors.
Acknowledgments
Funding Support: Arnold Chavkin and Laura Chang, Department of Defense CDMRP Grant W81XWH-13-1-0192, NIH/NCI Grant P30CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC, Jr, Beral V, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15:668–679. doi: 10.1038/nrc4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akeson M, Jakobsen AM, Zetterqvist BM, Holmberg E, Brannstrom M, Horvath G. A population-based 5-year cohort study including all cases of epithelial ovarian cancer in western Sweden: 10-year survival and prognostic factors. Int J Gynecol Cancer. 2009;19:116–123. doi: 10.1111/IGC.0b013e3181991b13. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120:612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 5.Barlin JN, Long KC, Tanner EJ, Gardner GJ, Leitao MM, Jr, Levine DA, et al. Optimal (</=1 cm) but visible residual disease: is extensive debulking warranted? Gynecol Oncol. 2013;130:284–288. doi: 10.1016/j.ygyno.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Mironov S, Iyer RB, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol. 2014;134:455–461. doi: 10.1016/j.ygyno.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33:1460–1466. doi: 10.1200/JCO.2014.55.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlin JN, Yu C, Hill EK, Zivanovic O, Kolev V, Levine DA, et al. Nomogram for predicting 5-year disease-specific mortality after primary surgery for epithelial ovarian cancer. Gynecol Oncol. 2012;125:25–30. doi: 10.1016/j.ygyno.2011.12.423. [DOI] [PubMed] [Google Scholar]
- 9.Lachance JA, Choudhri AF, Sarti M, Modesitt SC, Jazaeri AA, Stukenborg GJ. A nomogram for estimating the probability of ovarian cancer. Gynecol Oncol. 2011;121:2–7. doi: 10.1016/j.ygyno.2010.12.365. [DOI] [PubMed] [Google Scholar]
- 10.Kohn EC, Romano S, Lee JM. Clinical implications of using molecular diagnostics for ovarian cancers. Ann Oncol. 2013;24(Suppl 10):x22–x26. doi: 10.1093/annonc/mdt464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldron L, Haibe-Kains B, Culhane AC, Riester M, Ding J, Wang XV, et al. Comparative meta-analysis of prognostic gene signatures for late-stage ovarian cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlin JN, Jelinic P, Olvera N, Bogomolniy F, Bisogna M, Dao F, et al. Validated gene targets associated with curatively treated advanced serous ovarian carcinoma. Gynecol Oncol. 2013;128:512–517. doi: 10.1016/j.ygyno.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Verhaak RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123:517–525. doi: 10.1172/JCI65833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet Gynecol. 2015;126:491–497. doi: 10.1097/AOG.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]