Abstract
Objective
To examine the feasibility and efficacy of a home-based gait observation intervention for improving walking in Parkinson disease (PD).
Design
Participants were randomized to an intervention or control condition. A baseline walking assessment, a training period at home, and a post-training assessment were conducted.
Setting
The laboratory and participants' home and community environments.
Participants
23 non-demented individuals with PD experiencing walking difficulty.
Intervention
In the Gait Observation (intervention) condition, participants viewed videos of healthy and parkinsonian gait. In the Landscape Observation (control) condition, participants viewed videos of moving water. These tasks were completed daily for eight days.
Main Outcome Measures
Spatiotemporal walking variables were assessed using accelerometers in the laboratory (baseline and post-training assessments) and continuously at home during the training period. Variables included daily activity, walking speed, stride length, stride frequency, leg swing time, and gait asymmetry. Questionnaires including the 39-item Parkinson's Disease Questionnaire (PDQ-39) were administered to determine self-reported change in walking, as well as feasibility.
Results
At post-training assessment, only the Gait Observation group reported significantly improved mobility (PDQ-39). No improvements were seen in accelerometer-derived walking data. Participants found the at-home training tasks and accelerometer feasible to use.
Conclusion
Participants found procedures feasible and reported improved mobility, suggesting that observational training holds promise in the rehabilitation of walking in PD. Observational training alone, however, may not be sufficient to enhance walking in PD. A more challenging and adaptive task, and the use of explicit perceptual learning and practice of actions, may be required to effect change.
Keywords: Rehabilitation, Locomotion, Visual Perception, Activities of Daily Living, Neurodegenerative Diseases, Movement Disorders
Parkinson disease (PD) causes dysfunction in walking and gait.1–3 Physical interventions (e.g., treadmill walking) as well as sensory approaches (e.g., metronomes) are effective in improving gait in PD,4–8 though deficits often persist. Action observation training, in addition to physical practice, may be an effective adjunctive treatment. This training consists of repetitive visual perception of biological motion (the movement of human bodies). Biological motion perception depends on activity in the superior temporal sulcus and the mirror neuron system (premotor cortex, supplementary motor area),9 regions that are dysfunctional in PD.10–13 Previous data from our group (Jaywant et al., unpublished observations) have shown that perception of walking from biological motion is impaired in PD, which may contribute to walking impairments in this disorder.14 If individuals with PD can improve their perception of biological motion through repeated observations, motor aspects of gait and walking may improve via neuroplastic changes in motor/mirror neuron regions.
There is preliminary evidence for the usefulness of action observation in PD. A single session of observing finger movements reduced bradykinesia and enhanced spontaneous finger movement rate.15 Observation and practice of everyday actions improved functional independence.16 Observation of actors depicting strategies to overcome freezing of gait (and practicing those strategies) led to fewer self-reported episodes of freezing in comparison to a control condition, an effect that persisted at four-weeks follow-up.17 While these studies suggest that action observation training is beneficial in PD, several questions remain. First, it is unknown whether observation of biological motion alone is sufficient for improving motor function (as in Pelosin et al.15), or if practice of the observed movements is required (as in Buccino et al.16 and Pelosin et al.17). Second, previous studies have used control conditions such as sequences of static landscape images16 that make it difficult to isolate treatment effects to the observation of biological motion rather than non-biological motion. Third, it is unknown if the effects of action observation training lead to objective changes in spatiotemporal aspects of walking that generalize to naturalistic settings.
The goal of the current study was to examine the efficacy and feasibility of a home-based action observation (gait observation) intervention for walking in PD. We sought to determine the effect of observing biological motion alone, without physical practice. We also used a stringent control condition with videos of non-human motion (moving water) in a natural environment, which allowed us to isolate treatment effects to biological motion (processed in posterior superior temporal sulcus and premotor cortex9) and eliminate non-biological motion (processed in middle/inferior temporal cortex18,19) or other visual features of the scene as drivers of any intervention effect. We assessed self-reported mobility and objectively measured spatiotemporal walking in the laboratory and at home, to determine whether training effects generalized to a natural setting. We predicted that the home-based action observation intervention would be feasible and would result in self-reported and objective changes in walking.
Method
Participants
Twenty-three individuals with PD were enrolled from January 2014 to February 2015 (Figure 1). For analyses to yield a medium size effect with power=80% and alpha=.05, a sample of 18 was required. Participants were recruited through Boston Medical Center, Boston University's Center for Neurorehabilitation, and the Fox Foundation Trial Finder. Inclusion criteria included idiopathic PD (Hoehn & Yahr stage 1–3; U.K. Parkinson's Disease Society Brain Bank diagnostic criteria20), ≥ 1 on the Unified Parkinson Disease Rating Scale gait item (42), were native speakers of English, had at least 12 years of education, and lived independently at home. Exclusion criteria included orthopedic injuries impacting walking; use of an assistive device for walking; previous intracranial surgery; traumatic brain injury with loss of consciousness greater than a few seconds; substance abuse; and eye pathologies that impaired vision. This study was approved by the Boston University Institutional Review Board. All participants provided informed consent.
Figure 1.
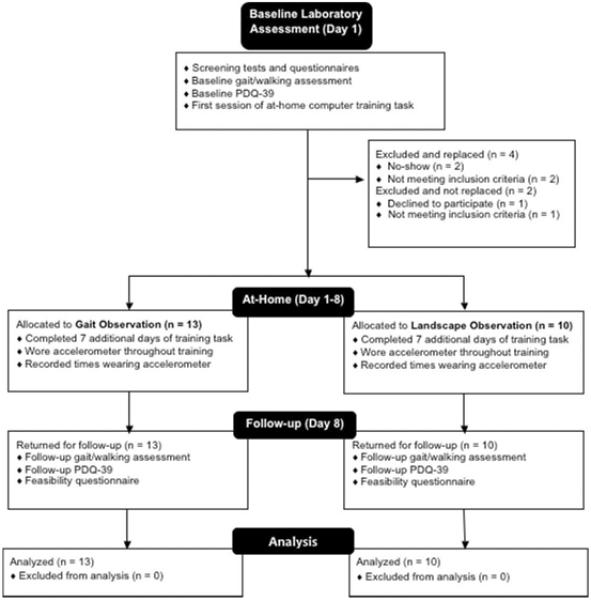
Flow diagram of study procedures.
Random Assignment of Participants
Following initial telephone screening, a staff member randomly assigned participants to the Gait Observation (intervention) condition or the Landscape Observation (control) condition using a computer-generated block randomization procedure (block size of four). An independent examiner was blind to group assignment for the baseline walking assessment (assignment kept in a sealed envelope), but was unblinded for the at-home and post-training assessment. Participants in both groups were naïve to the focus on improving walking.
Training Conditions
Gait Observation (Intervention)
Participants viewed videos of actors with and without PD. We filmed novel videos of actors walking in a hallway from lateral and anterior/posterior views, which allowed observation from multiple perspectives to facilitate motor learning. Eight to ten walking trials were filmed and edited for each actor, and entered into a perceptual experiment using SuperLab 5.0 presentation softwarea. A total of 112 videos were created: 56 of PD actors with unhealthy gait patterns, 56 of actors without PD with healthy gait patterns. Study participants judged if the walking in each video appeared healthy or resembled a PD-like gait pattern.
Landscape Observation (Control)
Participants viewed videos (freely available at www.mothernaturevideos.com) of landscapes with moving water in oceans, rivers, lakes, and waterfalls. To our knowledge, these videos have not previously been used as a control condition in an action observation study. Motion was isolated to water moving with different speeds and strengths, with no biological motion. A total of 112 video clips (56 with water moving roughly, and 56 with water moving calmly) were taken from several different landscapes. Participants judged if the water was moving “roughly” or “calmly.”
For both conditions, participants took home a laptop computer. They judged the videos via keyboard press. Feedback (“correct” or “incorrect”) was presented on the computer screen after each trial. The same videos appeared daily, in a randomized order.
Procedure
Figure 1 provides a flowchart of the study procedures. Participants were in the “on” medication state for assessments and training. One participant was not on anti-parkinsonian medication.
Laboratory-Based Walking Assessment
This assessment was administered at baseline and repeated seven days after completion of the home-based training. Participants wore tri-axial accelerometersb on each ankle while walking in the laboratory. Data were collected at a sampling frequency of 100 Hz (dynamic range: +/− 8g; resolution: 12 bit [3.9 mg]). Walking trials included: (a) Straight Line Walking (two trials each of 10m and 20m); (b) Walking with Turns (one trial to walk up to a cabinet [16m], two trials of walking to sit in a chair [18.8m and 13.8m], one trial walking up to a water cooler and drinking a glass of water [13.8m]); and (c) Dual-Task Walking (one trial of walking while holding a mug [16m]). Straight Line Walking trials closely matched the videos in the Gait Observation training task. Walking with Turns and Dual-Task Walking conditions were included to determine if potential intervention effects would generalize to more complex walking. Participants were familiarized with the environment and tasks before performing the walking trials. They were instructed to walk at their natural, comfortable walking pace.
Acceleration data were extracted using EMG Works softwarec. Spatiotemporal gait parameters were calculated using automatic peak detection functions that identified maximal (heel-strike) and secondary (toe-off) peaks from the accelerometer,21–24 using the x-axis oriented in the sagittal plane of the right ankle. One stride was defined as two successive heel strikes of the same foot. Swing time was quantified as the time between the initial toe-off and subsequent heel-strike for each stride. The spatiotemporal walking variables included walking speed (distance of the walking trial / time to complete the trial); stride length (distance of the walking trial / number of strides to complete the trial); stride frequency (number of strides / time to complete the walking trial); and percent leg swing time (100 × [swing time / stride time] for each stride).
Accelerometer data from both legs were used to determine gait asymmetry, following Yogev et al.3 For each walking trial, mean swing time was calculated for the left and right leg. Gait asymmetry was calculated as the natural log (leg with longer swing time / leg with shorter swing time), where higher values reflect greater degrees of asymmetry. The mean and standard deviation (as an index of variability) of gait parameters were computed separately for trials of Straight Line Walking, Walking with Turns, and Dual-Task Walking.
Home Walking Assessment
Participants wore an accelerometer (sampling rate of 75 Hz) on the right leg during waking hours throughout each day of training. To calculate walking parameters, we used a well-established algorithm that reliably differentiates walking from other activities, and identifies stride frequency (mean and standard deviation), number of walking periods, and duration of each walking period from an ankle-mounted tri-axial accelerometer.25 The algorithm defines a walking period as at least three strides occurring within five seconds.
Self-Report Questionnaires
The 39-item Parkinson's Disease Questionnaire (PDQ-39) (modified to rate quality of life over the past week) was self-administered on Day 1 (baseline) and on Day 8 (post-training assessment). Each question is scored on a 0–4 scale, for a maximum possible total of 156. The PDQ-39 has shown excellent psychometric properties.26 We examined the total PDQ-39 score and the Mobility subscale. At post-training, participants were also administered a 10-item questionnaire to rate feasibility and self-perceived improvement.
Feasibility
We examined feasibility using participants' self-reported ability to understand instructions and to use the computer and accelerometer. We also assessed the number of computer training sessions completed, days in which the accelerometer was worn, and number of hours per day wearing the accelerometer.
Statistical Analyses
Changes in walking based on Group (Gait Observation, Landscape Observation), Time (Baseline, Post-Training), and Walking Type (Straight Line, Walking with Turns) were analyzed using mixed-design ANOVA. Primary outcome measures were walking speed, stride length, and stride frequency; secondary outcome measures were leg swing time and gait asymmetry. 95% confidence intervals are presented in square brackets. For simple main effects and interaction effects, we report effect size using eta-squared (η2), the proportion of the total variability in the data accounted for by that effect. To explore significant interaction effects we conducted post-hoc t-tests. We report effect size using Cohen's d (.20 = small effect, .50 = medium effect, .80 = large effect), with the pooled standard deviation as the standardizer. Results of the training task and home walking assessment were analyzed using linear mixed effects modeling with Group, Day (1–8), and Group × Day as fixed effects, and participants as a random effect.
Results
Demographic Characteristics and Feasibility
There were no group differences in age, education, male:female ratio, disease severity, medication dosage, visual acuity, cognitive status, depression, or anxiety (Table 1). Participants found study procedures highly feasible and demonstrated a high rate of adherence to the study protocol (Table 2). No adverse events were reported.
Table 1.
Participant Characteristics at Baseline
| Gait Observation (N=13) | Landscape Observation (N=10) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Measure | M | SD | Range | M | SD | Range | Significance |
| Age (years) | 63.7 | 6.2 | 51–74 | 65.8 | 8.7 | 52–80 | ns |
| Education (years) | 17.2 | 1.4 | 16–20 | 16.0 | 2.4 | 12–20 | ns |
| Male:Female Ratio | 6:7 | - | - | 4:6 | - | - | ns |
| UPDRS Motor Score | 18.8 | 4.9 | 12–31 | 19.5 | 8.2 | 7–32 | ns |
| H&Y Stage (median) | 2.0 | - | 1.5–3 | 2.0 | - | 1–3 | ns |
| LED (mg/day) | 519 | 293 | 0–900 | 447 | 268 | 100–880 | ns |
| Acuity (logMAR) | 0.08 | 0.11 | −0.1–0.2 | 0.06 | 0.08 | −0.1–0.2 | ns |
| MMSE | 28.6 | 0.8 | 27.24–29.71 | 28.5 | 1.0 | 27.24–29.71 | ns |
| GDS | 5.8 | 4.4 | 0–15 | 6.8 | 4.1 | 3–14 | ns |
| BAI | 6.4 | 6.3 | 0–24 | 3.6 | 3.5 | 0–9 | ns |
M = mean; SD = standard deviation; UPDRS = Unified Parkinson's Disease Rating Scale; H&Y = Hoehn & Yahr stage; LED = Levodopa equivalent dosage; logMAR = logarithm of mean angle of resolution; MMSE = Mini-Mental State Examination; GDS = Geriatric Depression Scale; BAI = Beck Anxiety Inventory. Values presented are means (standard deviations) unless otherwise indicated.
Table 2.
Feasibility and Adherence to Study Protocol
| Gait Observation | Landscape Observation | Significance | |
|---|---|---|---|
| Ability to understand study instructions * | 8.5 (0.5) | 8.4 (0.7) | ns |
| Ability to use computer equipment * | 8.3 (0.9) | 8.4 (0.7) | ns |
| Ability to use/wear accelerometer while going about daily life * | 8.5 (.52) | 8.6 (1.0) | ns |
| Participants who completed all computer training sessions † | 10/13 | 7/10 | -- |
| Participants who wore the accelerometer on all training days ‡ | 11/13 | 9/10 | -- |
| Mean hours wearing accelerometer per day | 12.4 (2.0) | 13.7 (1.4) | ns |
Participants answered the questions by circling a number on a 0–9 visual analog scale, where 0 = very difficult and 9 = very easy. Values are means (standard deviations).
The remaining three participants in the Gait Observation group and the Landscape Observation group each missed one training session.
Two participants in the Gait Observation group and one participant in the Landscape Observation group missed one day of wearing the accelerometer.
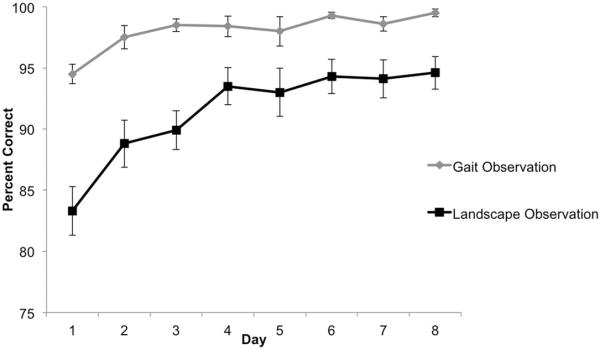
Performance on Training Task
There were significant effects of Group, Day, and Group × Day (Table 3). Baseline performance on the computer task was better for Gait Observation than Landscape Observation by 10.6%. Both groups showed significant improvement in training task performance across days (Figure 2), though the rate of improvement was significantly less for Gait Observation (.89% less gain per day) than Landscape Observation.
Table 3.
Results of Linear Mixed Effects Modeling for the Computer Training Task
| Effect | df | F | p |
|---|---|---|---|
| Tests of Fixed Effects | |||
| Intercept | (1, 44.4) | 13630.1 | < .001 |
| Group | (1, 44.4) | 46.7 | < .001 |
| Day | (1, 44.7) | 94.0 | < .001 |
| Group × Day | (1, 44.7) | 20.0 | < .001 |
| Estimates of Fixed Effects | β | 95% CI | t | p |
|---|---|---|---|---|
| Intercept* | 85.1 | [82.8, 87.4] | 73.0 | < .001 |
| Group = Gait Observation | 10.6 | [7.5, 13.7] | 6.8 | < .001 |
| Day* | 1.4 | [1.1, 1.7] | 9.4 | < .001 |
| Group = Gait Observation | −0.9 | [−0.5, −1.3] | −4.5 | < .001 |
Note. The dependent variable is accuracy (percent correct)
Intercept and Day refer to the reference group (Landscape Observation).
Figure 2. Performance on the at-home computer training task for the Gait Observation and Landscape Observation groups.
The task in the Gait Observation condition was to discriminate between healthy and parkinsonian gait/walking. The task in the Landscape Observation group was to discriminate between water moving roughly and calmly. The outcome variable is accuracy (percent correct). Both groups improved significantly over subsequent days, though the rate of change (slope) was smaller in the Gait Observation group. Error bars represent standard error of the mean.
Laboratory-Based Walking Assessment
Primary Outcome Measures
Means and standard deviations of walking variables are displayed in Table 4. No main or interaction effects emerged for walking speed (mean and standard deviation) or mean stride length (all Fs(1,21)<2.74, ps>.05). For stride length (standard deviation), only a significant main effect of Walking Type emerged (F(1,21)=7.72, p<.05, η2=.11), with stride length being more variable in trials of Walking With Turns than trials of Straight Line Walking (mean difference=.02 [.01, .03], p<.05, d=.63).
Table 4.
Mean (Standard Deviation) of Gait Characteristics by Group (Gait Observation, Landscape Observation) and Time (Baseline, Follow-up)
| Gait Observation (N = 13) | Landscape Observation (N = 10) | |||
|---|---|---|---|---|
|
|
||||
| Walking Parameter | Baseline | Follow-up | Baseline | Follow-up |
| Straight Line Walking Trials | ||||
| Walking Speed (m/s) | 1.19 (.15) | 1.19 (.15) | 1.13 (.14) | 1.18 (.08) |
| Walking Speed Variability (SD) | .07 (.04) | .07 (.04) | .07 (.04) | .05 (.03) |
| Stride Length (m) | 1.34 (.18) | 1.35 (.21) | 1.3 (.15) | 1.34 (.12) |
| Stride Length Variability (SD) | .06 (.04) | .07 (.03) | .06 (.04) | .05 (.03) |
| Stride Frequency (strides/s) | .86 (.06) | .89 (.06) | .87 (.08) | .89 (.06) |
| Stride Frequency Variability (SD) | .04 (.02) | .03 (.01) | .03 (.01) | .03 (.01) |
| Swing Time (% of stride) | 46.0 (1.6) | 45.6 (1.6) | 44.5 (1.4) | 44.8 (1.7) |
| Swing Time % Variability (SD) | 1.7 (.4) | 1.9 (.4) | 1.8 (.5) | 1.5 (.2) |
| Gait Asymmetry | .03 (.02) | .03 (.02) | .02 (.01) | .02 (.01) |
| Walking With Turns Trials | ||||
| Walking Speed (m/s) | 1.18 (.15) | 1.19 (.13) | 1.16 (.12) | 1.19 (.08) |
| Walking Speed Variability (SD) | .08 (.04) | .06 (.03) | .07 (.03) | .06 (.04) |
| Stride Length (m) | 1.35 (.20) | 1.36 (.20) | 1.33 (.15) | 1.35 (.11) |
| Stride Length Variability (SD) | .09 (.04) | .07 (.03) | .08 (.03) | .07 (.04) |
| Stride Frequency (strides/s) | .88 (.06) | .89 (.07) | .88 (.08) | .88 (.06) |
| Stride Frequency Variability (SD) | .02 (.01) | .03 (.01) | .02 (.01) | .02 (.02) |
| Swing Time (% of stride) | 45.8 (1.5) | 45.3 (1.3) | 44.6 (1.5) | 44.7 (1.6) |
| Swing Time % Variability (SD) | 2.1 (.4) | 1.9 (.4) | 2.0 (.5) | 1.9 (.3) |
| Gait Asymmetry | .03 (.02) | .03 (.01) | .02 (.01) | .03 (.01) |
| Dual Task Trial | ||||
| Walking Speed (m/s) | 1.13 (.14) | 1.17 (.18) | 1.10 (.10) | 1.17 (.15) |
| Stride Length (m) | 1.30 (.19) | 1.34 (.23) | 1.26 (.13) | 1.34 (.14) |
| Stride Frequency (strides/s) | .88 (.07) | .88 (.07) | .87 (.09) | .88 (.08) |
| Swing Time (% of stride) | 45.4 (1.8) | 45.3 (1.7) | 44.4 (1.4) | 44.6 (1.9) |
| Swing Time % Variability (SD) | 2.0 (.7) | 2.0 (1.0) | 2.1 (.9) | 1.7 (.5) |
| Gait Asymmetry | .03 (.02) | .03 (.03) | .02 (.02) | .03 (.02) |
Note. Values presented are mean (standard deviation). m = meters; s = seconds; SD = standard deviation.
A significant Group × Time × Walking Type interaction emerged for mean stride frequency (F(1,21)=5.62, p<.05, η2=.04); however, post-hoc t-tests showed no significant between-group or within-group differences (all ps>.05). For stride frequency (standard deviation), there was only a significant interaction between Time and Walking Type (F(1,21)=7.7, p<.05, η2=.09). Regardless of group, stride frequency (standard deviation) decreased from baseline to post-training assessment for Straight Line Walking (mean difference=.006 [.001, .01], t(22)=2.65,p<.05, d=.42), but did not change for Walking With Turns (mean difference=.004 [−.002, .01], t(22)=1.53, p>.05, d=.38).
The dual-task walking trial was analyzed separately. A significant main effect of Time emerged for mean walking speed (F(1,21)=8.48, p<.01), which increased from baseline to post-training assessment (mean difference=.06 [.02,.1], p<.05, d=.38) regardless of group, suggesting a possible practice effect. A significant main effect of Time emerged for mean stride length (F(1,21)=8.75, p<.01, η2=.29), which increased after training (mean difference=.06 [.02, .1], p<.05, d=.33) regardless of group, again suggesting a practice effect. No main or interaction effects emerged for mean stride frequency.
Secondary outcome measures
No main or interaction effects emerged for mean percent swing time (all Fs(1,21)<3.41, ps>.05). For percent swing time (standard deviation), a significant Group × Time × Walking Type interaction emerged (F(1,21)=11.25, p<.05, η2=.06). In Straight Line Walking only, there was no change from baseline to post-training assessment in the Gait Observation group (mean difference=.25 [−.1, .60], t(12)=1.56, p>.05, d=.63), while there was a trend towards decreased percent swing time (standard deviation) in the Landscape Observation group (mean difference=.35 [−.03, .72], t(9)=2.09, p=.07, d=.92). For gait asymmetry, the only significant effect was a main effect of Group (F(1,21)=4.94, p<.05), where regardless of Time and Walking Type, the Gait Observation group had more gait asymmetry than the Landscape Observation group (mean difference=.01 [.001, .02], p<.05, d=.64). On the dual task walking trial, no main or interaction effects emerged for percent swing time (mean or standard deviation) or gait asymmetry.
Home Walking Assessment
No significant main or interaction effects emerged for walking periods per hour, mean duration of each walking period, or stride frequency (all Fs<3.44, ps>.05).
Self-Report Questionnaires
There was a significant Group × Time interaction on the PDQ-39 Mobility subscale (F(1,21)=9.44, p<.01, η2=.31) (Figure 3). Post-hoc t-tests revealed that although there was no significant difference between Gait Observation and Landscape Observation at baseline (mean difference=.75 [−5.5, 6.99], t(21)=.54,p>.05, d=.11) or post-training (mean difference=3.08 [−2.97, 9.12], t(21)=1.06, p>.05, d=.45), the Gait Observation group had a significant decrease in score (increase in self-reported mobility) post-training (Figure 3; mean difference=−1.92 [−3.64, −.21], t(12)=−2.44, p<.05, d=−.25). The score decreased by 4.8%, which exceeded the threshold for a clinically meaningful change.27 The Landscape Observation group's score did not change post-training (mean difference=1.9 [−.32, 4.12], t(9)=1.93, p>.05, d=.31). No significant main or interaction effects emerged on the PDQ-39 Total score (all Fs(1,21)<2.19, ps>.05).
Figure 3. Change in self-reported mobility by Group (Gait Observation, Landscape Observation) and Time (Baseline, Post-Training).
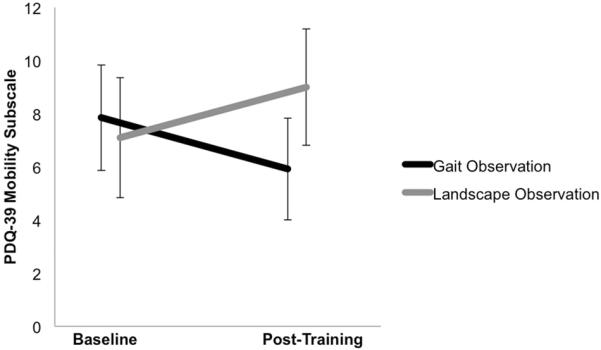
The outcome variable is score on the 39-item Parkinson's Disease Questionnaire (PDQ-39), Mobility subscale. Higher scores indicate worse mobility. Error bars represent standard error of the mean. The Gait Observation group had significantly improved self-reported mobility (lower score) at post-training compared to baseline (t(12)=−2.44, p<.05, d=−.25). The score decreased by 4.8%, which exceeded the threshold for a clinically meaningful change. The Landscape Observation group's score did not significantly change from baseline to post-training.
Participants in the Gait Observation group reported greater improvement in walking speed and stride length than the Landscape Observation group (Table 5); though not statistically significant, the magnitude of the effects were medium. The Gait Observation group reported strategies they learned through the training, including increased attention to one's own gait, keeping one's head up, swinging the arms, and visualizing gait patterns of others while walking.
Table 5.
Self-Reported Change in Gait and Walking
| Gait Observation | Landscape Observation | Significance | Cohen's d | |
|---|---|---|---|---|
| Walking Speed | 3.6 (3.0) | 1.7 (1.9) | p = .08 | .76 |
| Stride Length | 3.5 (2.8) | 1.8 (1.9) | p = .11 | .73 |
| Arm Swing and Arm/Leg Coordination | 3.2 (2.8) | 2.2 (2.5) | ns | .39 |
| Learning differences between PD-like and healthy walking | 5.1 (2.9) | -- | -- | -- |
| Ability to perceive differences in PD-like and healthy walking | 6.0 (2.9) | -- | -- | -- |
Note. Participants answered the questions by circling a number on a 0–9 visual analog scale, where 0 = “no improvement at all” and 9 = “improved a lot.” Higher values reflect greater improvement. Participants in the Landscape Observation group were not asked the last two questions. Values are means (standard deviations).
Discussion
The present study investigated the efficacy of home-based gait observation training to enhance walking in PD. With repeated perceptual training, PD participants in the Gait Observation group showed an improved ability to discriminate between healthy and parkinsonian gait (biological motion). This improvement did not result in objective changes in walking measured in the lab or home, but did result in increased self-perceived mobility (small effect) following the training.
There are a number of possible reasons for the lack of change in objective walking results. First, individuals with PD have impaired perception of biological motion (Jaywant et al., submitted) and may require more practice than healthy adults28,29 to benefit from action observation training; therefore, it is possible that an insufficient dose of training was provided to see objective changes in gait. Second, our training task may not have been sufficiently challenging. Participants in the Gait Observation condition were 95% accurate in discriminating between healthy and PD gait on day 1 of training. Our training intervention used the same videos daily for one week. A perceptual training intervention that employs a wider range of stimuli, more than two response choices (i.e., a broader array of gait types to discriminate between), or an adaptive procedure that increases in difficulty with participant improvement, may prove more efficacious than our intervention.
Third, the nature of our training task relied on implicit learning. We expected that participants with PD would implicitly perceive and discriminate between healthy and impaired aspects of gait during observational learning, resulting in the adoption of healthier gait patterns.30–33 Observation-based training may require more explicit strategies, such as directing attention toward specific aspects of gait (e.g., stride length, gait speed), or specific parts of the body (e.g., feet) rather than observing the whole task with a general focus. Such directed attention could elicit stronger activity in the mirror neuron system (premotor cortex), because premotor cortex activates in a somatotopic manner when observing actions.34 Objective gait changes may also require directed practice of such strategies in combination with the training task (i.e., by drawing conscious attention to one's own gait and implementing strategies repeatedly during training), similar to previous paradigms that have paired action observation with physical training.16,17
Our results are in line with previous studies on action observation training that have shown self-reported improvement in motor function.17,35 The Gait Observation intervention led to a self-perceived increase in functional walking ability in natural environments (PDQ-39 Mobility subscale) that was clinically meaningful,27 despite the fact that participants were not told that the purpose of the training was to improve walking. It is possible that walking assessed with accelerometers did not capture the types of functional improvements represented on the PDQ-39 Mobility subscale. Self-perceived improvement is important for participants to remain motivated to engage in such interventions, and may also increase participation in walking-based activities of daily living.
Participants found study procedures feasible: They reported the study instructions were easy to understand, and the computer equipment and accelerometer were easy to operate. The majority of participants completed all training sessions and wore the accelerometer each day, for several hours during the day. All participants returned for post-training assessment. These findings suggest that future studies of home-based interventions with continuous activity monitoring using accelerometers are feasible in the PD population.
Our landscape observation condition was a novel addition to an action-observation paradigm as we controlled for the effects of observing non-biological motion while providing challenge (<85% correct performance at baseline) and motivation/engagement (of ten participants, seven completed all sessions; three missed only one session). We recommend such a control condition in future investigations because it will strengthen the claim that treatment effects are attributable to perception of biological motion per se.
Study Limitations
Limitations of the study included the small sample size with consequent constraints on the generalizability of the results. This study also did not have a long-term follow-up to determine maintenance of self-reported increased mobility over time. As mentioned above, the gait training discrimination task may have been insufficiently challenging to evaluate the full potential impact of this approach.
Conclusions
Despite the lack of objective change, the Gait Observation intervention holds promise in the rehabilitation of walking in PD, particularly given the self-reported increase in functional mobility. Participants found our home-based intervention to be highly feasible. Accelerometers allowed us to assess walking in a naturalistic setting at home, which is important in understanding treatment effects in real world settings. Our data inform the design of future research investigating the benefits of action observation treatments to improve gait in PD.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS), including a Ruth L. Kirschstein National Research Service Award to AJ (grant number F31 NS078919) and an R01 grant to ACG (grant number R01 NS067128). NINDS was not involved in conducting this research or preparing this article. The authors have no conflict of interest to declare. We thank Marie Saint-Hilaire, M.D., Cathi Thomas, R.N., Katy Hendron, D.P.T., and the Michael J. Fox Foundation for their assistance in participant recruitment. We also thank Dr. Saint-Hilaire for neurological consultation regarding individual participants. We are grateful to Laura Pistorino, B.A., Gretchen Reynolds, M.A., Michael Otto, Ph.D., and Todd Farchione, Ph.D., for assistance with experimental design and participant randomization. We dedicate this paper to the memory of our colleague and friend, Dr. Robert Wagenaar, who provided invaluable guidance in developing the study.
Abbreviations
- PD
Parkinson Disease
- PDQ-39
39-item Parkinson's Disease Questionnaire
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suppliers
Cedrus, San Pedro, CA
ActivInsights Ltd., Cambridgeshire, UK
Delsys Inc., Natick, MA
References
- 1.Carpinella I, Crenna P, Calabrese E, et al. Locomotor function in the early stage of Parkinson's disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15(4):543–551. doi: 10.1109/TNSRE.2007.908933. doi:10.1109/TNSRE.2007.908933. [DOI] [PubMed] [Google Scholar]
- 2.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22(5):1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. doi:10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 3.Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff JM. Gait asymmetry in patients with Parkinson's disease and elderly fallers: when does the bilateral coordination of gait require attention? Exp Brain Res. 2007 doi: 10.1007/s00221-006-0676-3. doi:10.1007/s00221-006-0676-3. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson CL, Patel S, Meek C, et al. Cochrane Libr. 2013. Physiotherapy versus placebo or no intervention in Parkinson's disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med. 2012;366:511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132–143. doi: 10.1177/1545968311421614. doi:10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78(2):134–140. doi: 10.1136/jnnp.200X.097923. doi:10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochester L, Baker K, Hetherington V, et al. Evidence for motor learning in Parkinson's disease: Acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res. 2010;1319:103–111. doi: 10.1016/j.brainres.2010.01.001. doi:10.1016/j.brainres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Iseki K, Hanakawa T, Shinozaki J, Nankaku M, Fukuyama H. Neural mechanisms involved in mental imagery and observation of gait. Neuroimage. 2008;41(3):1021–1031. doi: 10.1016/j.neuroimage.2008.03.010. doi:10.1016/j.neuroimage.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Herz DM, Siebner HR, Hulme OJ, Florin E, Christensen MS, Timmermann L. Levodopa reinstates connectivity from prefrontal to premotor cortex during externally paced movement in Parkinson's disease. Neuroimage. 2014;90:15–23. doi: 10.1016/j.neuroimage.2013.11.023. doi:10.1016/j.neuroimage.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Jubault T, Gagnon J-F, Karama S, et al. Patterns of cortical thickness and surface area in early Parkinson's disease. Neuroimage. 2011;55(2):462–467. doi: 10.1016/j.neuroimage.2010.12.043. doi:10.1016/j.neuroimage.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 12.Suppa A, Iezzi E, Conte A, et al. Dopamine influences primary motor cortex plasticity and dorsal premotor-to-motor connectivity in Parkinson's disease. Cereb Cortex. 2010;20:2224–2233. doi: 10.1093/cercor/bhp288. doi:10.1093/cercor/bhp288. [DOI] [PubMed] [Google Scholar]
- 13.Zarei M, Ibarretxe-Bilbao N, Compta Y, et al. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2013;84(8):875–881. doi: 10.1136/jnnp-2012-304126. doi:10.1136/jnnp-2012-304126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller Koop M, Hill BC, Bronte-Stewart HM. Perceptual errors increase with movement duration and may contribute to hypokinesia in Parkinson's disease. Neuroscience. 2013;243:1–12. doi: 10.1016/j.neuroscience.2013.03.026. doi:10.1016/j.neuroscience.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Pelosin E, Bove M, Ruggeri P, Avanzino L, Abbruzzese G. Reduction of bradykinesia of finger movements by a single session of action observation in Parkinson disease. Neurorehabil Neural Repair. 2013;27(6):552–560. doi: 10.1177/1545968312471905. doi:10.1177/1545968312471905. [DOI] [PubMed] [Google Scholar]
- 16.Buccino G, Gatti R, Giusti MC, et al. Action observation treatment improves autonomy in daily activities in Parkinson's disease patients: Results from a pilot study. Mov Disord. 2011;26(10):1963–1964. doi: 10.1002/mds.23745. doi:10.1002/mds.23680. [DOI] [PubMed] [Google Scholar]
- 17.Pelosin E, Avanzino L, Bove M, Stramesi P, Nieuwboer A, Abbruzzese G. Action observation improves freezing of gait in patients with Parkinson's disease. Neurorehabil Neural Repair. 2010;24(8):746–752. doi: 10.1177/1545968310368685. doi:10.1177/1545968310368685. [DOI] [PubMed] [Google Scholar]
- 18.Beauchamp MS, Lee KE, Haxby JV, Martin A. fMRI responses to video and point-light displays of moving humans and manipulable objects. J Cogn Neurosci. 2001;15(7):991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- 19.Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34:149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 20.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J-A, Cho S-H, Lee Y-J, Yang H-K, Lee J-W. Portable activity monitoring system for temporal parameters of gait cycles. J Med Syst. 2010;34(5):959–966. doi: 10.1007/s10916-009-9311-8. doi:10.1007/s10916-009-9311-8. [DOI] [PubMed] [Google Scholar]
- 22.Willemsen ATM, Bloemhof F, Boom HBK. Automatic stance-swing phase detection from accelerometer data for peroneal nerve stimulation. IEEE Trans Biomed Eng. 1990;37(12):1201–1208. doi: 10.1109/10.64463. doi:10.1109/10.64463. [DOI] [PubMed] [Google Scholar]
- 23.Boutaayamou M, Schwartz C, Stamatakis J, et al. Validated extraction of gait events from 3D accelerometer recordings. 2012 International Conference on 3D Imaging; 2012. pp. 1–4. doi:10.1109/IC3D.2012.6615147. [Google Scholar]
- 24.Heiden T, Burnett A. International Society of Biomechianics in Sports. 2004. Determination of heel strike and toe-off in the running stride using an accelerometer: Application to field-based gait studies; pp. 98–101. [Google Scholar]
- 25.Zhang Y, Beenakker KGM, Butala PM, et al. Monitoring walking and cycling of middle-aged to older community dwellers using wireless wearable accelerometers. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS; 2012. pp. 158–161. doi:10.1109/EMBC.2012.6345895. [DOI] [PubMed] [Google Scholar]
- 26.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well-being for individuals with Parkinson's disease. Qual Life Res. 1995;4(3):241–248. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 27.Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ-39 Parkinson's disease questionnaire. Age Ageing. 2001;30:299–302. doi: 10.1093/ageing/30.4.299. doi:10.1093/ageing/30.4.299. [DOI] [PubMed] [Google Scholar]
- 28.Swinnen SP, Steyvers M, Van Den Bergh L, Stelmach GE. Motor learning and Parkinson's disease: refinement of within-limb and between-limb coordination as a result of practice. Behav Brain Res. 2000;111(1–2):45–59. doi: 10.1016/s0166-4328(00)00144-3. doi:10.1016/S0166-4328(00)00144-3. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwboer A, Rochester L, Müncks L, Swinnen SP. Motor learning in Parkinson's disease: limitations and potential for rehabilitation. Park Relat Disord. 2009;15(S3):S53–S58. doi: 10.1016/S1353-8020(09)70781-3. doi:10.1016/S1353-8020(09)70781-3. [DOI] [PubMed] [Google Scholar]
- 30.Abbruzzese G, Trompetto C, Marinelli L. The rationale for motor learning in Parkinson's disease. Eur J Phys Rehabil Med. 2009;45(2):209–214. [PubMed] [Google Scholar]
- 31.Marinelli L, Crupi D, Di Rocco A, et al. Learning and consolidation of visuo-motor adaptation in Parkinson's disease. Park Relat Disord. 2009;15(1):6–11. doi: 10.1016/j.parkreldis.2008.02.012. doi:10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gobel EW, Blomeke K, Zadikoff C, Simuni T, Weintraub S, Reber PJ. Implicit perceptual-motor skill learning in mild cognitive impairment and Parkinson's disease. Neuropsychology. 2013;27(3):314–321. doi: 10.1037/a0032305. doi:10.1037/a0032305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behrman AL, Cauraugh JH, Light KE. Practice as an intervention to improve speeded motor performance and motor learning in Parkinson's disease. J Neurol Sci. 2000;174(2):127–136. doi: 10.1016/s0022-510x(00)00267-7. doi:10.1016/S0022-510X(00)00267-7. [DOI] [PubMed] [Google Scholar]
- 34.Buccino G, Binkofski F, Fink GR, et al. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur J Neurosci. 2001;13(2):400–404. doi:10.1046/j.1460-9568.2001.01385.x. [PubMed] [Google Scholar]
- 35.Ertelt D, Small S, Solodkin A, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36:T164–T173. doi: 10.1016/j.neuroimage.2007.03.043. doi:10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]