Abstract
Accurate sampling of cancer suspicious locations is critical in targeted prostate biopsy, but can be complicated by the motion of the prostate. We present an open-source software for intra-procedural tracking of the prostate and biopsy targets using deformable image registration. The software is implemented in 3D Slicer and is intended for clinical users. We evaluated accuracy, computation time and sensitivity to initialization, and compared implementations that use different versions of the Insight Segmentation Toolkit (ITK). Our retrospective evaluation used data from 25 in-bore MRI-guided prostate biopsy cases (343 registrations total). Prostate Dice similarity coefficient improved on average by 0.17 (p < 0.0001, range 0.02–0.48). Registration was not sensitive to operator variability. Computation time decreased significantly for the implementation using the latest version of ITK. In conclusion, we presented a fully functional open-source tool that is ready for prospective evaluation during clinical MRI-guided prostate biopsy interventions.
Keywords: Prostate cancer, Image-guided interventions, Magnetic resonance imaging, Image registration, Software evaluation, 3D slicer
1 Introduction
Prostate cancer (PCa) remains a leading cause of cancer mortality in the USA and worldwide [1]. A critical question in management of PCa is in distinguishing aggressive cancer from indolent disease. Characterization of tumor aggressiveness relies on histopathological analysis of biopsy samples [2]. In the recent years, targeted sampling of suspected cancer areas have emerged as an effective personalized alternative to the systematic sextant biopsy [3]. Such targeted approaches require multiparametric MRI (mpMRI) for localizing suspected regions, which are then re-identified by means of image registration in the intra-procedural imaging. MRI can also be used as the intra-procedural imaging modality, as it provides superior visualization of the needle, anatomy and suspicious regions [4]. However, prostate motion during the course of biopsy, which can last for over an hour for in-bore procedures, can complicate localization of the suspected lesion. Continuous tracking of the prostate may thus be required to enable accurate targeting. In this paper we present an open-source platform to facilitate re-identification of the cancer targets and their tracking throughout the course of the procedure.
The most commonly used approach to targeted prostate biopsy relies on intra-procedural transrectal ultrasound (TRUS) registered (fused) with the diagnostic MRI [5] for target definition. In an alternative approach, the patient is positioned inside the MR scanner bore throughout the procedure, potentially allowing for improved targeting accuracy [4]. In this paper we focus on the latter approach. The clinical procedure can be subdivided into a pre-procedural planning, and an intra-procedural biopsy phase. The tumor-suspicious biopsy targets are defined using the pre-procedural mpMRI. At the time of the biopsy, a lower resolution T2 image is obtained to visualize prostate anatomy and biopsy needle. In order to spatially correlate biopsy targets and the intra-procedural scan, we use deformable image registration to compensate for the high deformation of the prostate that occurs when an endorectal coil is used during the pre-procedural imaging. Applying deformable registration and visually evaluate the results is a time-consuming and complex task, requiring specialized expertise and remains challenging for clinical staff.
Our contribution is the development and integration of a software solution to support in-bore MR-guided biopsy, developed for a clinical operator. Some of the individual algorithms and components we used were presented and evaluated elsewhere. Furthermore, some of these components, such as the deformable registration between pre- and intra-procedural MRI proposed by Fedorov et al. [6], have been used to support over a hundred of clinical research cases as discussed in [4]. However, these existing components are not designed for the end user (such as a nurse or technologist supporting the procedure), and are utilizing versions of the foundation tools that are no longer maintained (i.e., 3D Slicer1 [7] version 3 and Insight Segmentation and Registration Toolkit version 32 (ITKv3) [8]). These issues affect clinical utility of the registration tools, and complicate their validation, improvement and maintenance. Here we present an end-to-end open-source platform that utilizes the currently supported, widely used versions of both 3D Slicer (version 4) and Insight Toolkit version 4 (ITKv4).
This work has potentially wider impact to support accurate sampling of suspected cancer tissue and accurate correlation of the pathology findings with the pathology, genomics and emerging radiomics biomarkers. Our contribution is novel: while in-bore MRI-guided prostate biopsy is used by several groups, the existing workflows typically rely on visual re-identification of the cancer suspicious targets, which may affect accuracy and reproducibility of the procedure [9, 10].
2 Methods
We first present the overall setup and clinical workflow of the targeted in-bore MRI-guided prostate biopsy to establish the requirements for the software development and discuss the details of image acquisition. We follow with the description of our approach to the development of the platform. Finally, we present our evaluation approach, which is concerned with the accuracy, consistency and computational performance of the registration.
In-bore Targeted MRI-Guided Prostate Biopsy
In-bore transperineal targeted MRI-guided biopsy protocol involves two stages. First, mpMRI is acquired prior to the procedure and the cancer suspicious targets are localized using 3D Slicer. Prostate gland is contoured on the T2-weighted image. During the procedure, the patient is immobilized on the table top with velcro wrap and sedated. Imaging involved two types of imaging sequences: (1) axial T2w MRI (voxel size 0.5 × 0.5 × 3 mm, imaging time ~ 4 min) obtained in the beginning of the biopsy procedure for the purposes of target identification and (2) a series of lower resolution T2w MRI needle confirmation images (voxel size 0.75 × 0.75 × 3 mm, imaging time ~ 1 min) collected after needle placement to visually assess targeting accuracy. The purpose of image registration is to assist the interventionalist in re-identification of the cancer suspicious targets in the intra-procedural images.
Requirements
In order to support the clinical workflow described above, the developed software needs to meet the four following requirements: (1) Well-defined workflow A proper guided software process must indicate in which working step the user is currently situated and what task must successfully be accomplished to continue. No user action should allow to break out of the workflow. (2) High user transparency Crucial steps in the workflow should directly give feedback to the user to confirm their functionality. (3) High registration quality Registration results should be accurate, robust towards changing image quality, require short computation time, and be reproducible. Hence, effects of inter-user variability should be minimized. (4) High failure transparency Registration failures and subsequent errors in the results should be visible to the user to allow subsequent troubleshooting.
Image Registration
The most critical component of the workflow is the registration step, since its result may have direct effect on the accuracy of biopsy sampling. Our custom deformable image registration strategy requires limited user interaction and is based on the earlier developed methodology [6, 11]. As described in Fig. 1, prostate gland is contoured manually in the higher resolution T2w scan as part of intra-procedural workflow, but registration of subsequent needle confirmation images is done automatically. Registration step is implemented in the BRAINSFit3 module of 3D Slicer, which is using a hierarchical approach that includes 6, 9, and 12 degrees of freedom transformations, followed by b-spline deformable transformation, with mutual information as the similarity metric and is based on ITK [6]. In the case of registration failure due to large prostate motion, software workflow allows for manual segmentation of the needle image.
Fig. 1.
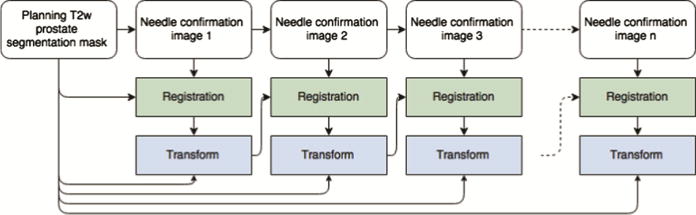
Outline of the registration process. Initial T2w prostate image is contoured semi-automatically, and the result is propagated to the subsequent needle confirmation images using the chain of transformations.
Description of the Software
Figure 2 shows the clinical workflow implemented in our software. As a pre-procedural step, the patient is selected from the database and the diagnostic data can be reviewed in order to confirm target positions. Data connection between the research workstation and the clinical workstation is established in order to receive intra-procedural DICOM data. After the first planning scan is received, a coarse manual segmentation of the prostate gland is prepared using semi-automated procedure (this segmentation is used for the initialization of the registration algorithm and does not need to be very accurate [6]). Upon completion of the registration, registered images and pre-procedural targets are examined side-by-side with the capability to switch between different registration stages. Intra-procedural registration and evaluation are applied every time a needle confirmation scan is received.
Fig. 2.
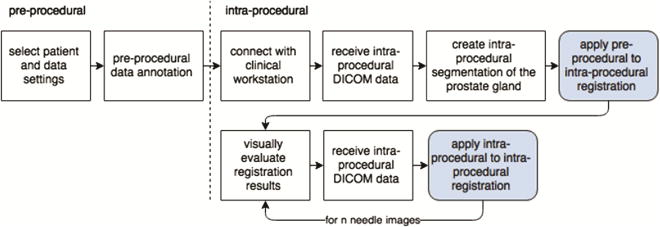
Steps of the procedural workflow. The developed software platform provides support for the intra-procedural phase of the workflow.
Evaluation
Our evaluation included three components. First, we evaluated registration accuracy and computational performance. Registration quality was first evaluated using visual assessment for each image pair, since annotation of images for quantitative assessment would be very time-consuming due to the large number of images. Quantitative evaluation was done using Dice Similarity Coefficient (DSC) between the manual segmentation of the high-resolution T2w image propagated to the last needle confirmation image through the chain of registration transformations, and the manual segmentation of the last needle image. The segmentations used for DSC assessment were prepared by an expert radiologist with specialization in abdominal imaging, and were not used in the registration process. Second, we evaluated sensitivity of registration to the variability in segmentation of the prostate gland. This was done by comparing registration results performed by two readers with different level of training. Neither of the readers had medical training. First reader had multi-year hands-on experience in prostate gland contouring, while the second reader had a brief training and no prior experience. Finally, we compared the results obtained using ITKv3 and the current ITKv4. This is important for our application, because components of the workflow currently used during clinical procedures are based on ITKv3. Evaluation was done retrospectively using datasets collected during clinical MR-guided prostate biopsy procedures.
3 Results
The software was implemented as a module within the 3D Slicer extension SlicerProstate4, which provides a collection of modules to facilitate (1) processing and management of prostate image data, (2) utilizing prostate images in image-guided interventions and (3) development of the imaging biomarkers of prostate cancer. Functionality and user interface are not discussed here due to the lack of space, and demonstrated in the videos available online5. Imaging data was collected in compliance with the human subject protection regulations. Informed consent was obtained from each patient in advance of the procedure. A total number of 343 needle confirmation images from N = 25 clinical cases with the median of 15 needle confirmation images (range 2–26) were used in the evaluation of the registration functionality. In 8 clinical cases large prostate motion for at least one of the needle confirmation images caused registration failure and manual segmentation of the needle confirmation image was required (i.e., in 15 out of 343 needle confirmation images). Improved alignment of the prostate gland between the registered planning scan and the needle confirmation images was confirmed visually for all 343 registrations. We provide an interactive website that can be used to visually assess the registration quality for each of the image pairs6. Registration quality was characterized as excellent in 227 images (example is case 18 needle image 6, or c18-n6), good in 88 images (e.g., c19-n7), moderate in 26 images (e.g., c12-n14) and poor in 2 images (e.g., c15-n9). Figure 3a shows assessment of the gland segmentation overlap before and after registration for the final needle confirmation image. We observed improved DSC in all 25 cases, with the average improvement by 0.17 (p < 0.0001) and a range from 0.02 (Case 22) to 0.48 (Case 28). Figure 3b shows the summary of gland segmentation overlap (DSC) before and after registration for the final needle confirmation image using two different sets of non-expert segmentations with different levels of training (average difference in DSC of 0.01 and maximum difference of 0.06 (p > 0.05)). Mean ± standard deviation (SD) of the computation time for registration of one needle confirmation image was 17.83 ± 6.98 s (range 5.22–43.92), which is compatible with the clinical constraints of the workflow. Figure 4a shows the distribution of computation times across 25 cases and Fig. 4b illustrates the computation time for every needle confirmation image comparing ITKv3 and ITKv4 implementations. DSC improved from 0.68 ± 0.13 (range 0.31–0.89) before registration to 0.84 ± 0.06 (range 0.67–0.93) for ITKv4 and 0.84 ± 0.06 (range 0.68–0.93) for ITKv3. No significant difference in DSC was observed between ITKv3 and ITKv4 results.
Fig. 3.
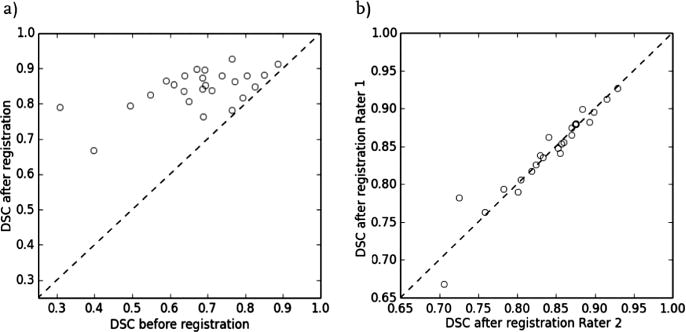
a: Summary of gland segmentation overlap (DSC) before and after registration for the final needle confirmation image; b: Gland segmentation overlap (DSC) after registration for the final needle confirmation image comparing two sets of non-expert segmentations with different levels of training.
Fig. 4.
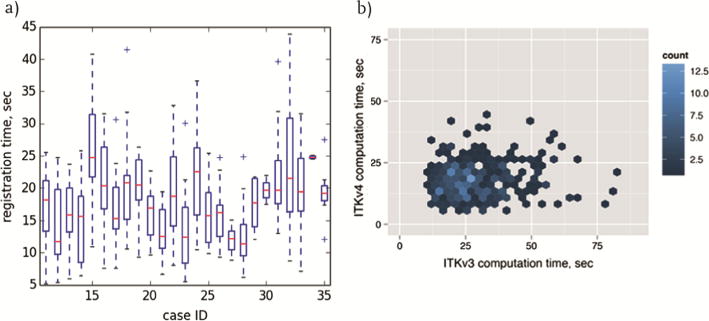
a: Summary of the computational time for registration of all needle confirmation images across all 25 cases using ITKv4 (median, lower and upper quartiles (bottom and top 25 % of the data) and the extreme values within the 1.5 × interquartile range); b: computation time for registration of all needle confirmation images comparing ITKv3 and ITKv4 implementations.
4 Discussion and Conclusions
Our study was motivated by the practical need for a tool to support intra-procedural biopsy workflow and enable motion compensation for cancer suspicious targets tracking, addressing clinical users. We presented an open-source end-user solution, implemented as an extension to the widely used 3D Slicer software, for intra-procedural tracking of the prostate gland and the biopsy targets throughout the procedure. We evaluated the underlying registration approach and demonstrated an improvement of gland alignment in all 25 cases. Our evaluation showed that the registration approach is not sensitive to the differences in initialization due to the variability in segmentation of the prostate gland by different readers. Furthermore, we demonstrated that the use of ITKv4 led to significant reduction in the computation time as compared to the earlier implementation that was based on ITKv3. We note however, that registration results with ITKv4 showed moderate to high irregularities in a subset of cases (approximately 28 of 343 images, e.g., see c15-n9). We could sharply reduce those irregularities by using a dilated version of the propagated mask for deformable registration phase within our ITKv4 implementation.
Evaluation of registration results is always a challenging problem. The two commonly used approaches rely on evaluation of the overlap of the segmented structures, captured by DSC or a similar metric, and on the Landmark Registration Error (LRE). It has been recognized that the use of structure overlap may not characterize the performance of a registration method well [12]. Manual annotation of the images with anatomical landmarks is time-consuming (especially when hundreds of images need to be annotated), and is particularly difficult in the prostate that has limited number of salient points. Neither DSC nor LRE can capture unrealistic or inaccurate deformations within the outlined regions or in the areas with no landmarks. These observations motivated us to develop an online resource that enables visualization of the registration results for each of the 343 registrations.
To illustrate the points above, the results website can be used to observe that c11-n34 shows good alignment in the peripheral zone based on the alignment of the dark spots corresponding to the brachytherapy seeds (specifically, see the last image in the middle row with and without registration). Improvement in DSC of the total gland segmentation is large: from 0.49 to 0.79. However, alignment of the anterior portion of the gland is not perfect, and would be difficult to quantify due to the lack of clear landmark points.
Our study has several limitations. Although design of the software was performed in coordination with the target clinical users, we have not evaluated it prospectively during biopsy procedures. Our evaluation was limited to the intra-procedural motion compensation step. As with any open-source software, the functionality will be refined in the course of its applications in clinical trials.
In conclusion, we presented a fully functional open-source tool, that we believe is ready for prospective evaluation during clinical research MRI-guided prostate biopsy procedures. Further studies evaluating the complete workflow in a prospective setting under the guidance of a clinical operator is warranted. Although the motivating application for this development was prostate biopsy, we aim to investigate other use cases to make the software more generic for other procedures that require intra-procedural motion compensation.
Acknowledgments
This work was supported in part by the National Institutes of Health through grants U24 CA180918, R01 CA111288 and P41 EB015898.
Footnotes
Contributor Information
Peter A. Behringer, Email: peterbehringer@gmx.de.
Andriy Fedorov, Email: andrey.fedorov@gmail.com.
References
- 1.Boyle P, Levin B. World cancer report, 2008. IARC Press, International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Andrén O, Fall K, Franzén L, Andersson SO, Johansson JE, Rubin MA. How well does the Gleason score predict prostate cancer death? a 20-year followup of a population based cohort in Sweden. J Urol. 2006;175:1337–1340. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 3.Delongchamps NB, Peyromaure M, Schull A, Beuvon F, Bouazza N, Flam T, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189:493–499. doi: 10.1016/j.juro.2012.08.195. [DOI] [PubMed] [Google Scholar]
- 4.Penzkofer T, Tuncali K, Fedorov A, Song SE, Tokuda J, Fennessy FM, et al. Transperineal in-bore 3-T MR imaging-guided prostate biopsy: a prospective clinical observational study. Radiology. 2015;274:170–180. doi: 10.1148/radiol.14140221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23:43–50. doi: 10.1097/MOU.0b013e32835ad3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedorov A, Tuncali K, Fennessy FM, Tokuda J, Hata N, Wells WM, et al. Image registration for targeted MRI-guided transperineal prostate biopsy. J Magn Reson Imaging. 2012;36:987–992. doi: 10.1002/jmri.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibanez L, Schroeder W, Ng L, Cates J. The ITK software guide: the insight segmentation and registration toolkit. Kitware Inc. 2003;5 [Google Scholar]
- 9.Hambrock T, Fu JJ, Fütterer JJ, Huisman HJ, Hulsbergen-vandeKaa C, van Basten JP, et al. Thirty-two-channel coil 3T magnetic resonance-guided biopsies of prostate tumor suspicious regions identified on multimodality 3T magnetic resonance imaging: technique and feasibility. Invest Radiol. 2008;43:686–694. doi: 10.1097/RLI.0b013e31817d0506. [DOI] [PubMed] [Google Scholar]
- 10.Franiel T, Stephan C, Erbersdobler A, Dietz E, Maxeiner A, Hell N, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding-multiparametric MR imaging for detection and biopsy planning. Radiol Radiol Soc N Am. 2011;259:162–172. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 11.Fedorov A, Tuncali K, Penzkofer T, Tokuda J, Song SE, Hata N, et al. Quantification of intra-procedural gland motion during transperineal MRI-guided prostate biopsy. Proceeding of ISMRM 2013. 2013 [Google Scholar]
- 12.Rohlfing T. Image similarity and tissue overlaps as surrogates for image registration accuracy: widely used but unreliable. IEEE Trans Med Imaging. 2012;31:153–163. doi: 10.1109/TMI.2011.2163944. [DOI] [PMC free article] [PubMed] [Google Scholar]


