Abstract
Objective
Conflict in early life family environments is known to affect psychosocial functioning and coping styles into adulthood and is reported to negatively affect access to psychosocial resources that are critical to the management of stress. However, it remains unknown whether early life family conflict similarly affects subclinical cardiovascular disease (CVD) in adulthood. We predicted that family conflict in early life would be associated with greater mean Intima-Media thickness (IMT), a subclinical marker of CVD risk, in adulthood.
Methods
Data were collected in a community sample of 503 adults (47.4 % male, mean age: 42.8 years [SD=7.3]). Associations between family conflict in early life with IMT (assessed using B-mode ultrasound) in adulthood were examined using regression analysis. We also tested for indirect effects of early life family conflict on mean IMT through ecological momentary assessment (EMA) reports of social interactions, diversity of social roles, and perceived social support.
Results
Linear regression analyses adjusted for demographics and physiological risk factors showed conflict in early life associated with greater mean IMT (β=0.08, t(447)=2.13, p=0.034, R2=0.46). Early life conflict was significantly related to diversity of social roles, perceived social support, and EMA reports of pleasant and social conflict interactions. Significant indirect effects of early life conflict on mean IMT were observed through fewer pleasant social interactions and more frequent social conflict interactions in adulthood (β = 0.001, 95% CI, 0.0001–0.0014 and β=0.001, 95% CI, 0.0002–0.0015, respectively).
Conclusions
These findings provide initial evidence that family conflict in early life heightens CVD risk in adulthood, in part by shaping the quality of adulthood social interactions.
Keywords: early life family conflict, social interactions, ecological momentary assessment, intima-media thickness, cardiovascular disease
Introduction
A growing body of research documents a relationship between our early life experiences and physical health in adulthood (1–4). More specifically, evidence suggests that early life family environments characterized by high levels of conflict account for variability in cardiovascular risk factors in adulthood (5–6). How might conflict in early life family environments exert enduring effects on one’s risk for cardiovascular disease (CVD) across the lifespan?
Anger, aggression, and conflict in the childhood familial environment associate with behavioral and emotional problems in children, including aggression and antisocial behavior (7–8). Additionally, adolescents raised in these environments are less accurate in their reading of the emotions of others (9), have stronger emotional reactions to conflict (10), and favor coping strategies that are focused on reducing tension and escaping the situation at hand (11).
In addition to its impact on childhood behavior, evidence suggests that early life family conflict negatively affects adult psychosocial functioning (6), associating with more negative emotions in adulthood (5) and poor coping styles, including a tendency to overreact to unavoidable stressors (12). Importantly, one’s early environment also affects the development of psychosocial resources that are critical to managing stress (e.g., social support). Consequently, individuals raised in environments characterized by conflict are likely to have fewer psychosocial resources available to them, and as such, may not only experience stress more often, but may experience a more pronounced physiological effect when stressors are encountered (13–14).
A recent longitudinal study provides evidence that early family environments characterized by conflict predict higher rates of hostility and anger over a period of 27 years (15). Collectively, anger, aggression and hostility are the most studied psychosocial risk factors for CVD, and the majority of research supports this association (16–19). This documented relationship could play an important role in the association between early life conflict and increased risk of heart disease in later life.
The majority of the extant research on early life stress and CVD risk examines endpoints of cardiovascular disease without focus on biological precursors of disease risk. Subclinical markers of CVD risk allow for investigation of how the quality of early life environments correlate with future risk of CVD in healthy samples, with the advantage of identifying emerging risk before the occurrence of disease. Carotid artery Intima-Medial thickness (IMT) can be measured by high-resolution B-mode ultrasonography and is considered a marker of early atherosclerosis and a predictor of future CVD. (20–21). Specifically, greater IMT relates to unfavorable levels of cardiovascular risk factors (22), is associated with atherosclerosis at other arterial sites (22), and shows a consistent, graded association with risk of future cardiovascular disease (23). Preclinical changes in arterial morphology occur over time and begin to emerge long before obstructive atherosclerotic plaques that contribute to late-stage CVD outcomes (20).
A recent investigation found that women who were sexually abused in early life exhibited higher mean IMT in midlife than those without a history of sexual abuse (24). While this finding highlights the importance of examining the role of early life stressors in shaping subclinical risk for CVD in adulthood, to date no research has examined whether a family dynamic characterized by high levels of conflict similarly affects subclinical risk for CVD in adulthood.
Here, in a sample of mid-life healthy adults, we examine whether high levels of conflict in early life environments associates with greater carotid IMT as a marker of risk for future CVD. Further, we examine relationships between conflict in early life family environments and social relationship characteristics in adulthood measured using both standard assessments and Ecological Momentary Assessment (EMA) reports (25). We hypothesized that reports of high levels of conflict in early life family environments would associate with greater mean IMT, greater EMA reports of social conflict in adulthood, lower EMA reports of pleasant social interactions, less diverse social roles and lower perceived social support. Importantly, we examined whether these relationships were independent of previously documented biological risk factors, hostility and neuroticism. Lastly, we examined whether the relationship between early life conflict and mean IMT was mediated by social interactions, social role diversity, or total perceived social support as assessed in adulthood.
Methods
503 participants were drawn from the Adult Health and Behavior Project- Phase 2 (AHAB-II), a study of psychosocial factors, behavioral and biological risk factors, and subclinical CVD. AHAB-II was approved by the University of Pittsburgh Institutional Review Board.
In order to be eligible for participation in AHAB-II, individuals had to be between 30 and 54 years of age and working outside the home for at least 25 hours per week. Individuals were not eligible for participation if they a) had a history of CVD, schizophrenia or bipolar disorder, chronic hepatitis, renal failure, neurological disorder, lung disease requiring drug treatment, or stage 2 hypertension, b) excessively consumed alcohol (≥ 5 portions 3–4 times per week); c) used fish oil supplements (in line with requirements for a sub-study not described here), were prescribed medications with autonomic effects, or used insulin, glucocorticoid, antiarrhythmic, anti-hypertensive, lipid-lowering, psychotropic, or prescription weight-loss medication, e) were pregnant, f) had less than eighth grade reading skills, or g) were shift workers. Participants were recruited between March 2008 and October 2011 through mass mailings of recruitment letters to individuals randomly selected from voter registration and other public domain lists. Participants signed informed consent upon enrollment and were compensated up to $410, depending on the extent of their participation and their compliance with the study protocol.
Procedure
AHAB-II consisted of seven visits, some of which are not relevant to this investigation. Demographic variables were collected at visit 1 along with a fasting blood draw to assess blood levels of physiologic risk factors (e.g. glucose, HDL, LDL). Clinic blood pressure was collected at visits 2 and 3. A carotid artery ultrasound was completed at visit 4.
EMA measures were completed over a 4-day monitoring period (3 working days and 1 non-working day), as described previously (26). The monitoring protocol consisted of two 2-day monitoring periods, with at least one non-monitoring day in between. During monitoring days participants carried a PDA (Palm Z22, software: Satellite forms). They also wore other ambulatory devices that are not relevant to this report. During waking hours on monitoring days, participants completed an hourly 43-item EMA questionnaire on the PDA, many of which have been used in previous investigations (26–27). Participants completed extensive training before entering the field for ambulatory monitoring. In addition, they received feedback on compliance after completing one practice day. During monitoring, participants received four scheduled telephone calls from study staff, and staff was always available by phone for support or assistance. By obtaining reports of daily life environments in real time as they occur, EMA offers an important advantage, as the measurement is likely more proximal to fluctuating biological and behavioral mechanisms that may promote disease risk (28). Furthermore, EMA avoids potential recall biases associated with retrospective report (29).
Measures
Early life Conflict
Degree of conflict was indexed by the conflict subscale of the widely used Family Environment Scale (FES; 30), as modified by Plomin, McLearn, Pederson, Nesselroade, and Bergeman (31) to assess self-reported childhood rearing environment among adults (Range= 5–25, M= 13.95 SD=4.16; α=0.82). The scale was modified by converting the response format from true-false to 5-point Likert scales (anchored by end ratings of strongly disagree and strongly agree). In addition, the subscale was shortened from the original nine items to the five items with the strongest factor loadings for the scale (31). The conflict subscale consists of five items referencing competition, criticism, expressed anger, and fighting within the family. Each item in the subscale begins with the phrase, “When you were growing up”. As such this measure reflects an overall subjective impression of how much conflict was present across childhood. Prior research demonstrates that high levels of early life family conflict (as measured by this subscale) relate to childhood trauma (e.g., abuse and neglect) and to psychological problems (32–33).
Carotid IMT
B-mode ultrasound assessments were conducted at the University of Pittsburgh Ultrasound Research Laboratory using an Acuson Sonoline Antares high resolution duplex scanner (Acuson-Siemens, Malvern, PA). Trained, certified sonographers identified the borders of the intima and medial layers of the left and right carotid artery, using the intima-lumen interface and the media-adventitial interface as markers. B-mode images were obtained from the following four locations of the left and right carotid arteries: the near and far walls of the distal common carotid artery, 1 cm proximal to the carotid bulb; the far walls of the carotid bulb, from the point where the near and far walls are no longer parallel and extending down to the flow divider; and the internal carotid artery from the flow divider to 1 cm distal from the flow divider. Studies were read using an automated edge detection algorithm (Artery Measurement System, Goteborg University, Gothenburg, Sweden). (34). The software generates two lines: one that goes along the lumen-intima interface and one that goes along the media-adventitia interface. The distances between the interfaces are measured in 1 cm increments, generating one measurement (in mm) per pixel in each region. Mean IMT was computed as the average of all intima-media distance values in mm across both carotid arteries. The intraclass correlation coefficient between readers was calculated each year of the study and ranged from 0.89 to 0.97. Two extreme outliers (>4 standard deviations (SDs) above the mean) were eliminated from our analyses. Both before and after elimination of the outliers, the scores were not normally distributed. To reduce skewness, we applied log transformation to IMT scores.
EMA Measure of Social Interactions
EMA measures of pleasant social interactions and interactions marked by social conflict were created using 4 items from the hourly electronic questionnaire. During each assessment, participants were administered several items inquiring about their most recent social interaction. From each interview, scores were extracted for the current or most recent social interaction. Only interactions that occurred within the 10 minutes prior to the EMA report were utilized in our analyses.
Interaction quality was assessed using four Likert-scale items. Item responses (NO! No no yes, Yes YES!) were converted to a 1–6 point rating scale. Two items assessed positive aspects of interactions (i.e. “agreeable interaction?” and “pleasant interaction?”) and two items assessed conflict in interactions (i.e. “someone in conflict with you?” and “someone treated you badly?”) (see 26). Although the positive and negative items were inversely correlated, confirmatory factor analysis revealed that they are best treated as indicators of two distinct constructs, utilizing a two-factor model. The comparative fit index (CFI) for the one-factor model was 0.80, while the CFI for the two-factor model was 0.99, reflecting a better fit of the two-factor model to the data. The AIC for the two-factor model was 92.60 and the RMSEA was 0.06, again reflecting an excellent fit to the data in this research.
Social Role Diversity
The social network index assesses participation in 12 types of social roles (35). These relationships include those with a spouse, parents, parents-in-law, children, other close family members, close neighbors, friends, colleagues at work, schoolmates, fellow volunteers, members of groups without religious affiliations, and members of religious groups. For the measure of social role diversity, one point is designated for each type of role (12 possible points) in which there is interaction (in person or on the phone) at least once every 2 weeks. Social role diversity has previously been related to susceptibility to the common cold (35) and risk of ischemic heart disease (36). As a measure of social network size, we also tallied the total number of persons listed as interaction partners in the measured roles.
Perceived Social Support
The interpersonal support evaluation list-12 (ISEL-12) (37) was used to measure perceptions about the support available to the participant. The ISEL-12 is a shorter version of the 40-item Interpersonal Support Evaluation List and consists of three subscales (appraisal, belonging and tangible), assessing the perceived availability of three types of social support functions. Respondents indicate how accurately each item describes them on a 4-point scale ranging from 1 (“definitely false”) to 4 (“definitely true”). (M=30.15, SD=4.81) and scores are summed across items within each subscale. For this report, global scores on the ISEL-12 were employed (summed across these 3 subscales).
Demographics
Participants self-reported their age, race/ethnicity, sex, highest level of education, and parental education as an indicator of early life socioeconomic status.
Neuroticism and Hostility
Neuroticism and hostility were measured and considered as covariates that may affect retrospective report of early life experiences. These factors were also measured given previous documentation of their association with CVD risk (e.g., 17–19, 38) and given their associations with the frequency and intensity of social interactions (39).
We assessed neuroticism using the NEO Personality Inventory-Revised (40–41). The NEO Personality Inventory-Revised is a 240-item self-report questionnaire that measures each of the “big five” personality dimensions, including neuroticism. Sum scores for the neuroticism scale were computed, comprised of 48 items measuring anxiety, angry hostility, depression, self-consciousness, impulsiveness, and vulnerability to stress (range=24–147, M=74.97, SD=22.31, α=0.92). It is believed that this subscale captures a common construct of negative affect. Internal consistency of the neuroticism subscale is high, with reliability coefficients ranging from 0.89 to 0.95 in non-psychiatric populations (39). Further, the neuroticism scale has demonstrated validity based on its relation with similar constructs and its convergence with peer reports (39).
We used the Cook-Medley Hostility Inventory (42) as a measure of the cognitive components of hostility. A considerable body of research supports a positive association between hostility and cardiovascular disease (e.g., 17–19). The inventory consists of 50 true-false items derived empirically from the Minnesota Multiphasic Personality inventory (MMPI). Global hostility scores were calculated from responses to these items and ranged from 1–41 (M= 16.90, SD=7.92, α=0.86).
Physiological Risk Factors
Clinic blood pressure was assessed at visits 2 and 3 by trained research assistants using a manual Baumanometer mercury sphygmomanometer (desk model; W.A. Baum Co Inc, Copiague, NY) and a standard protocol. At each of the two visits, two readings were taken. Clinic systolic blood pressures for each visit were calculated as the average of the two readings, and the mean systolic blood pressure across the two visits was calculated. A standard lipid panel (total cholesterol, high-density lipoprotein, low-density lipoprotein, and fasting serum glucose) was assessed. Fasting serum glucose was assayed via standard calorimetry, and participants’ waists were measured in inches. Finally, smoking status was considered (0=past smoker/non-smoker, 1= current smoker). These measures are standard covariates in investigations examining carotid IMT, given their documented relationship with IMT (26, 43–44).
Statistical Analyses
Analyses were conducted using SPSS. Multiple linear regressions were used to conduct most analyses. Continuous covariates were centered before being used in analyses. Race/ethnicity was recoded into a dummy variable representing Non-Hispanic White (1 vs. 0). Education was recoded into 4 categories (1=High school diploma or lower, 2=some college, 3= bachelors degree, 4= graduate degree or professional degree). Using the same categorization described above, we measured parental education as an indicator of childhood socioeconomic status.
We tested our primary hypothesis across two regression models predicting mean IMT. In the first model, we entered the early life conflict measure along with age, sex, race, education, and physiological risk factors. In our second model, we included psychosocial covariates and early life socioeconomic status (i.e. parental education) to see if these factors accounted for any significant relationship between early life conflict and mean IMT. Finally, we explored potential indirect effects of early life conflict on mean IMT. To test for indirect effects, following suggestions by Preacher & Hayes (45), we used a bootstrapping approach in which a point estimate of the indirect effect was derived from the mean of the 5000 estimates of the indirect pathways, and 95% percentile based confidence intervals were computed using the cut-offs for the 2.5% highest and lowest scores of the distribution. Indirect effects were considered significant when the confidence interval did not include zero. For these analyses, demographics, physiological risk factors, hostility, and neuroticism were included as covariates.
Results
Sample Characteristics
Participants were middle-aged (M=42.76, SD=7.34), predominantly Non-Hispanic White (82% Non-Hispanic White, 15.8% African American, 1.4% Asian, 0.4% multi-racial, and 0.4% other) and well-educated (70% had at least a Bachelor’s degree). Descriptive statistics of the study sample are summarized in Table 1.
Table 1.
Select Characteristics of the Analytic Sample.
| Characteristic | |
|---|---|
| Age y, M (SD) | 42.76 (7.34) |
| Male sex, % (n) | 47.4 (234) |
| Race, % (n): | |
| Non-Hispanic White | 80.5 (405) |
| African-American | 15.5 (78) |
| Asian | 1.4 (7) |
| Multi-Racial | 0.4 (2) |
| Other | 0.4 (2) |
| Education, % (n): | |
| High school diploma or lower | 9.9 (50) |
| Some college | 18.1 (91) |
| Bachelor’s degree | 37.2 (187) |
| Graduate or professional degree | 33 (166) |
| Early life conflict | 13.94 (4.16) |
Early life family conflict was not significantly related to age, sex, race, education level, or physiological risk factors. We ran partial correlations between early life conflict and variables of interest, adjusting for age, sex, race, and education. Partial correlations are listed in Table 2.
Table 2.
Correlations between Early life Family Conflict and Variables of interest (controlling for age, sex, race, education).
| Early life Family Conflict | |
|---|---|
| Parental Education | −0.11* |
| Cook-Medley Hostility | 0.25** |
| Neuroticism | 0.30** |
| Social Network Diversity | −0.11* |
| Perceived Social Support | −0.23** |
| Social conflict interactions | 0.13** |
| Systolic Blood Pressure | −0.03 |
| Waist Circumference | −0.04 |
| HDL | 0.03 |
| LDL | −0.003 |
| Cholesterol | 0.01 |
| Fasting glucose | −0.01 |
| Smoking Status (0=non-smoker, 1=smoker) | 0.04 |
Early Life Family Conflict and IMT
Demographics and physiological risk factors accounted for 45% of the variance in mean IMT. Addition of early life family conflict to the model showed that individuals with higher levels of early life family conflict had higher mean IMT, (β=0.08, t(447)=2.13, p=0.034, R2=0.46, R2 change= 0.01). This association remained after adjustment for hostility, neuroticism and early life socioeconomic status (β=0.09, t(416)=2.26, p=0.024, R2=0.46).
Social Interactions and IMT
Role of Pleasant Social Interactions
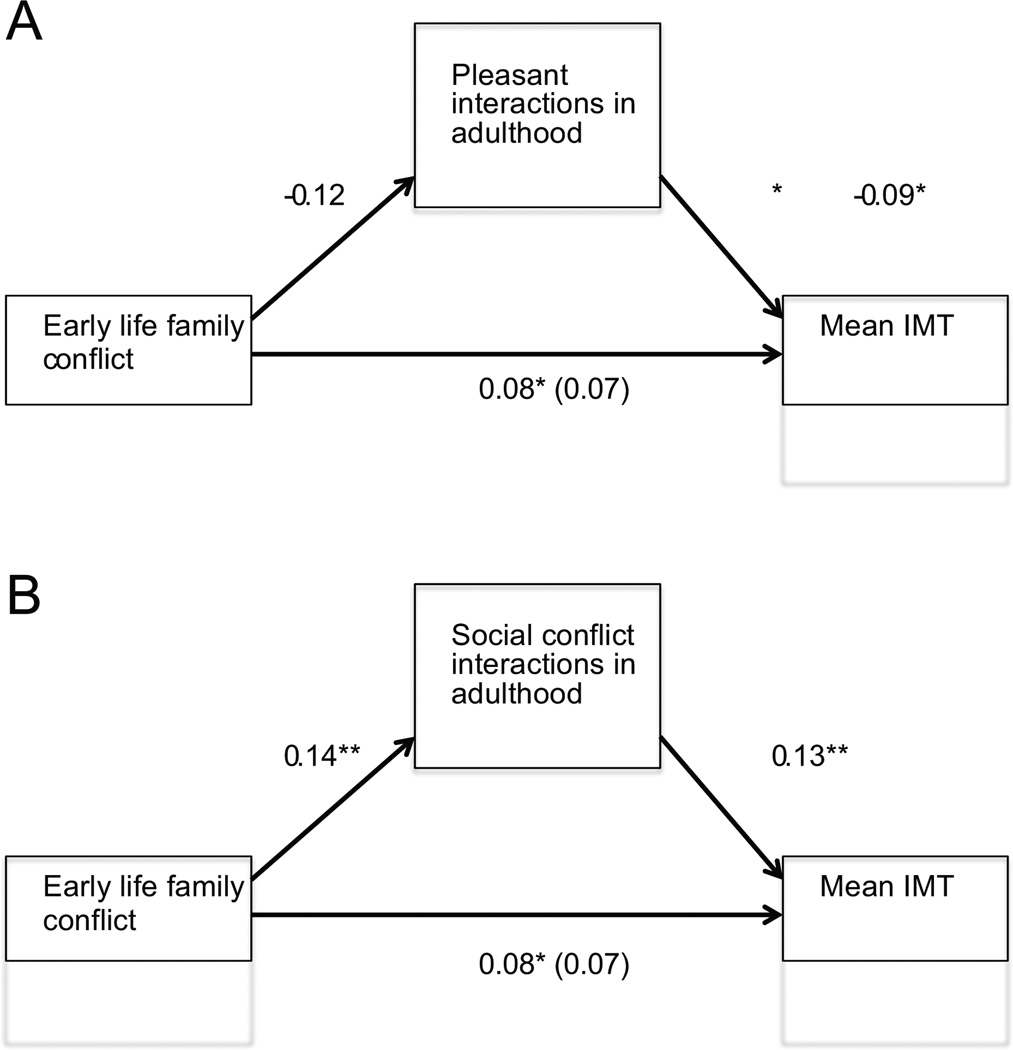
We examined whether early life family conflict predicted EMA reports of pleasant social interactions. Individuals who reported more conflict in their early life family environments experienced significantly fewer pleasant social interactions (β=−0.16, t(462)= −3.57, p<0.001), adjusting for neuroticism and hostility. Using the previously described method (47), we found a significant indirect effect of early life conflict on mean IMT through pleasant social interactions. As figure 1a illustrates, the standardized regression coefficient between early life conflict and pleasant social interactions in adulthood and the standardized regression coefficient between pleasant social interactions in adulthood and mean IMT were inverse and statistically significant. The standardized indirect effect of early life family conflict on mean IMT was also significant (95% CI [.0001, .0014]). The bootstrapped unstandardized indirect effect and confidence interval are reported in Table 3. This analyses controlled for demographics, physiological risk factors, early life socioeconomic status, neuroticism and hostility.
Figure 1.
Standardized regression coefficients for direct and indirect effects of early life family conflict on mean IMT in adulthood. Figure 1a displays model for pleasant interactions, and Figure 1b displays model for social conflict interactions. The standardized regression coefficients for the direct effect between early life conflict and mean IMT is in parentheses.
Table 3.
Indirect effects of early life family conflict on mean IMT through social pleasant social interactions, social conflict interactions, social network diversity, and total perceived social support, controlling for age, sex, race, physiological risk factors, and psychosocial covariates using Preacher & Hayes method (2008).
| Mediators | Indirect Effects | Bootstrapping Percentile 95% |
||
|---|---|---|---|---|
| Coefficient | SE | Lower | Upper | |
| Pleasant interactions | 0.001* | 0.0003 | 0.0001 | 0.0014 |
| Social conflict Interactions | 0.001* | 0.0003 | 0.0002 | 0.0015 |
| Social role diversity | 0.0003 | 0.0002 | 0.0000 | 0.0010 |
| Perceived social support | 0.0003 | 0.0004 | −0.0004 | 0.0012 |
p<0.05
Role of Social Conflict Interactions
Next, we tested whether early life conflict predicted EMA reports of social interactions marked by conflict. Individuals who reported more conflict in their early life environment experienced significantly more social conflict interactions as adults than those from low conflict family environments (β=0.14, t(466)=3.00, p=0.003), once again adjusting for neuroticism and hostility. Furthermore, similar to our findings for pleasant social interactions, we found a significant indirect effect of early life conflict on mean IMT through social conflict interactions in adulthood. As figure 1b illustrates, the standardized regression coefficient between early life conflict and social conflict interactions in adulthood and the standardized regression coefficient between social conflict interactions in adulthood and mean IMT were statistically significant. The standardized indirect effect of early life family conflict on mean IMT was also significant (95% CI [0.0002, 0.0015]). The bootstrapped unstandardized indirect effect and confidence interval are reported in Table 3. Once again, this analyses controlled for demographics, physiological risk factors, early life socioeconomic status, neuroticism and hostility.
Early Life Conflict and Psychosocial Resources
Social Role Diversity
As predicted, individuals who reported more early life family conflict reported having less diversity in their social roles in adulthood, β=−0.11, t(466)= −2.36, p=0.020. However, the indirect pathway was not significant (see Table 3).
Perceived Social Support
In a similar manner, early life family conflict predicted lower perceived social support in adulthood β=−0.23, t(466)= −5.06, p<0.001. Similar to social role diversity, the indirect pathway was not significant (see Table 3).
Discussion
Past work has demonstrated that conflict in early life family environments associates with disruptions in physiological regulatory systems (e.g., the hypothalamic-pituitary-adrenocortical axis and the sympathetic-adrenal-medullary axis) and health-threatening behaviors such as smoking and alcohol and drug abuse (6). This is the first investigation to report that early life family conflict associates with higher IMT, independent of physiological risk factors, early life socioeconomic status and potential psychological confounders. These findings are in line with a growing body of research highlighting the importance of our early life experiences in shaping risk for disease across the life-span.
Furthermore, while our findings showed that reports of early family conflict was associated with more conflictual social interactions and fewer pleasant social interactions, less diverse social roles, and less perceived social support in adulthood, only social interactions (both pleasant and social conflict interactions) were associated with significant indirect effects of early life family conflict on mean IMT. This suggests that early life family conflict could plausibly exert its effects on this outcome, in part, by shaping the nature of social interactions in adulthood.
A notable strength of this research is the use of IMT as a measure of subclinical CVD risk. Carotid artery IMT is a widely used and well-validated subclinical CVD measure that predicts future CVD events even among low-risk populations (46). Examination of these relationships once disease is evident gives rise to the possibility that the occurrence of symptomatic disease may shape retrospective reports of early life experiences. As such, use of IMT offers an advantage as it allows for indexing preclinical vascular disease.
We established that these relationships were independent of early life socioeconomic status by including parental education in our regression models. Additionally, we considered several physiological risk factors that are related to IMT, allowing us to test for independent associations between early life conflict and IMT and to explain variance not otherwise accounted for. Separately, our use of EMA reports of social interactions allowed us to capture social interactions in real-time, avoiding retrospective recall bias.
Relatedly, an important limitation of this research is the retrospective measure of early life family conflict, which could be affected by personality and psychological factors. To address this limitation, we included neuroticism and hostility in our complete regression models, with the assumption that these measures would effectively capture effects of affective bias on reports of early life conflict, and the relationships remained significant. Given the cross-sectional design of this investigation, causality cannot be inferred. To address this limitation, longitudinal work should be conducted, beginning in childhood and extending into adulthood. Such a study would more thoroughly address the impact of reverse causality and allow for a clearer picture of how these effects may unfold over time. Finally, given that our sample included primarily non-Hispanic white, well-educated individuals, the generalizability of our findings to other populations is unknown.
The magnitude of the observed relationship between early life family conflict and mean IMT in adulthood is small, accounting for about 1% of variance in IMT in this sample left unaccounted for by demographic covariates and other CVD risk factors). The clinical significance of this effect is difficult to quantify with certainty. However, a recent meta-analysis concluded that a 0.1 mm increase in carotid IMT associated with a 22% increased risk for future myocardial infarction or stroke (47). Based on this estimate, individuals in the top quartile of early life family conflict in our sample would have a 2.2% increased risk of future myocardial infarction or stroke, compared to individuals in the lowest quartile of early life family conflict.
In addition, ultrasound measurement of IMT cannot differentiate the intima layer of the artery from the media layer. As such, it is important to note that there are other factors, such as hypertension, (48) that could contribute to hypertrophy of the media layer of the artery, and thus could affect IMT measurement. Lastly, we are unable to account for the contribution of family history of cardiovascular disease to the observed relationships in this research.
The biological embedding of childhood adversity model (2,49) proposes two central pathways through which early environments may affect future disease risk. Physiologically, it proposes that when stress occurs during sensitive periods of development it shapes function and response tendencies of stress-regulatory physiological systems (50–51). Behaviorally, early life stress is thought to encourage heightened vigilance for threat and an increased mistrust of others. Not surprisingly, these tendencies shape encounters in the social world, fostering a proclivity for eliciting conflict and making these individuals less likely to have access to social support (2). While our findings in this report provide support for the possibility that social interactions in adulthood may play a role in these associations, our findings cannot speak to whether the relationship observed here between early life family conflict and IMT is in part accounted for by early life programming of physiological systems.
Overall, these findings add to a growing literature highlighting the enduring effects of early life experiences on health and risk for disease across the life span (e.g., 1–5, 52). More specifically, we extend prior work showing that sexual abuse in childhood predicts greater IMT in healthy middle-aged women (24), in showing that conflict in early life environments similarly associate with greater IMT. Furthermore, these findings suggest that in addition to factors such as socioeconomic status, abuse, or adversity in early life, the social dynamics of early life environments are important in our study of the effects of early life experiences on subclinical CVD risk in adulthood.
Acknowledgments
source of funding: This work was supported by National Institutes of Health grant P01 HL040962 (SBM)
Abbreviations
- IMT
intima-media thickness
- EMA
ecological momentary assessment
- CVD
cardiovascular disease
- SES
socioeconomic status
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 2.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving towards a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav Immun. 2011;25:1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shonkoff JP, Boyce T, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 5.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychol. 2009;28:338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 7.Ingoldsby EM, Shaw DS, Winslow E, Schonberg M, Gilliom M, Criss MM. Neighborhood disadvantage, parent-child conflict, neighborhood peer relationships, and early antisocial behavior problem trajectories. J Abnorm Child Psychol. 2006;34:303–319. doi: 10.1007/s10802-006-9026-y. [DOI] [PubMed] [Google Scholar]
- 8.Grych J, Fincham FD. Marital conflict and children’s adjustment: A cognitive-contextual framework. Psychol Bull. 108:267–290. doi: 10.1037/0033-2909.108.2.267. [DOI] [PubMed] [Google Scholar]
- 9.Dunn J, Brown J, Slomkowski C, Tesla C, Youngblade L. Young children’s understanding of other people’s feelings and beliefs: individual differences and their antecedents. Child Dev. 1991;62:1352–1366. [PubMed] [Google Scholar]
- 10.Davies PT, Cummings EM. Exploring children’s emotional security as a mediator of the link between marital relations and child adjustment. Child Dev. 1998;69:124–139. [PubMed] [Google Scholar]
- 11.Valentiner DP, Holahan CJ, Moos RH. Social support, appraisals of event controllability and coping: An integrative model. J Pers Soc Psychol. 1994;66:1094–1102. [Google Scholar]
- 12.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol responses by psychosocial resources. J Pers Soc Psychol. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin KA, Kubzansky LD, Dunn EC, Waldinger R, Vaillant G, Koenen KC. Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depress anxiety. 2010;27:1087–1094. doi: 10.1002/da.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakulinen C, Jokela M, Hinstsanen M, Pulkki-Raback L, Hintsa T, Merjonen P, Josefsson K, Kahonen M, Raitakari OT, Keltikanga-Jarvinen L. Childhood family factors predict developmental trajectories of hostility and anger: a prospective study from childhood into middle adulthood. Psychol Med. 2013;43:2417–2426. doi: 10.1017/S0033291713000056. [DOI] [PubMed] [Google Scholar]
- 16.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 17.Smith TW, Ruiz JM. Psychosocial influences on the development and course of coronary heart disease: Current status and implications for research and practice. J Consult Clin Psychol. 2002;7:548–568. doi: 10.1037//0022-006x.70.3.548. [DOI] [PubMed] [Google Scholar]
- 18.Krantz DS, McCeney MK. Effects of psychological and social factors on organic disease: A critical assessment of research on coronary heart disease. Annu Rev Psychol. 2002;53:341–369. doi: 10.1146/annurev.psych.53.100901.135208. [DOI] [PubMed] [Google Scholar]
- 19.Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med. 1999;61:476–487. doi: 10.1097/00006842-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Bots ML, Sutton-Tyrrell K. Lessons from the past and promises for the future for carotid intima-media thickness. J Am Coll Cardiol. 2012;60:1600–1604. doi: 10.1016/j.jacc.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 21.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eikjemans MJ, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML. Common carotid intima-media thickness measurements in cardiovascular risk prediction. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 22.Peters SA, Grobbee DE, Bots ML. Carotid intima-media thickness: a suitable alternative for cardiovascular risk as outcome? Eur J Cardiovasc Prev Rehabil. 2011;18:167–74. doi: 10.1177/1741826710389400. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 24.Thurston RC, Chang Y, Derby CA, Bromberger JT, Harlow SD, Janssen I, Matthews KA. Abuse and subclinical cardiovascular disease among midlife women. Stroke. 2014;45:2246–2251. doi: 10.1161/STROKEAHA.114.005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Ann Behav Med. 1994;16:199–202. [Google Scholar]
- 26.Joseph NT, Kamarck TW, Muldoon MF, Manuck SB. Daily marital interaction quality and Carotid Artery Intima-Medial thickness in healthy middle-aged adults. Psychosom Med. 2014;76:347–354. doi: 10.1097/PSY.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connor TS, Barrett LF. Trends in ambulatory self-report: the role of momentary experience in psychosomatic medicine. Psychosom Med. 2012;64:327–337. doi: 10.1097/PSY.0b013e3182546f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 29.Gump BB, Polk DE, Kamarck TW, Shiffman SM. Partner interactions are associated with reduced blood pressure in the natural environment: ambulatory monitoring evidence from a healthy, multiethnic adult sample. Psychosom Med. 2001;63:423–433. doi: 10.1097/00006842-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Moos RJ, Moos BS. Family environment Scale. Palo Alto, CA: Consulting Psychologists’ Press; 1981. [Google Scholar]
- 31.Plomin R, McClearn GE, Pederson NL, Nesselroade JR, Bergeman CS. Genetic influence on childhood family environment perceived retrospectively from the last half of the life span. Dev Psychol. 1988;24:738–745. [Google Scholar]
- 32.Matherne M, Thomas A. Family environment as a predictor of adolescent delinquency. Adolescence. 2001;36:655–665. [PubMed] [Google Scholar]
- 33.Hastings T, Kern JM. Relationships between bulimia, child sexual abuse and and family environment. Int J Eat Disorder. 1994;15:103–111. doi: 10.1002/1098-108x(199403)15:2<103::aid-eat2260150202>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Wendelhag I, Liang Q, Gustavsson T, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke. 1997;28:2195Y200. doi: 10.1161/01.str.28.11.2195. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- 36.Barefoot JC, Gronbaek M, Jensen G, Schnohr P, Prescott E. Social network diversity and risks of ischemic heart disease and total mortality: Findings from the Copenhagen city heart study. Am J Epidemiol. 2005;161:960–967. doi: 10.1093/aje/kwi128. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Memelstein R, Kamarck T, Hoberman H. Measuring the functional components of social support. In: Sarason IG, Sarason B, editors. Social Support: Theory, research and application. The Hague: Martinus Nijhoff; pp. 73–94. [Google Scholar]
- 38.Hagger-Johnson G, Roberts B, Boniface D, Sabia S, Batty GD, Elbaz A, Singh-Manoux A, Deary IJ. Neuroticism and cardiovascular disease mortality: socioeconomic status modifies the risk in women (UK health and lifestyle survey) Psychosom Med. 2012;74:596–603. doi: 10.1097/PSY.0b013e31825c85ca. [DOI] [PubMed] [Google Scholar]
- 39.Brandolo E, Rieppi R, Erickson SA, Bagiella E, Shapiro PA, McKinley P, Sloan RP. Hostility, Interpersonal interactions and ambulatory blood pressure. Psychosom Med. 2003;65:1003–1011. doi: 10.1097/01.psy.0000097329.53585.a1. [DOI] [PubMed] [Google Scholar]
- 40.Costa PT, McCrae RR. NEO PI-R. Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc.; [Google Scholar]
- 41.McCrae RR, John OP. An introduction to the five-factor model and its applications. J Pers. 1992;60:175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 42.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J App Psychol. 1954;38:414–418. [Google Scholar]
- 43.Whipple MO, Lewis TT, Sutton-Tyrrell K, Matthews KA, Barinas-Mitchell E, Powell LH, Everson-Rose SA. Hopelessness, depressive symptoms, and carotid atherosclerosis in women: The Study of Women’s Health Across the Nation (SWAN) Heart Study. Stroke. 2009;40:3166–3172. doi: 10.1161/STROKEAHA.109.554519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janicki-Deverts D, Cohen S, Matthews KA, Jacobs DR, Adler NE. Occupational mobility and carotid intima-media thickness: findings from the Coronary Artery Risk Development In Young Adults (CARDIA) study. Psychosom Med. 2011;73:795–802. doi: 10.1097/PSY.0b013e3182365539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preacher KJ, Hayes AF. Asymptomatic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 46.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. American society of echocardiography carotid intima-media thickness task force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine, J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 47.van den Oord S, Sijbrands EJG, ten Kate GL, van Klaveren D, van Domburg RT, van der Steen AFW, Schinkel AFL. Carotid intima-media thickness for cardiovascular risk assessment: Systematic review and meta-analysis. Atherosclerosis. 2013;228:1–11. doi: 10.1016/j.atherosclerosis.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka H, Dinenno FA, Monahan KD, DeSouza CA, Seals DR. Carotid artery wall hypertrophy with age is related to local systolic blood pressure in healthy men. Arterioscler Thromb Vasc Biol. 2001;21:82–87. doi: 10.1161/01.atv.21.1.82. [DOI] [PubMed] [Google Scholar]
- 49.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann NY Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 50.Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci. 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- 51.Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci Biobehav Rev. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66(4):553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]