Abstract
Aim
Impairment in neuropsychological functioning is common in major depressive disorder (MDD), but it is not clear to what degree these deficits are related to risk (e.g., trait), scar, burden, or state effects of MDD. The objective of this study was to use neuropsychological measures, with factor scores in verbal fluency, processing speed, attention, set-shifting, and cognitive control in a unique population of young, remitted, un-medicated, early course individuals with a history of MDD in hopes of identifying putative trait markers of MDD.
Methods
Youth aged 18-23 in remission from MDD (rMDD; n = 62) and healthy controls (HC; n = 43) were assessed with neuropsychological tests at two time points. These were from four domains of executive functioning, consistent with previous literature as impaired in MDD; verbal fluency and processing speed, conceptual reasoning and set-shifting, processing speed with interference resolution, and cognitive control.
Results
rMDD youth performed comparably to healthy controls on verbal fluency and processing speed, processing speed with interference resolution, and conceptual reasoning and set-shifting, reliably over time. Individuals with rMDD demonstrated relative decrements in cognitive control at Time 1, with greater stability than HC participants.
Conclusion
MDD may be characterized by regulatory difficulties that do not pertain specifically to active mood state or fluctuations in symptoms. Deficient cognitive control may represent a trait vulnerability or early course scar of MDD that may prove a viable target for secondary prevention or early remediation
Introduction
Major depressive disorder (MDD) is a chronic and disabling disorder associated with significant impairment in functioning and high rates of relapse. Cognitive dysfunction is one illness feature particularly important for understanding course and impairment in MDD, as it has been linked to increased susceptibility to relapse, poor occupational functioning, and reduced quality of life1-3. Active state MDD has been consistently associated with a wide range of cognitive deficits, including attention4-11, processing speed9-13, visuospatial abilities4, 11, 14, memory4, 8, 9, 15, 16, and executive dysfunction4, 10, 17-19. Importantly, performance in several of these neuropsychological domains may be linked to symptom severity20 and diminished treatment response18, 21-25. Despite broad understanding that cognition is reduced during depression, there is still a lack of consensus regarding the specificity of these deficits.
Several other illness features likely contribute to the lack of specificity, or variability in cognitive functioning among depressed individuals26; features that many previous MDD studies of cognition in the active state have failed to measure. For instance, in most studies, patient populations are heterogeneous with respect to number or duration of MDD episodes, severity of MDD episodes or current symptoms, age of illness onset, length of illness, current age, treatment and medication status, psychiatric co-morbidity, and even primary diagnosis27. Relatively few studies of neuropsychological functioning exist among early course samples of adolescents or young adults28. Furthermore, most studies of neuropsychological performance in MDD are small, cross-sectional studies that have compared cognitive abilities in symptomatic patients with those of matched controls27 offering limited power to consider many of these MDD features outlined above. A recent meta-analysis of 113 studies that revealed significantly impaired performance in MDD across several domains of executive functioning, also examined the role of potential moderating variables such as symptom severity/remission status, age, medication, and psychiatric co-morbidities29. Some of these variables were related to the degree of impairment in domains of cognitive functioning (e.g. processing speed, verbal fluency, verbal memory, shifting, inhibition), however the analysis was underpowered to fully dissociate these effects in other domains (e.g. visual working memory, planning, updating).
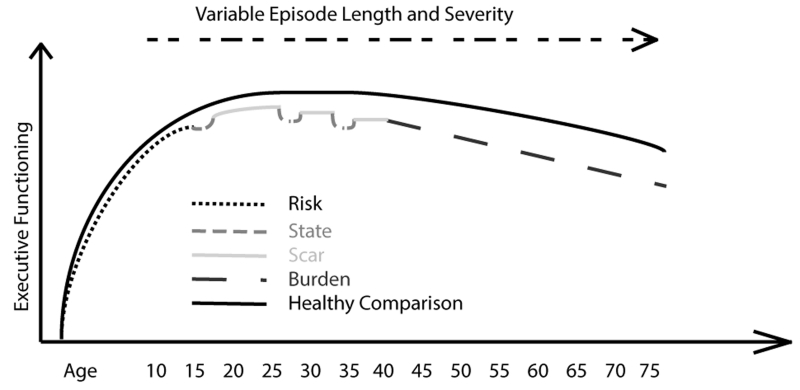
One strategy to avoid past challenges regarding participant heterogeneity is to study a more homogeneous set of individuals within a more restricted window of MDD characteristics. To optimize understanding of trait features of MDD, it may be advantageous to study those with MDD with few episodes, currently in remission, at a point of both developmental and mood stability. This is a point in the illness when cognitive systems are unlikely to demonstrate changes associated with increasing illness burden or state effects30. To understand the methodological advantages of studying cognition in this epoch of MDD, it is helpful to divide illness factors affecting cognition in depression into four broad categories: (1) risk/trait (2) state (3) scar and (4) burden (See Figure 1). Risk/trait effects refer characteristics present prior to illness onset. State effects of MDD include severity of symptoms, duration of current episode, co-morbid psychiatric conditions, and treatment related to the current episode. Scar effects are decrements in abilities and functioning after an episode, inferring a potential failure to achieve complete inter-episode recovery. Burden refers to the repetitive and possibly cumulative effects of illness characteristics over time. This framework suggests that studying cognitive functioning early, in a remitted state, offers a unique window into possible trait vulnerability factors and early neurobiological abnormalities in depression31.
Figure 1.
Several existing studies have assessed cognitive functioning in the remitted state. A recent review of adults concluded that neuropsychological deficiencies persists in remission relative to healthy controls (HCs), particularly in the domains of sustained and selective attention, memory, and executive function32. These patients were in remission from depression as defined by cut-off scores on clinician-rated depression scales, however, variability in illness features including subsyndromal symptoms, duration of remission, chronicity, and medication status, made it difficult to estimate the magnitude of cognitive deficits observed during remission. An additional problem with studying risk traits in MDD is that few studies have collected repeated measurements of cognitive functioning; a method that is ideal for evaluating reliability of any impairment. Few longitudinal studies have addressed the stability of neuropsychological deficits over time in active state MDD and longitudinal studies are notably scarce in remitted MDD (for a review see33). None of the existing longitudinal studies reviewed were conducted in youth samples (lowest mean age was 41 years16) and 14 of these studies were actually in late-life depression, limiting the conclusions that can be drawn about the early stages of illness. Moreover, many are limited by variability in assessment windows that could be overly vulnerable to practice effects, with repeat testing ranging from 1 week34 to one year later35-37. It is also worth noting that only 18 of the 30 studies reviewed included a healthy comparison group33.
To address these key methodological gaps in the literature, we investigated for trait or scar risk factors in cognitive functions among un-medicated, late-adolescents in remission from depression with repeated assessments. Based on previous research29, 32 we hypothesized that executive functions (processing speed with interference resolution, conceptual reasoning/set-shifting, and cognitive control) would be impaired in the rMDD group relative to HCs, and stable over time. By contrast, we hypothesized that verbal fluency and processing speed would be comparable in the rMDD and HC groups and stable over time. Positive results in this sample would indicate that deficits in executive functions are not exclusively due to state and chronic burden effects.
Method
Participants
Study participants were English-speaking young adults between the ages of 18 - 23 with a history of 1-3 episodes of MDD who are currently in remission (rMDD; n = 62) and similarly aged HCs (n = 43). Participants were recruited from the community surrounding two study sites; University of Michigan (n = 40) and the University of Illinois at Chicago (n = 65).
rMDD participants met criteria for the study if they, (a) currently scored seven or below on the Hamilton Depression Rating Scale, 17-item (HAM-D38), and (b) reported between one and three prior episodes of MDD. rMDD participants could enroll with current or past co-morbid anxiety disorders, but were excluded if they met criteria for a substance use disorder (last two years) or childhood-onset ADHD. HC participants could not meet current or past criteria for any Axis I or Axis II psychiatric disorder and could not have any first-degree relatives with a history of psychiatric illness. In addition, all enrolled participants were free of any psychiatric medication for 90 days, did not have head injury with loss of conscious greater than 10 minutes, and did not suffer from any significant birth complications or chronic medical conditions that would affect cognitive functioning.
Procedure
After the initial phone screen, participants completed a diagnostic interview and clinician-rated measures of depression. Previous MDD was established using the Diagnostic Interview for Genetic Studies39, with single-blind confirmation by phone with a parent/guardian/older sibling using a modified Family Interview for Genetic Studies39. Depression was assessed using the HAM-D38, by a trained interviewer. Anxiety was assessed using the Hamilton Anxiety Rating Scale (HAM-A40). Following diagnostic confirmation, participants completed a battery of neuropsychological assessments. This test battery was repeated, spanning 3-15 weeks later. Ninety-one percent of HC participants (n = 39) and 92% of rMDD participants (n = 57) completed the follow-up battery.
Neuropsychological Test Battery
Neuropsychological tests focused heavily upon areas known to be impaired in active state MDD, including memory, processing speed, attention, and executive functioning. Specific tasks included the Stroop Color and Word Test41, the Controlled Oral Word Association Test42, Digit Symbol from the Wechsler Adult Intelligence Scale-IV43, the Trail Making Test–Parts A and B44, and the Parametric Go/No-Go Task45-47. The Parametric Go/No-Go Task is a measure of cognitive control. It has demonstrated reliability and validity in previous studies 11, 46. The task consists of three conditions or levels that ascend in difficulty. For all three levels, a series of sequential letters are presented rapidly on a computer screen, and participant responses were recorded on a designated computer keyboard key. In the first level of the task, the “Go” condition, participants respond to three target letters every time they are presented. In levels 2 and 3, “Go/No-Go” conditions, the participant is expected to keep track of the last target to which they had responded and inhibit responding to that target until they had seen and responded to either 1 or 2 alternate targets (non-repeating rule), respectively.
Data Analytic Approach
All analyses were conducted in SPSS with an alpha threshold of .05. Primary analyses sought to assess differences between rMDD and HC participants on neuropsycholgical domains. Next, we used factor analysis to obtain more reliable estimates of underlying cognitive constructs, minimizing measurement error and consistent with prior convention11, 48, 49. Standard data reduction techniques (confirmatory principal axis factor analysis with oblique rotation) were used to reduce the tests using conceptually and theoretically categorized variables, consistent with our prior studies50, 51. Any scores with negative scale properties were inverted; as a result, lower factor scores reflect poorer performance.
Mixed-effects regression models52 (MRMs) were conducted to examine changes in neuropsychology functions over time, group differences between rMDD and HC participants in performance and, group × time interactions in performance. MRMs are well suited for repeated measures: they are robust to the data dependency that occurs with repeated assessments of individuals over time. MRMs are efficient in handling missing data by using all available data for a given participant to estimate group trends at each time point. Models for each neuropsychological domain as a dependent variable, included both fixed (time, diagnosis [coded HC =0, rMDD = 1]) and random (patient) effects. Chronbach’s alpha and intraclass correlation coefficients were computed to evaluate the stability of performance over time.
Results
Sample Composition
Participants were an average age of 21.14 (SD 1.70), 65% female (n = 68), with approximately 14.63 years of education (SD = 1.50). Additional descriptive statistics for demographic and clinical characteristics of the sample are presented in Table 1. rMDD and HC groups were of similar age, IQ, years of education, sex distribution, racial distribution, and time between neuropsychological assessments. Participants from UIC and UM were of comparable age, race, sex, and education. Participants recruited from UM had higher IQ (UM: M = 111.21, SD = 8.96; UIC: M = 103.66, SD = 8.96), p < .001) and lower levels of anxiety (UM: M = 1.31, SD = 2.01; UIC: M = 2.63, SD = 3.40), t(50) = −2.10, p = .036.
Table 1.
Clinical and Demographic Characteristics of rMDD and HC participants
| Variable | rMDD (n = 62) | HC (n = 43) |
|---|---|---|
| Age | 20.92 (1.61) | 20.73 (1.66) |
| Shipley Verbal IQ | 106.25 (9.65) | 106.73 (9.30) |
| Years of education | 14.31 (1.38) | 14.53 (1.41) |
| Depressive severity (HAM-D)** | 2.71 (3.43) | .42 (1.03) |
| Anxiety severity (HAM-A)** | 3.20 (3.35) | .65 (1.56) |
| Female (%) | 47 (72.3) | 23 (57.5) |
| Caucasian (%) | 34 (53.1) | 28 (70.0) |
| Days between neuropsychological assessments | 50.79 (25.97) | 55.88 (36.56) |
| Age of onset | 16.53 (3.38) | |
| Years since most recent MDD episode | 2.68 (2.94) | |
| Medication naïve (%) | 28 (70.0) | |
| Never hospitalized (%) | 46 (90) | |
| Longest MDD duration (weeks) | 32.23 (36.71) |
() denotes SD unless otherwise noted, percentages are calculated based on percent of available cases
p<.05,
p<.01
Though in the remitted state, rMDD participants had higher depression and anxiety rating scores than HCs. All rMDD participants scored seven or below on these measures (range = 0 - 7); the average score for both ratings in the rMDD group was substantially lower than this cutoff. rMDD participants were medication free for a minimum of 6 months, 70% were medication nai̇ve. rMDD participants were on average of 2.68 (SD = 2.94) years since the end of the last episode. Modal number of previous depressive episodes was 1 and 90% were never hospitalized. Average age of onset was 16.53 (SD = 3.38).
Factor Scores
The resulting factor scores included verbal fluency and processing speed, conceptual reasoning and set-shifting, processing speed with interference resolution, and cognitive control. Factor loadings are reported in Table 2.
Table 2.
Confirmatory Factor Analysis of Neuropsychological Test Scores in rMDD and HC
| Time 1 | Time 2 | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Factor | Test | rMDD Raw Score |
HC Raw Score | Factor Loading |
rMDD Raw Score |
HC Raw Score | Factor Loading |
| Verbal Fluency and Processing Speed |
Phonemic and Category Fluency Stroop Color Word Test |
45.56 (11.92) | 46.81 (9.87) | .67 | 50.81 (12.65) | 48.42 (10.29) | .56 |
| Stroop Word Condition | 105.33 (22.65) | 110.54 (17.26) | .88 | 109.67 (15.79) | 110.75 (20.75) | .90 | |
| Stroop Color Condition | 78.45 (14.84) | 76.78 (25.08) | .84 | 83.77 (10.96) | 83.58 (12.15) | .89 | |
|
| |||||||
| Conceptual Reasoning and Set- Shifting |
Parametric Go/No-go* | ||||||
| Level 2 Accuracy Target Trials | 95.98% | 97.17% | .79 | 96.66% | 98.09% | .88 | |
| Level 3 Accuracy Target Trials | 88.37% | 90.58% | .85 | 91.09% | 91.91% | .80 | |
|
| |||||||
| Processing Speed with Interference Resolution |
Trail Making Test B Stroop Color Word Test |
53.35 (17.92) | 51.51 (16.78) | .43 | 51.92 (19.44) | 50.88 (12.92) | .51 |
| Interference Condition | 57.20 (7.02) | 56.33 (7.18) | .29 | 59.13 (7.58) | 57.67 (8.84) | .62 | |
| Parametric Go/No-go* | |||||||
| Level 2 Target Response Time^ | −422.99 (46.79) | −415.32 (41.59) | .90 | −429.91 (49.30) | −414.47 (42.41) | .70 | |
| Level 3 Target Response Time^ | −495.44 (51.37) | −489.10 (48.35) | .88 | −490.30 (57.11) | −499.97 (92.24) | .70 | |
|
| |||||||
| Cognitive Control | Parametric Go/No-go | ||||||
| Level 2 Inhibitory Accuracy | 74.68% | 78.26% | .85 | 72.44% | 74.95% | .81 | |
| Level 3 Inhibitory Accuracy | 59.87% | 66.19% | .84 | 64.77% | 61.48% | .80 | |
() denotes mean(SD) unless otherwise noted
Time permitting, participants received a practice administration of the PGNG. rMDD and HC groups did not differ in proportion of participants completing practice at Time 1 (79% vs. 70%, x2 = .98; p =.322) or at Time 2 (84% vs. 78%, x2 = .47; p =.492)
Neuropsychological Functioning
Statistical parameters for each model reported below are presented in Table 3. rMDD participants demonstrated domain-specific decrement in cognitive control relative to HCs at Time 1. At time 2, performance of HC’s declined (low stability in this sample), such that the between group performance difference in cognitive control (stable performance) no longer remained significant. rMDD and HC participants demonstrated comparable performance on verbal fluency and processing speed, processing speed with interference resolution, and conceptual reasoning and set-shifting. Performance on these domains was stable over time in both groups.
Table 3.
Effects of Time and Diagnosis on Neuropsychological Factor Scores in rMDD and HC
| Mixed Effects Regression Models | Effect Sizes (d) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | b | SE | p | Time 1 | Time 2 | |
| Verbal Fluency and Processing Speed |
Diagnosis | −.07 | .24 | .755 | .18 | .03 |
| Time | −.09 | .20 | .653 | |||
| Time × Diagnosis | −.02 | .12 | .847 | |||
|
| ||||||
| Conceptual Reasoning and Set- Shifting |
Diagnosis | −.43 | .23 | .063 | .38 | .28 |
| Time | −.17 | .22 | .434 | |||
| Time × Diagnosis | .13 | .13 | .301 | |||
|
| ||||||
| Processing Speed with Interference Resolution |
Diagnosis | −.23 | .31 | .462 | .16 | .11 |
| Time | −.03 | .33 | .928 | |||
| Time × Diagnosis | −.08 | .19 | .676 | |||
|
| ||||||
| Cognitive Control | Diagnosis | −.66 | .32 | .042 | .38 | .01 |
| Time | −.47 | .31 | .134 | |||
| Time × Diagnosis | .31 | .19 | .095 | |||
Reliability
Table 4 reports the internal consistency values for neuropsychological performance across domains among all participants and according to diagnosis. Alpha and intra-class correlation coefficients were generally in the acceptable to excellent range. Overall internal consistency was excellent for verbal fluency and processing speed (α = .92), good for processing speed with interference resolution (α = .80), acceptable for conceptual reasoning and set-shifting (α = .63) and cognitive control (α = .67).
Table 4.
Internal Consistency and Test-Retest Reliability of Neuropsychological Domains in HC and rMDD Participants
| All Participants | Healthy Controls | rMDD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Alpha | ICC | Alpha | ICC | Alpha | ICC | |
| Verbal Fluency and Processing Speed | .92 | .91 | .90 | .90 | .93 | .92 |
| Conceptual Reasoning and Set-Shifting | .63 | .64 | .60 | .60 | .82 | .81 |
| Processing Speed with Interference Resolution |
.80 | .80 | .66 | .66 | .86 | .86 |
| Cognitive Control | .67 | .66 | .58 | .59 | .74 | .74 |
Notably, internal consistency in the rMDD group was higher than HC’s across all domains. In particular, the rMDD deficit in cognitive control was more reliable over time (α = .74) than cognitive control among HC’s (α = .58), which was poor.
Clinical Correlates of Cognitive Control
Illness characteristics of rMDD, such as residual symptoms or scar effects from prior episodes may contribute to the observed relative deficit in cognitive control in rMDD at Time 1. Therefore, we evaluated the association between the cognitive control domain and clinical attributes specific to MDD among the rMDD group. Residual depressive symptoms (HAM-D; r = −.04, p = .773.), residual anxiety symptoms (HAM-A; r = −.08, p = .574.), number of prior depressive episodes (r = −.06, p = .862), age at onset (r = .07, p = .666), number of hospitalizations (r = −.02, p = .905), longest episode duration (r = .08, p = .631), years since last episode (r = .20, p = .219), and being medication nai̇ve (r = .11, p = .521) were unrelated to the rMDD deficit in cognitive control.
Discussion
In the current study, deficits in inhibitory regulatory processes persisted during remission from depressive episodes in rMDD. rMDD participants demonstrated poorer cognitive control relative to HCs. This is the first study to show that these cognitive control markers were reliable and stable over time in rMDD, and unrelated to residual depressive symptoms or chronicity of illness. That cognitive control was unrelated to sub-threshold symptoms or illness burden rules out the possibility that active illness is the sole cause of poor inhibition regulation. If deficits in inhibition were associated with symptom severity, prior illness characteristics, or vulnerable to state fluctuations in depression, then interference in cognitive performance could be interpreted as temporal repercussions or concomitants of depressive symptoms, and would be minimally informative about underlying mechanisms or vulnerabilities. In contrast, deficits in cognitive control were present independent of current severity in rMDD, suggesting a more robust signature, or intermediate phenotype, of MDD exists. This intermediate phenotype is similar to that observed in bipolar disorder (impairment in executive functioning, attention, memory, fine motor function53), though the intermediate phenotype of rMDD constitutes a more specific domain of executive functioning.
rMDD participants demonstrated more stable performance in cognitive control relative to HC’s, of a small to medium effect size. Although HC’s converged with rMDD on cognitive control performance at Time 2, declining performance among HC’s over time is common with repeat performance of neuropsychological tests and likely representative of distraction and suspect effort rather than true abnormalities in cognitive performance54, 55. The HC group may also be more prone to boredom in a study with no direct or long term benefits and only being compensated for their time. In contrast, the higher reliability scores of this relative deficit in rMDD suggest the possibility it is a more stable and robust measure of a potential trait illness characteristic. It is unclear whether the effect observed at Time 1 translates to observable clinical impairment in the real world, highlighting that neuropsychological screenings provide can provide valuable, and potentially otherwise undetectable information about illness characteristics that may constitute vulnerabilities. This distinction could be clarified in future studies by incorporating neuropsychological assessments in a longitudinal high-risk design to evaluate whether the same differences are present before the first onset of MDD, and whether the differences are related to the clinical outcomes in the long-term.
rMDD participants did not differ from HCs in processing speed with interference resolution, verbal fluency and processing speed, or conceptual reasoning and set-shifting. Even within the umbrella of executive functions, relatively lower order cognitive processes, such as sustained or divided attention, may remain intact in the early course of MDD, and that challenges in these areas is an artifact of either active symptoms or chronic illness burden. In contrast, the higher order process of responding flexibly to new information or inhibiting pre-potent impulses in response to changing goals may uniquely represent either an early course scar or risk factor for MDD. In this sense, the failure of the higher order ability to manage and direct lower order cognitive processes or impulses, may constitute a vulnerability in the cognitive system that precedes impairment in more basic processes with prolonged persistence of depression. This possibility is consistent with a prior comprehensive review of cognition among young adults with internalizing disorders that suggests executive dysfunction is present in early course MDD, but that other domains of cognition are not consistently impaired56.
A key strength of this study is that detection of cognitive deficits was optimized by restricting the sample to individuals early in their illness course whose performance is not affected by a chronic illness burden. However, results of this study cannot fully dissociate whether observed differences constitute trait risk for the illness, or potential early scar effects on brain structure and function deriving from a less than full recovery from the index episode. An additional limitation of the study is that although no participants were informed of the specific hypotheses of the study, rMDD participants were aware that they were recruited based on a past history of depression, which could have operated as a demand characteristic or stereotype threat leading them to perform more poorly in cognitive control. It would be more likely, though, to have broader based cognitive difficulties if stereotype threat were at play in this sample. Further, while it is generally considered that executive functioning development asymptotes between 14-15 and peaks around age 1857, brain regions that support executive functions continue to consolidate and myelinate/prune through the early-to-mid twenties58-60. Thus, it is a critical future endeavor to follow rMDD individuals in longitudinal, developmental designs to dissociate points of impairment and whether this impairment in cognitive control persists or resolves. Last, despite the need for studies of cognition in depression that are not confounded by repeated episodes or complex treatment histories, it deserves emphasizing that these findings cannot, at this point, be generalized beyond a relatively high-functioning group of young individuals early in their illness course. Individuals outside this window may demonstrate more severe impairments across more domains of cognition. In addition, those who were unable to reach remission by our strict criteria may have been more likely to exhibit cognitive difficulties.
Nonetheless, our findings have important implications for the pathoetiology of MDD. Active state MDD is characterized by altered inhibition-related activity most prominently in the rostral anterior cingulate cortex (ACC) and the dorsal lateral prefrontal cortex (dlPFC)61. The ACC is thought to play an essential role in shifting flexibly between cognitive tasks and response sets, whereas the lateral structures of the dlPFC are recruited when competing responses need to be inhibited62, 63. These regions operate within a cognitive control network that maintains goals by flexibly adjusting attention and working memory to changing environments and demands64. Indeed, increased activity in these areas has been linked with successful inhibition trials on a Go/No-go task18, suggesting potential compensatory mechanisms, and with impairment on interference resolution tasks such as the Stroop or continuous performance tasks65, 66. Thus, the direction of inhibition related activity may differ depending on the particular nature of the task, or potentially clinical confounds such as depressive severity and chronicity67. Evaluating the circuitry involved in regulatory deficits among early course, remitted individuals may help to clarify the nature of these abnormalities by reducing confounds of active illness, complex treatment histories, or neural scarring resulting from decades of illness.
These findings have important clinical implications. Patterns of inflexible, maladaptive, and ruminative thinking styles common in depression may be related, in part, to decreased attentional resources and cognitive control68. Advances in neurobehavioral training strategies, such as computer-based cognitive control exercises, to recruit the networks and resources necessary for executive control via repeated behavioral exercises, suggest that it is possible to strengthen cognitive and emotional functions. Actively depressed participants who have received cognitive control training exhibited reduced negative affect and rumination, and improved concentration69. Given that cognitive control deficits persist in remission of MDD, the application of cognitive control training during the euthymic phase may prove useful in reducing vulnerability to MDD relapse and warrant future study.
Acknowledgments
Funding provided by NIMH grant RO1 081911 (SAL).
References
- 1.Jaeger J, Berns S, Uzelac S, Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry research. 2006;145:39–48. doi: 10.1016/j.psychres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 2.McCall WV, Dunn AG. Cognitive deficits are associated with functional impairment in severely depressed patients. Psychiatry research. 2003;121:179–84. doi: 10.1016/j.psychres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Naismith SL, Longley WA, Scott EM, Hickie IB. Disability in major depression related to self-rated and objectively-measured cognitive deficits: a preliminary study. BMC psychiatry. 2007;7:32. doi: 10.1186/1471-244X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter RJ, Gallagher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. The British journal of psychiatry : the journal of mental science. 2003;182:214–20. doi: 10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- 5.Cohen R, Lohr I, Paul R, Boland R. Impairments of attention and effort among patients with major affective disorders. The Journal of neuropsychiatry and clinical neurosciences. 2001;13:385–95. doi: 10.1176/jnp.13.3.385. [DOI] [PubMed] [Google Scholar]
- 6.Koetsier GC, Volkers AC, Tulen JH, Passchier J, van den Broek WW, Bruijn JA. CPT performance in major depressive disorder before and after treatment with imipramine or fluvoxamine. Journal of psychiatric research. 2002;36:391–7. doi: 10.1016/s0022-3956(02)00026-2. [DOI] [PubMed] [Google Scholar]
- 7.Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry research. 2008;159:7–17. doi: 10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landro NI, Stiles TC, Sletvold H. Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry, neuropsychology, and behavioral neurology. 2001;14:233–40. [PubMed] [Google Scholar]
- 9.Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R. Cognitive deficits in major depression. Scandinavian journal of psychology. 2002;43:239–51. doi: 10.1111/1467-9450.00292. [DOI] [PubMed] [Google Scholar]
- 10.Lampe IK, Sitskoorn MM, Heeren TJ. Effects of recurrent major depressive disorder on behavior and cognitive function in female depressed patients. Psychiatry research. 2004;125:73–9. doi: 10.1016/j.psychres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Langenecker SA, Caveney AF, Giordani B, et al. The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry research. 2007;152:143–54. doi: 10.1016/j.psychres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Hammar A, Lund A, Hugdahl K. Selective impairment in effortful information processing in major depression. Journal of the International Neuropsychological Society : JINS. 2003;9:954–9. doi: 10.1017/S1355617703960152. [DOI] [PubMed] [Google Scholar]
- 13.Singh MK, DelBello MP, Fleck DE, Shear PK, Strakowski SM. Inhibition and attention in adolescents with nonmanic mood disorders and a high risk for developing mania. Journal of clinical and experimental neuropsychology. 2009;31:1–7. doi: 10.1080/13803390801945038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asthana HS, Mandal MK, Khurana H, Haque-Nizamie S. Visuospatial and affect recognition deficit in depression. Journal of affective disorders. 1998;48:57–62. doi: 10.1016/s0165-0327(97)00140-7. [DOI] [PubMed] [Google Scholar]
- 15.Fossati P, Coyette F, Ergis AM, Allilaire JF. Influence of age and executive functioning on verbal memory of inpatients with depression. Journal of affective disorders. 2002;68:261–71. doi: 10.1016/s0165-0327(00)00362-1. [DOI] [PubMed] [Google Scholar]
- 16.Vythilingam M, Vermetten E, Anderson GM, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biological psychiatry. 2004;56:101–12. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Naismith SL, Hickie IB, Turner K, et al. Neuropsychological performance in patients with depression is associated with clinical, etiological and genetic risk factors. Journal of clinical and experimental neuropsychology. 2003;25:866–77. doi: 10.1076/jcen.25.6.866.16472. [DOI] [PubMed] [Google Scholar]
- 18.Langenecker SA, Kennedy SE, Guidotti LM, et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biological psychiatry. 2007;62:1272–80. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elderkin-Thompson V, Moody T, Knowlton B, Hellemann G, Kumar A. Explicit and implicit memory in late-life depression. The American Journal of Geriatric Psychiatry. 2011;19:364–73. doi: 10.1097/JGP.0b013e3181e89a5b. [DOI] [PubMed] [Google Scholar]
- 20.Basso M, Combs D, Purdie R, Candilis P, Bornstein R. Neuropsychological correlates of symptom dimensions in inpatients with major depressive disorder. Psychiatry research. 2013;207:61–7. doi: 10.1016/j.psychres.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. Journal of affective disorders. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 22.Mohr DC, Epstein L, Luks TL, et al. Brain lesion volume and neuropsychological function predict efficacy of treatment for depression in multiple sclerosis. Journal of consulting and clinical psychology. 2003;71:1017–24. doi: 10.1037/0022-006X.71.6.1017. [DOI] [PubMed] [Google Scholar]
- 23.Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:2266–71. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- 24.Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:117–36. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas KM, Porter RJ, Knight RG, Maruff P. Neuropsychological changes and treatment response in severe depression. The British journal of psychiatry : the journal of mental science. 2011;198:115–22. doi: 10.1192/bjp.bp.110.080713. [DOI] [PubMed] [Google Scholar]
- 26.McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24:9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi MH, Greer TL. Cognitive dysfunction in unipolar depression: Implications for treatment. Journal of affective disorders. 2014;152-154:19–27. doi: 10.1016/j.jad.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Baune BT, Fuhr M, Air T, Hering C. Neuropsychological functioning in adolescents and young adults with major depressive disorder--a review. Psychiatry research. 2014;218:261–71. doi: 10.1016/j.psychres.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 29.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Molecular psychiatry. 2013;18:595–606. doi: 10.1038/mp.2012.33. [DOI] [PubMed] [Google Scholar]
- 31.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 32.Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. Journal of affective disorders. 2011;134:20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. The Australian and New Zealand journal of psychiatry. 2009;43:1105–17. doi: 10.3109/00048670903279887. [DOI] [PubMed] [Google Scholar]
- 34.Nebes RD, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of psychiatric research. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 35.Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2006;14:419–27. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 36.Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. International psychogeriatrics / IPA. 2007;19:125–35. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- 37.Portella MJ, Marcos T, Rami L, Navarro V, Gasto C, Salamero M. Residual cognitive impairment in late-life depression after a 12-month period follow-up. International journal of geriatric psychiatry. 2003;18:571–6. doi: 10.1002/gps.895. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nurnberger JI, Jr., Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of general psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. discussion 63-4. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton M. The assessment of anxiety states by rating. British journal of medical psychology. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 41.Stoelting GC. Stroop Color and Word Test. Chicago: 1978. [Google Scholar]
- 42.Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: Reliability and updated norms. Archives of Clinical Neuropsychology. 1996;1996;11:329–38. [PubMed] [Google Scholar]
- 43.Wechsler D. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV) NCS Pearson; San Antonio, TX: 2008. [Google Scholar]
- 44.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8:271–6. [Google Scholar]
- 45.Langenecker SA, Caveney AF, Giordani B, et al. The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry research. 2007;152:143–54. doi: 10.1016/j.psychres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Langenecker SA, Zubieta JK, Young EA, Akil H, Nielson KA. A task to manipulate attentional load, set-shifting, and inhibitory control: convergent validity and test-retest reliability of the Parametric Go/No-Go Test. Journal of clinical and experimental neuropsychology. 2007;29:842–53. doi: 10.1080/13803390601147611. [DOI] [PubMed] [Google Scholar]
- 47.Votruba KL, Langenecker SA. Factor structure, construct validity, and age- and education-based normative data for the Parametric Go/No-Go Test. Journal of clinical and experimental neuropsychology. 2013;35:132–46. doi: 10.1080/13803395.2012.758239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bleiberg J, Kane RL, Reeves DL, Garmoe WS, Halpern E. Factor analysis of computerized and traditional tests used in mild brain injury research. The Clinical neuropsychologist. 2000;14:287–94. doi: 10.1076/1385-4046(200008)14:3;1-P;FT287. [DOI] [PubMed] [Google Scholar]
- 49.Rund BR, Sundet K, Asbjornsen A, et al. Neuropsychological test profiles in schizophrenia and non-psychotic depression. Acta psychiatrica Scandinavica. 2006;113:350–9. doi: 10.1111/j.1600-0447.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 50.Ryan KA, Vederman AC, McFadden EM, et al. Differential executive functioning performance by phase of bipolar disorder. Bipolar disorders. 2012;14:527–36. doi: 10.1111/j.1399-5618.2012.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langenecker SA, Saunders EF, Kade AM, Ransom MT, McInnis MG. Intermediate: cognitive phenotypes in bipolar disorder. Journal of affective disorders. 2010;122:285–93. doi: 10.1016/j.jad.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 53.Langenecker SA, Saunders EFH, Kade AM, Ransom MT, McInnis MG. Intermediate cognitive phenotypes in bipolar disorder. Journal of affective disorders. 2010;122:285–93. doi: 10.1016/j.jad.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Binder LM, Iverson GL, Brooks BL. To err is human: “abnormal” neuropsychological scores and variability are common in healthy adults. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2009;24:31–46. doi: 10.1093/arclin/acn001. [DOI] [PubMed] [Google Scholar]
- 55.Schretlen DJ, Testa SM, Winicki JM, Pearlson GD, Gordon B. Frequency and bases of abnormal performance by healthy adults on neuropsychological testing. Journal of the International Neuropsychological Society : JINS. 2008;14:436–45. doi: 10.1017/S1355617708080387. [DOI] [PubMed] [Google Scholar]
- 56.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. Journal of affective disorders. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Luna B. Developmental changes in cognitive control through adolescence. Advances in child development and behavior. 2009;37:233–78. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of child psychology and psychiatry, and allied disciplines. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 59.De Luca CR, Wood SJ, Anderson V, et al. Normative data from the CANTAB. I: development of executive function over the lifespan. Journal of clinical and experimental neuropsychology. 2003;25:242–54. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- 60.Taylor SJ, Barker LA, Heavey L, McHale S. The typical developmental trajectory of social and executive functions in late adolescence and early adulthood. Developmental psychology. 2013;49:1253–65. doi: 10.1037/a0029871. [DOI] [PubMed] [Google Scholar]
- 61.Rubia K, Russell T, Overmeyer S, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 62.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Human brain mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 64.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 65.Holmes AJ, MacDonald A, 3rd, Carter CS, Barch DM, Andrew Stenger V, Cohen JD. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophrenia research. 2005;76:199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 66.Wagner G, Sinsel E, Sobanski T, et al. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biological psychiatry. 2006;59:958–65. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 67.Matthews S, Simmons A, Strigo I, Gianaros P, Yang T, Paulus M. Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry research. 2009;172:1–6. doi: 10.1016/j.pscychresns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Joormann J, Quinn ME. Cognitive processes and emotion regulation in depression. Depression and anxiety. 2014;31:308–15. doi: 10.1002/da.22264. [DOI] [PubMed] [Google Scholar]
- 69.Calkins AW, McMorran KE, Siegle GJ, Otto MW. The Effects of Computerized Cognitive Control Training on Community Adults with Depressed Mood. Behavioural and cognitive psychotherapy. 2014:1–12. doi: 10.1017/S1352465814000046. [DOI] [PubMed] [Google Scholar]