Abstract
Hypothermic machine perfusion (HMP) is increasingly used in deceased-donor kidney transplantation, but controversy exists regarding the value of perfusion biomarkers and pump parameters for assessing organ quality. We prospectively determined associations between perfusate biomarkers [neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18) and liver-type fatty acid-binding protein (L-FABP)] and pump parameters (resistance and flow) with outcomes of delayed graft function (DGF) and 6-month estimated glomerular filtration rate (eGFR). DGF occurred in 230/671 (34%) recipients. Only 1-hour flow was inversely associated with DGF. Higher NGAL or L-FABP concentrations and increased resistance were inversely associated with 6-month eGFR, while higher flow was associated with higher adjusted 6-month eGFR. Discarded kidneys had consistently higher median resistance and lower median flow than transplanted kidneys, but median perfusate biomarker concentrations were either lower or not significantly different in discarded compared with transplanted kidneys. Notably, most recipients of transplanted kidneys with isolated “undesirable” biomarker levels or HMP parameters experienced acceptable 6-month allograft function, suggesting these characteristics should not be used in isolation for discard decisions. Additional studies must confirm the utility of combining HMP measurements with other characteristics to assess kidney quality.
Introduction
Since the original work by Belzer and colleagues in the late 1960s (1), hypothermic machine perfusion (HMP) has seen a resurgence as an effective method to preserve deceased-donor kidneys that otherwise would be at increased risk for poor transplant outcomes. In the US, by 2008, nearly half of the kidney transplants from expanded criteria donors (ECD) and 70% of those from donors after circulatory determination of death (DCD) were machine perfused (2). With over 100,000 patients on the kidney waiting list in the US, the transplant community will continue to rely upon deceased donors with a range of organ quality, and HMP of procured kidneys will likely continue to increase (3).
HMP is employed with the goal of reducing the rate of DGF and improving allograft function by minimizing the ischemic damage to deceased-donor kidneys that occurs with static cold storage (3–5). In a follow-up study of the Eurotransplant HMP trial, Moers et al. reported that three-year graft survival remained significantly better in recipients of kidneys preserved by HMP compared with static cold storage (91% vs. 87%) (6). The favorable effects of HMP may not be universal, however, as no clear benefit was noted for the subgroup of DCD kidneys within the Eurotransplant trial nor in an earlier trial by Watson et al. that randomized 90 DCD kidneys to HMP or static cold storage (7). Also, a recent study by Jochman et al. showed that while machine-perfused DCD kidneys had reduced risk of DGF and improved one-year graft function vs. kidneys transported with cold storage, they found no significant differences in one-year graft and patient survival between groups (8).
In addition to its potentially modest therapeutic benefits, HMP offers the opportunity to assess kidney viability, which is critical to optimal organ allocation. Accurate determination of kidney quality may decrease the number of viable kidneys that are discarded as well as the number of poor-quality kidneys with unacceptable survival that are transplanted. Multiple risk models that rely primarily on donor characteristics and pre-implantation biopsy have been developed to evaluate kidney quality. These models are limited, however, in their ability to predict individual graft outcomes (9, 10). Many factors not included in these donor models may affect kidney quality, including inflammatory injury induced by brain-death, hemodynamic instability during the donor hospitalization, traumatic injury caused during organ procurement, and ischemic damage to the kidney throughout transport.
HMP continuously flushes the kidney with cold preservation solution (perfusate) to provide nutrients, carry away toxic metabolites, and reduce lactic acid build-up (11). There are conflicting reports, however, regarding the ability of biomarkers in perfusate to assess kidney quality and predict graft outcomes (12). Our group recently demonstrated the utility of perfusate glutathione S-transferase (GST) iso-enzymes for predicting DGF (13), but there are no large studies focused on more novel biomarkers of kidney ischemia-reperfusion injury in perfusate. HMP also generates real-time pump parameter measurements that include perfusion pressure, perfusion flow, and renal resistance, which may reflect the effects of continued ischemia on the kidney.
Many institutions already assess kidney quality via these HMP parameters (14, 15). Promising subsequent analyses from the Eurotransplant trial mentioned above revealed that renal resistance was an independent risk factor for DGF and one-year graft failure (16). Though the trial authors argued against the use of pump parameters as kidney discard criteria, high renal resistance and low perfusate flow have been associated with higher rates of discard (17, 18). Overall, the literature remains mixed about the predictive utility of these physical measurements with regard to kidney viability and allograft outcomes (19).
To address continued equipoise regarding the use of pump parameters and to explore the association of perfusate biomarkers with recipient outcomes in the setting of real-world practice in the US, we conducted a prospective, observational cohort study across multiple organ procurement organizations. We sought to examine the associations of kidney injury biomarkers in perfusate and pump parameters with both DGF and kidney allograft function at six-months.
Materials and Methods
Study Design
Four organ procurement organizations (OPOs) and five academic institutions collaborated for this translational study. Yale University served as the sample and data coordinating center. The scientific review committees at each OPO and the institutional review boards at each academic site approved the study. Authorization for research was obtained from donor surrogates and registry data according to individual OPO guidelines.
Study Population
Participating OPOs enrolled deceased donors between May 2010 and December 2013. Donors were included if at least one kidney underwent HMP (initiated at the donor hospital) and transplanted. Recipients of single kidneys with at least one recorded flow and resistance value were included. Kidneys were excluded if no perfusate samples were obtained. We also excluded kidneys that were pumped en-bloc, since it would not be possible to determine the contribution of each individual kidney to the perfusate biomarker concentrations.
Data Sources and Data Collection
Detailed donor characteristics were extracted from OPO donor charts. This study also used data from the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN, and has been described elsewhere. The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN contractor. Recipient characteristics were obtained from OPTN data files and defined according to the United Network for Organ Sharing (UNOS), which is the current OPTN contractor.
Perfusate Collection and Storage
All kidneys were individually pumped using the LifePort Kidney Transporter (Organ Recovery Systems, Itasca, IL). OPO personnel managed the perfusion machines according to each OPO’s protocol. Kidneys were pumped using pulsatile flow without additives other than 1 L of KPS-1 solution at a targeted pressure of 30 mmHg and targeted temperature of 4°C. Perfusion flow and renal resistance, available for review by potential transplant centers as per usual clinical practice, were recorded in our study database at up to five time-points (1, 2, 4, 6, and 8 hours). These data were extracted from machine-generated perfusion reports or, in the case of one OPO, from the handwritten perfusion report maintained by the perfusion technician.
Perfusate samples were collected from the perfusion apparatus at two time points: one sample within minutes of starting perfusion (once the perfusionist was confident the pump setup was functioning properly, defined as “base”) and a second sample just before the OPO transferred management of the kidney (defined as “post”). The timing of sample collections was recorded by OPO personnel. Each sample was transported on ice to the OPO and stored at −80°C until monthly batch shipments to the coordinating center. One OPO temporarily stored samples at −20°C for less than 2 weeks prior to batch shipments. Samples were subsequently processed at the coordinating center following a single controlled thaw, separated into barcoded aliquots and stored at −80°C without the addition of protease inhibitors until biomarker measurement.
Perfusate Biomarker Measurements
Perfusate biomarker measurements were performed in a blinded fashion without additional freeze-thaw cycles. Neutrophil gelatinase-associated lipocalin (NGAL) measurement was performed with the Architect platform that is approved for clinical use in Europe (Abbott Diagnostics, Chicago, IL, USA). The upper limit of the assay was 6000 ng/mL and the coefficient of variation (CV) ranged from 1.9% to 7%. The concentrations of kidney injury molecule-1 (KIM-1) and interleukin-18 (IL-18) were measured via the Meso Scale Discovery platform (Meso Scale Diagnostics, LLC, Rockville, MD, USA), which employs proprietary electrochemiluminescence detection methods combined with patterned arrays and has an intra-assay CV of <10%. The inter-assay CVs for KIM-1 and IL-18 were 4.5–7.8% and 3.7–4.9%, respectively. The average lower limits of detection obtained from 24 runs were 0.39 pg/mL for KIM-1 and 0.12 pg/mL for IL-18. Liver-type fatty acid-binding protein (L-FABP) concentration was measured with clinical chemistry analyzers using a latex-enhanced immunoturbidimetry method that employs anti-human L-FABP mouse monoclonal antibodies (Sekisui Medical CO., LTD., Tokyo, Japan). The inter-assay CV was 1–3.5%, and the lower and upper limits of detection for L-FABP were 0.5 ng/ml and 250 ng/ml, respectively. Biomarkers concentrations measured below the lower limit of detection or above the upper limit of detection were imputed as equal to the lowest or highest measured value in the cohort, respectively.
Primary Outcomes
All study outcomes were ascertained from data reported by centers to the OPTN. The primary outcomes were DGF and 6-month estimated glomerular filtration rate (eGFR). DGF was defined as any dialysis in the first week of transplantation. Six-month eGFR was calculated from 6-month serum creatinine records using the Chronic Kidney Disease Epidemiology Collaboration formula (20). We imputed 6-month eGFR values for recipients who experienced graft failure or died prior to 6-months. Graft failures were assigned an eGFR of 10 ml/min/1.73m2, and deaths were assigned the last available eGFR carried forward. Deaths were ascertained both through center reports to the OPTN and the Social Security Death Master File.
Secondary Outcomes
We performed secondary analyses of the outcome of organ discard, which was defined as kidneys procured for the purpose of transplantation but not ultimately transplanted. These analyses included donors and kidneys in which at least one kidney underwent HMP and a) both kidneys from the same donor were transplanted, b) one kidney was transplanted and the other donated or c) both kidneys were discarded.
For additional secondary analyses, we evaluated the outcomes of primary non-function (PNF, defined by OPTN as failure of the allograft to function by 90 days after transplant and necessitating re-transplantation or continued dialysis support) as well as all-cause graft loss, death-censored graft failure and recipient death by 6 months post-transplant.
Statistical Analysis
Descriptive statistics were reported as mean (standard deviation) or median [interquartile range] for continuous variables and as frequency (percentage) for categorical variables. We calculated the kidney donor risk index (KDRI) based on the following donor characteristics: age, gender, race, height, weight, history of hypertension, history of diabetes, hepatitis C serostatus, stroke as the cause of death, DCD status, and terminal serum creatinine (21, 22). We calculated the 2010 kidney donor profile index (KDPI) from the KDRI as per convention (22).
The Wilcoxon rank sum test and the Kruskal-Wallis test were used to compare continuous variables between groups. Bivariate associations between categorical variables were assessed using Pearson’s chi-square test. Spearman correlations were calculated to quantify the association between base and post perfusate biomarkers as well as perfusate measures between kidneys from the same donor. Multivariable modified Poisson regression models were fit to estimate the relative risk of DGF per unit change in biomarkers and machine pump parameters (23).
For each biomarker, its relationship with the response was visually assessed by plotting the log risk of DGF versus biomarker via spline transformation. The c-statistic was estimated using the trapezoidal area under the ROC curve. We modeled 6-month eGFR using multivariable linear regression. In all regression models, perfusate biomarkers were log (base 2) transformed. To account for the possible correlation of outcomes for kidneys within the same donor, models were fit utilizing generalized estimating equations. The multivariable analyses were repeated for all secondary outcomes and for 6-month eGFR as a sensitivity analysis after excluding overall graft losses by 6 months (rather than using imputed eGFR values). For each biomarker and pump parameter, we incorporated multivariable adjustments into regression models as follows: Step 1) we regressed the response on a single biomarker or parameter at each time-point separately; Step 2) we added the following donor variables: age (years), black race, height (cm), weight (kg), hypertension, diabetes, stroke as cause of death, DCD status and terminal serum creatinine (mg/dl); Step 3) we added the following transplant and recipient variables: cold ischemia time (hours), number of human leukocyte antigen mismatches, age (years), black race, gender, previous kidney transplant, diabetes as the cause of end stage renal disease, panel reactive antibody, body mass index (kg/m2), and dialysis duration (vintage in months) before transplant. All analyses were performed using SAS 9.3 statistical software for Windows (SAS Institute, Cary, NC). Statistical tests and confidence intervals (CI) were two-sided with an alpha level of 0.05.
Results
Study Cohort
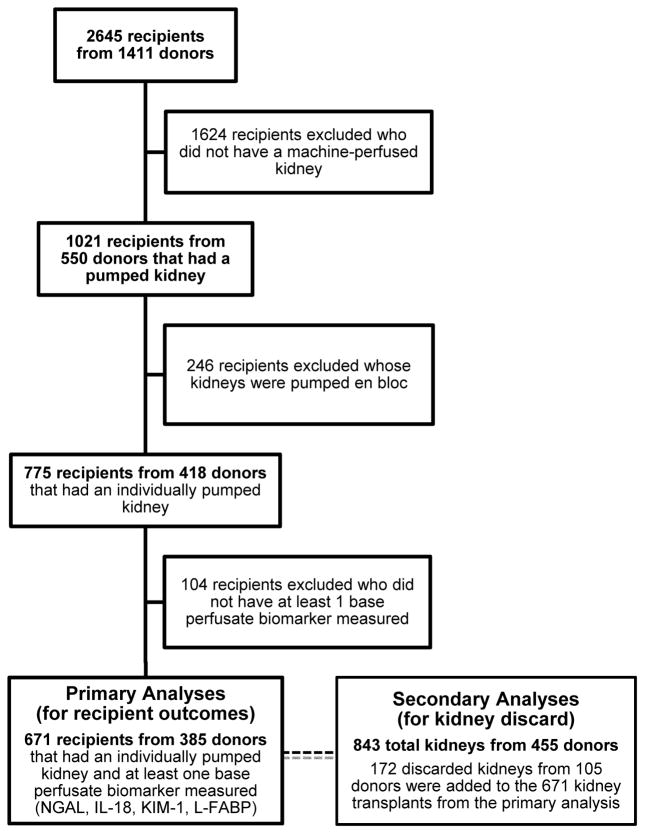
A total of 671 recipients of kidneys that were individually machine perfused from 385 deceased donors were included (Figure 1). There were significant differences in characteristics between machine-perfused kidneys and cold-stored kidneys that were excluded in the primary analyses (Supplemental Table S1). For those included, Table 1 shows baseline recipient and donor characteristics in groups defined by DGF, which occurred in 34% (n=230) of recipients. Recipients with DGF had a higher percentage of individuals who were male, black race, and receiving chronic dialysis pre-transplant. Recipients with DGF also tended to have slightly higher body mass index and longer dialysis vintage. Pump duration and cold ischemia time were significantly longer in the group with DGF. DCD status was the only donor characteristic that was significantly different (37% with DGF vs. 21% without DGF, p<0.001). Notably, the KDPI was similar between groups, with a median cohort value of 62 [40–79].
Figure 1.
Study flow diagram.
Table 1.
Characteristics of kidney transplant recipients and allografts by DGF status
| ALL (N=671) | DGF (N=230) | No DGF (N=441) | P 1 | ||
|---|---|---|---|---|---|
| Recipient Characteristics | |||||
| Age, years | 54.7 (15) | 55.1 (13.7) | 54.4 (15.6) | 0.938 | |
| Male | 420 (63%) | 157 (68%) | 263 (60%) | 0.028 | |
| Black race | 288 (43%) | 116 (50%) | 172 (39%) | 0.005 | |
| Previous kidney transplant | 74 (11%) | 23 (10%) | 51 (12%) | 0.539 | |
| Cause of ESRD | Other or unknown | 113 (17%) | 38 (17%) | 75 (17%) | 0.512 |
| Diabetes | 202 (30%) | 77 (33%) | 125 (28%) | ||
| Hypertension | 192 (29%) | 67 (29%) | 125 (28%) | ||
| Glomerulonephritis | 117 (17%) | 35 (15%) | 82 (19%) | ||
| Graft failure | 47 (7%) | 13 (6%) | 34 (8%) | ||
| Panel reactive antibody | 0% | 464 (69%) | 165 (72%) | 299 (68%) | 0.217 |
| 1–20% | 48 (7%) | 18 (8%) | 30 (7%) | ||
| 21–80% | 93 (14%) | 23 (10%) | 70 (16%) | ||
| >80% | 66 (10%) | 24 (10%) | 42 (10%) | ||
| Pre-transplant dialysis | 614 (92%) | 219 (95%) | 395 (90%) | 0.013 | |
| Body mass index | 28.4 (5.8) | 29.4 (5.7) | 27.9 (5.8) | 0.002 | |
| Duration of dialysis before transplant, months2 | 59.1 (35.8) | 62.1 (33.3) | 57.4 (37) | 0.017 | |
| HLA mismatch level | 5 [4–5] | 5 [4–5] | 5 [4–5] | 0.019 | |
| Cold ischemia time, hours | 18 [14–22] | 19 [15–23] | 17 [13–22] | 0.008 | |
| Pump duration, hours | 10 [6–15] | 11 [7–15] | 10 [6–14] | 0.015 | |
| Allograft (Donor) Characteristics | |||||
| Age, years | 46.8 (13.7) | 46.7 (13) | 46.9 (14.1) | 0.446 | |
| Male | 415 (62%) | 153 (67%) | 262 (59%) | 0.072 | |
| Black race | 102 (15%) | 43 (19%) | 59 (13%) | 0.069 | |
| ECD | 205 (31%) | 56 (24%) | 149 (34%) | 0.012 | |
| DCD | 175 (26%) | 84 (37%) | 91 (21%) | <0.001 | |
| Kidney donor risk index | 1.4 [1.1–1.7] | 1.4 [1.2–1.6] | 1.4 [1.1–1.7] | 0.844 | |
| Kidney donor profile index, % 3 | 62 [40–79] | 61 [45–76.5] | 63 [38–81] | 0.849 | |
| Hypertension | 262 (39%) | 81 (35%) | 181 (41%) | 0.142 | |
| Diabetes | 73 (11%) | 19 (8%) | 54 (12%) | 0.116 | |
| Height, cm | 170.4 (11.1) | 170.6 (11.6) | 170.3 (10.9) | 0.520 | |
| Weight, kg | 83.3 (22.5) | 84.8 (25.3) | 82.5 (21) | 0.387 | |
| Stroke as cause of death | 289 (43%) | 90 (39%) | 199 (45%) | 0.137 | |
| Terminal serum creatinine, mg/dL | 1.22 (1.01) | 1.4 (1.29) | 1.12 (0.82) | 0.207 | |
Values are mean (SD), median [interquartile range], or n (%). DGF, delayed graft function; ESRD, end-stage renal disease; HLA, human leukocyte antigen; DCD, donation after circulatory determination of death; ECD, expanded criteria donor (defined as donor age ≥60 years or 50–59 years with 2 or more of the following risk factors: terminal serum creatinine >1.5 mg/dl, history or hypertension, stroke as the cause of death).
Wilcoxon rank sum test for continuous variables and Chi-Square test for categorical variables
Among those with pre-transplant dialysis
Relative to all US deceased donors in 2010
Perfusate Biomarkers and Pump Parameters: Trends and Correlations
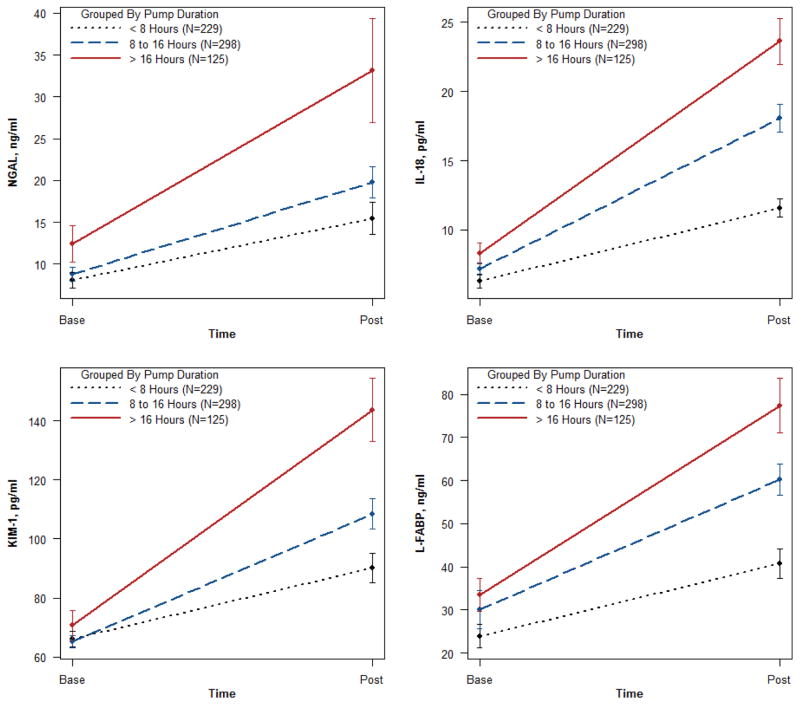
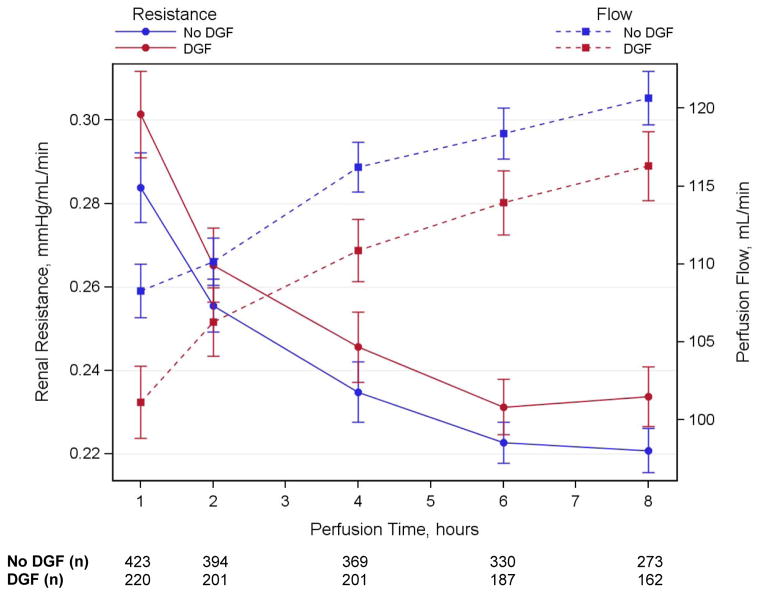
The concentrations of all biomarkers significantly increased during HMP from base to post time-points. This relationship was observed for varying lengths of pump durations, demonstrating a progressive release of all biomarkers into the perfusate during the pumping process (Figure 2). Spearman rank correlations between base and post perfusate concentrations for each biomarker were modest and ranged from 0.27 to 0.53, and the correlations were even more variable between different biomarkers, with a range from 0.01 to 0.79 (Supplemental Table S2). The length of time varied for the post perfusate collection, and Supplemental Figure S1 shows that the concentration for each biomarker was modestly correlated with HMP duration (range from 0.2 to 0.38). Renal resistance progressively decreased and perfusion flow increased during the period of HMP (Figure 3). The biomarker concentrations and renal resistances between kidneys from the same donor at each time-point were strongly correlated, with a range from 0.4 to 0.9 (Supplemental Table S2).
Figure 2.
Perfusate biomarker levels at the beginning (base) and end of perfusion (post) by strata of pump duration. Values are Mean ± SE.
Figure 3.
Mean renal resistance and perfusion flow over time by DGF. Error bars are ± 1 SE. DGF, delayed graft function.
Associations with DGF
Table 2 lists median perfusate biomarker concentrations. Base NGAL concentration was significantly higher in the DGF group. For KIM-1, approximately 90% of base values and 53% of post values were below the detection limits, and detectability did not differ by DGF status. For IL-18, base values trended higher among kidneys that developed DGF (p=0.055), whereas the post values were significantly higher in the DGF group. Both base and post perfusate concentrations of L-FABP were significantly higher in the DGF group. No independent relationships were detected, however, for any of the biomarkers after adjusting for donor, transport and recipient variables (Table 3). Delta concentration (“post” minus “base”) also failed to attain independent significance for any perfusate biomarker when compared between groups defined by DGF status.
Table 2.
Observed perfusate biomarker concentrations and pump parameters by DGF status
| Biomarker | Time Point (N) | ALL (N=671) | DGF (N=230) | No DGF (N=441) | P 1 |
|---|---|---|---|---|---|
| NGAL, ng/mL | Base (671) | 4.2 [1.1, 10.4] | 5.6 [1.7, 12.6] | 3.7 [0.9, 9.3] | 0.004 |
| Post (590) | 9.7 [5.3, 18.4] | 10.3 [5.5, 23] | 9.5 [5.2, 17.2] | 0.143 | |
| Delta (590) | 4.4 [1, 11.4] | 4.7 [1.2, 14.9] | 4.4 [1, 10.4] | 0.473 | |
| IL-18, pg/mL | Base (664) | 4.1 [2.6, 8.3] | 4.6 [2.6, 8.8] | 3.9 [2.6, 8.1] | 0.055 |
| Post (591) | 12.3 [7.27, 21.7] | 13.4 [8.51, 24.0] | 11.8 [6.6, 20.5] | 0.005 | |
| Delta (584) | 6.9 [2.73, 14.2] | 8.0 [3.8, 17.2] | 6 [1.91, 12.9] | 0.002 | |
| KIM-1, pg/mL | Base (626) | 59.0 [59.0, 59.0] | 59.0 [59.0, 59.0] | 59.0 [59.0, 59.0] | 0.466 |
| Post (590) | 59.0 [59.0, 129] | 60.4 [59.0, 133] | 59.0 [59.0, 124] | 0.219 | |
| Delta (549) | 0 [0, 67.6] | 2.96 [0, 75.7] | 0 [0, 61.5] | 0.243 | |
| L-FABP, ng/mL | Base (671) | 10.7 [3.7, 29.2] | 13.5 [5.1, 32.6] | 9.8 [3.4, 26.2] | 0.029 |
| Post (591) | 33.8 [14.9, 76.9] | 46.3 [15.8, 98.5] | 28.1 [14, 69.3] | 0.006 | |
| Delta (591) | 14.2 [5.3, 39.2] | 19.3 [6.8, 53.48] | 13.15 [4.8, 34.8] | 0.017 | |
| Resistance, mmHg/mL/min | 1 hr (643) | 0.25 [0.19, 0.35] | 0.27 [0.2, 0.37] | 0.25 [0.19, 0.33] | 0.039 |
| 2 hr (595) | 0.24 [0.18, 0.31] | 0.25 [0.19, 0.32] | 0.24 [0.17, 0.31] | 0.266 | |
| 4 hr (570) | 0.22 [0.17, 0.28] | 0.23 [0.18, 0.29] | 0.22 [0.16, 0.28] | 0.102 | |
| 6 hr (517) | 0.22 [0.16, 0.28] | 0.22 [0.17, 0.28] | 0.22 [0.16, 0.28] | 0.280 | |
| 8 hr (435) | 0.22 [0.16, 0.28] | 0.23 [0.16, 0.28] | 0.21 [0.16, 0.28] | 0.233 | |
| Delta 2–1 h (592) | −0.02 [−0.04, 0] | −0.02 [−0.05, 0] | −0.02 [−0.04, 0] | 0.130 | |
| Delta 4–1 h (567) | −0.03 [−0.07, −0.01] | −0.04 [−0.09, −0.01] | −0.03 [−0.07, −0.01] | 0.206 | |
| Delta 6–1 h (514) | −0.04 [−0.09, −0.01] | −0.05 [−0.11, −0.01] | −0.04 [−0.09, −0.01] | 0.318 | |
| Delta 8–1 h (431) | −0.05 [−0.1, −0.02] | −0.05 [−0.12, −0.02] | −0.05 [−0.1, −0.02] | 0.397 | |
| Flow, mL/min | 1 hr (643) | 103 [82, 128] | 98 [77, 124] | 106 [84, 134] | 0.011 |
| 2 hr (595) | 106 [88, 126] | 103 [84, 125] | 108 [89, 128] | 0.075 | |
| 4 hr (570) | 112 [94, 131] | 109 [92, 130] | 114 [96, 133] | 0.040 | |
| 6 hr (517) | 115 [97, 132] | 113 [94, 131] | 117 [100, 133] | 0.117 | |
| 8 hr (433) | 117 [101, 134] | 113 [98, 132] | 119 [103, 136] | 0.112 | |
| Delta 2–1 h (592) | 3.5 [−5, 13.5] | 4.5 [−3, 17] | 3 [−6, 11] | 0.082 | |
| Delta 4–1 h (567) | 7 [−4, 22] | 9 [−2.5, 24] | 6 [−5, 21] | 0.196 | |
| Delta 6–1 h (514) | 12 [−3, 30] | 15 [−1, 32] | 11 [−5, 28] | 0.190 | |
| Delta 8–1 h (429) | 17 [−1, 36] | 18 [0, 36] | 16 [−1.5, 36] | 0.322 |
Values are median [interquartile range]. DGF, delayed graft function; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid-binding protein. Delta for NGAL, KIM-1-, IL-18 and L-FABP calculated as Post minus Base; delta for perfusion parameters calculated as shown.
Wilcoxon rank-sum test (DGF vs. non-DGF)
Table 3.
Association of perfusate biomarkers and pump parameters with DGF
| Biomarker | Time Point (N) | Relative Risk (95% confidence interval) for DGF | ||
|---|---|---|---|---|
| Unadjusted | Adjusted for donor variables only 1 | Adjusted for donor, transport & recipient variables 2 | ||
| Log NGAL | Base (671) | 1.11 (1.04, 1.18) | 1.05 (0.99, 1.12) | 1.05 (0.98, 1.12) |
| Post (590) | 1.08 (1.00, 1.17) | 1.00 (0.91, 1.10) | 1.02 (0.93, 1.12) | |
| Delta (590) | 0.96 (0.89, 1.03) | 0.96 (0.89, 1.03) | 0.97 (0.90, 1.04) | |
| Log IL-18 | Base (664) | 1.10 (0.98, 1.23) | 1.04 (0.94, 1.15) | 1.03 (0.93, 1.14) |
| Post (591) | 1.17 (1.05, 1.30) | 1.08 (0.97, 1.22) | 1.08 (0.96, 1.21) | |
| Delta (584) | 1.09 (0.97, 1.23) | 1.05 (0.94, 1.17) | 1.05 (0.95, 1.16) | |
| Log KIM-1 | Base (626) | 1.27 (1.06, 1.52) | 1.12 (0.95, 1.34) | 1.13 (0.96, 1.33) |
| Post (590) | 1.07 (0.93, 1.22) | 0.96 (0.83, 1.11) | 0.95 (0.82, 1.10) | |
| Delta (549) | 1.02 (0.88, 1.19) | 0.95 (0.83, 1.10) | 0.94 (0.82, 1.08) | |
| Log L-FABP | Base (671) | 1.07 (1.01, 1.13) | 1.04 (0.98, 1.10) | 1.03 (0.97, 1.09) |
| Post (591) | 1.10 (1.02, 1.19) | 1.05 (0.97, 1.13) | 1.04 (0.96, 1.12) | |
| Delta (591) | 1.00 (0.93, 1.07) | 0.99 (0.93, 1.06) | 1.00 (0.94, 1.06) | |
| Resistance, 0.1 mmHg/mL/min | 1 hr (643) | 1.03 (0.98, 1.09) | 1.04 (0.98, 1.10) | 1.04 (0.98, 1.10) |
| 2 hr (595) | 1.03 (0.95, 1.12) | 1.03 (0.95, 1.13) | 1.03 (0.94, 1.12) | |
| 4 hr (570) | 1.03 (0.96, 1.10) | 1.04 (0.97, 1.12) | 1.06 (0.98, 1.14) | |
| 6 hr (517) | 1.07 (0.95, 1.21) | 1.09 (0.95, 1.25) | 1.08 (0.95, 1.23) | |
| 8 hr (435) | 1.11 (0.98, 1.27) | 1.16 (1.01, 1.33) | 1.14 (1.00, 1.30) | |
| Flow, 10 mL/min | 1 hr (643) | 0.96 (0.93, 1.00) | 0.96 (0.93, 1.00) | 0.96 (0.94, 0.99) |
| 2 hr (595) | 0.97 (0.94, 1.01) | 0.97 (0.94, 1.01) | 0.98 (0.94, 1.02) | |
| 4 hr (570) | 0.96 (0.93, 1.00) | 0.96 (0.93, 0.99) | 0.96 (0.93, 1.00) | |
| 6 hr (517) | 0.97 (0.93, 1.00) | 0.96 (0.93, 1.00) | 0.97 (0.93, 1.01) | |
| 8 hr (433) | 0.96 (0.92, 1.01) | 0.95 (0.91, 0.99) | 0.96 (0.93, 1.00) | |
Biomarkers were added individually to all models (i.e., biomarkers were not combined during multivariable adjustment). Perfusate biomarkers were log2-transformed. Delta indicates log2(Post/Base). DGF, delayed graft function; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein. Values in parenthesis in represent number of observations for unadjusted model.
Donor variables used for adjustment: age, circulatory death (rather than brain death), black race, hypertension, diabetes, height, weight, stroke as cause of death, and terminal serum creatinine.
Includes all variables listed above plus the following transport and recipient variables: cold ischemia time, age, black race, gender, previous kidney transplant, diabetes as the cause of end stage renal disease, number of human leukocyte antigen mismatches, body mass index, duration (vintage) of dialysis before transplant, and panel reactive antibody (%).
Pump parameters stratified by DGF status are presented in Table 2 and Figure 3. Multivariable analyses revealed that 1-hour perfusate flow was independently and inversely associated with DGF (Table 3). An increase in flow of 10 ml/min at 1 hour was associated with 4% lower risk of DGF after adjusting for donor, transport and recipient variables. Individual C-statistics for predicting DGF ranged from 0.52 to 0.57 for the perfusate biomarkers and pump parameters. The full clinical model for DGF (adjusting for donor, transport and recipient variables but without perfusate biomarkers or pump parameters) had a C-statistic of 0.71 and increased to 0.72 (95% CI: 0.67, 0.76) with the addition of 1-hour perfusate flow.
Associations with 6-month Allograft Function
Multivariable analyses of the associations between perfusate biomarkers and pump parameters with 6-month eGFR are presented in Table 4. Each doubling of post-perfusate NGAL concentration was independently associated with a 1.7 ml/min/1.73m2 lower adjusted eGFR at 6 months. Post-perfusate L-FABP was also independently associated with lower adjusted 6-month eGFR by 1.48 ml/min/1.73m2 for each doubling of the biomarker concentration. At neither time-point was perfusate IL-18 nor KIM-1 independently associated with 6-month allograft function.
Table 4.
Association of perfusate biomarkers and pump parameters with 6-month eGFR
| Biomarker | Time Point (N) | Linear Regression Coefficient (95% confidence interval) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted for donor variables only 1 | Adjusted for donor, transport & recipient variables 2 | ||
| Log NGAL | Base (668) | −0.23 (−1.32, 0.85) | −0.81 (−1.95, 0.33) | −0.68 (−1.78, 0.42) |
| Post (588) | −0.40 (−1.80, 1.00) | −1.45 (−2.98, 0.07) | −1.70 (−3.22, −0.18) | |
| Delta (588) | −0.35 (−1.44, 0.73) | −0.25 (−1.29, 0.79) | −0.58 (−1.62, 0.46) | |
| Log IL-18 | Base (661) | −0.11 (−2.23, 2.02) | −0.24 (−2.23, 1.74) | −0.15 (−2.06, 1.77) |
| Post (589) | −1.94 (−3.79, −0.09) | −1.76 (−3.61, 0.09) | −1.70 (−3.55, 0.15) | |
| Delta (582) | −1.91 (−3.66, −0.16) | −1.41 (−3.05, 0.24) | −1.62 (−3.27, 0.02) | |
| Log KIM-1 | Base (623) | −2.37 (−7.41, 2.66) | −1.89 (−6.78, 3.00) | −1.67 (−6.06, 2.72) |
| Post (588) | −0.94 (−3.14, 1.25) | −0.37 (−2.52, 1.78) | −0.38 (−2.67, 1.90) | |
| Delta (547) | −1.09 (−3.24, 1.06) | −0.36 (−2.40, 1.67) | −0.25 (−2.38, 1.87) | |
| Log L-FABP | Base (668) | −0.31 (−1.23, 0.60) | −0.56 (−1.41, 0.28) | −0.40 (−1.23, 0.43) |
| Post (589) | −1.65 (−2.87, −0.42) | −1.64 (−2.87, −0.40) | −1.48 (−2.69, −0.27) | |
| Delta (589) | −0.75 (−1.77, 0.28) | −0.35 (−1.44, 0.74) | −0.53 (−1.62, 0.55) | |
| Resistance, 0.1 mmHg/mL/min | 1 hr (640) | −1.61 (−2.80, −0.42) | −0.93 (−2.08, 0.21) | −1.11 (−2.25, 0.03) |
| 2 hr (592) | −2.76 (−4.79, −0.72) | −1.57 (−3.45, 0.31) | −1.63 (−3.45, 0.19) | |
| 4 hr (568) | −2.34 (−3.87, −0.81) | −1.06 (−2.32, 0.20) | −1.47 (−2.81, −0.14) | |
| 6 hr (515) | −3.81 (−5.96, −1.66) | −1.66 (−3.76, 0.44) | −2.48 (−4.55, −0.42) | |
| 8 hr (434) | −3.76 (−5.94, −1.58) | −2.01 (−4.17, 0.15) | −3.01 (−5.18, −0.83) | |
| Flow, 10 mL/min | 1 hr (640) | 0.89 (0.38, 1.41) | 0.63 (0.13, 1.13) | 0.71 (0.21, 1.20) |
| 2 hr (592) | 1.07 (0.49, 1.66) | 0.75 (0.19, 1.31) | 0.73 (0.18, 1.28) | |
| 4 hr (568) | 1.13 (0.51, 1.75) | 0.66 (0.08, 1.23) | 0.81 (0.25, 1.37) | |
| 6 hr (515) | 0.97 (0.30, 1.63) | 0.52 (−0.09, 1.12) | 0.65 (0.04, 1.27) | |
| 8 hr (432) | 1.18 (0.44, 1.91) | 0.86 (0.18, 1.55) | 0.94 (0.28, 1.59) | |
Perfusate biomarkers were log2-transformed. Biomarkers and pump parameters were added individually to all linear regression models (i.e., biomarkers were not combined during multivariable adjustment). Delta indicates log2(Post/Base). eGFR, estimated glomerular filtration rate; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein. Values in parenthesis in represent number of observations for unadjusted model.
Donor variables used for adjustment: age, circulatory death (rather than brain death), black race, hypertension, diabetes, height, weight, stroke as cause of death, and terminal serum creatinine.
Includes all variables listed above plus the following transport and recipient variables: cold ischemia time, age, Black race, gender, previous kidney transplant, diabetes as the cause of end stage renal disease, number of human leukocyte antigen mismatches, body mass index, duration (vintage) of dialysis before transplant, and panel reactive antibody (%).
Renal resistance and perfusate flow demonstrated strong trends as well as independent relationships with 6-month eGFR at several time-points. Each 0.1 mmHg/mL/min higher renal resistance at 4, 6 and 8 hours was associated with lower adjusted 6-month eGFR by 1.5, 2.5 and 3 ml/min/1.73m2, respectively. Each 10 ml/min faster perfusate flow at all time-points was also associated with statistically significantly higher adjusted 6-month eGFR, with increases in graft function ranging from 0.65 to 0.94 ml/min/1.73m2. When 1-hour flow was added to each adjusted biomarker model, the relationship with 6-month eGFR was attenuated and became non-significant for NGAL [adjusted regression coefficient (95% CI) from −1.7 (−3.22, −0.18) to −1.51 (−3.1, 0.08)] but remained significant for L-FABP [from −1.48 (−2.69, −0.27) to −1.46 (−2.69, −0.23)]. The regression coefficient for 1-hour flow was also attenuated at 0.54 (0.02, 1.05) and 0.55 (0.04, 1.06) after adjusting for NGAL and L-FABP, respectively.
Organ Discard Status
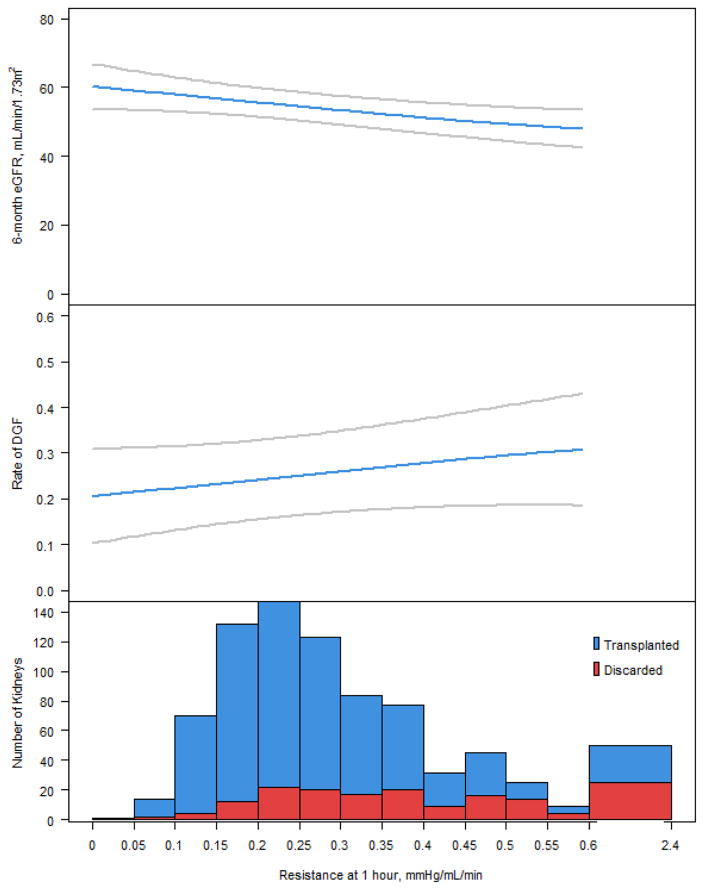
A total of 198 pumped kidneys from 124 enrolled donors were excluded from the primary analyses because of discard. Both kidneys were discarded from 74 donors, and 50 donors had one discarded kidney (while the other was transplanted). Of the discarded kidneys, 23 were not used because of anatomical reasons or increased risk of communicable disease, and biomarker measurements were not available for 3 discarded kidneys. Characteristics of the remaining 172 discarded kidneys are given in Table 5. As shown in Table 6, median resistance was significantly higher and median flow was significantly lower at all time-points in discarded kidneys when compared to transplanted kidneys either with or without DGF. For discarded kidneys, median perfusate biomarker concentrations were not demonstrably different (or were even lower in terms of base NGAL and base L-FABP) compared with transplanted kidneys. There was a step-wise increase in the proportion of kidneys with higher renal resistance values that were discarded; however, the relationship with DGF and 6-month eGFR was not robust (Figure 4).
Table 5.
Kidney (donor) characteristics by discard status
| Kidney Characteristic | Discarded (N=172) | Transplanted (N=671) | P 1 |
|---|---|---|---|
| Age, years | 56.4 (13.5) | 46.8 (13.7) | <0.001 |
| Male | 89 (52%) | 415 (62%) | 0.016 |
| Black race | 39 (23%) | 102 (15%) | 0.019 |
| ECD | 106 (62%) | 205 (31%) | <0.001 |
| DCD | 27 (16%) | 175 (26%) | 0.004 |
| Kidney donor profile index, % | 88 [69–96] | 62 [40–79] | <0.001 |
| Kidney donor risk index 1 | 1.90 [1.50–2.32] | 1.40 [1.12–1.70] | <0.001 |
| Hypertension | 116 (67%) | 262 (39%) | <0.001 |
| Diabetes | 7 (4%) | 73 (11%) | 0.007 |
| Height, cm | 169 (10) | 170 (11) | 0.026 |
| Weight, kg | 88.9 (26.8) | 83.3 (22.5) | 0.015 |
| Stroke as cause of death | 115 (67%) | 289 (43%) | <0.001 |
| Terminal serum creatinine, mg/dL | 1.5 (1.05) | 1.22 (1.01) | <0.001 |
| Pump Duration, hours | 9 [7–13] | 10 [6–15] | 0.373 |
| Discard reason provided to OPTN | |||
| Biopsy findings | 77 (45%) | ||
| No recipient located | 53 (31%) | ||
| Other 2 | 21 (12%) | ||
| Poor organ function | 10 (6%) | ||
| Too old on pump | 6 (3%) | ||
| Donor medical history | 2 (1%) | ||
| Warm ischemic time too long | 2 (1%) | ||
| Organ not as described | 1 (1%) | ||
Values are mean (SD), median [interquartile range], or n (%). ESRD, end-stage renal disease; DCD, donation after cardiac death; ECD, expanded criteria donor; OPTN, Organ Procurement and Transplantation Network.
Wilcoxon rank-sum test (discarded vs. transplanted)
Additional text field information available for 18 of these kidneys suggested 11 were discarded because of machine perfusion parameters.
Table 6.
Biomarkers and perfusion parameters, by organ discard and DGF status
| Biomarker | Time Point | Discarded Kidneys (N=172) | Transplanted & DGF (N=230) | Transplanted & Non-DGF (N=441) | P-value (Discard vs. DGF) | P-value (Discard vs. Non-DGF) |
|---|---|---|---|---|---|---|
| NGAL, ng/mL | Base (843) | 3.75 [0, 9.2] | 5.6 [1.7, 12.6] | 3.7 [0.9, 9.3] | 0.005 | 0.372 |
| Post (713) | 10.2 [6.1, 18.5] | 10.3 [5.5, 23] | 9.5 [5.2, 17.2] | 0.977 | 0.200 | |
| Delta (713) | 6.8 [1.7, 14.6] | 4.7 [1.2, 14.9] | 4.4 [1, 10.4] | 0.216 | 0.034 | |
| IL-18, pg/mL | Base (829) | 4.4 [2.58, 7.63] | 4.63 [2.58, 8.83] | 3.89 [2.58, 8.1] | 0.246 | 0.738 |
| Post (714) | 12.7 [8.7, 21.3] | 13.4 [8.5, 24.0] | 11.8 [6.6, 20.5] | 0.453 | 0.111 | |
| Delta (701) | 7.4 [3.5, 13.8] | 8.01 [3.8, 17.2] | 6 [1.9, 12.9] | 0.713 | 0.029 | |
| KIM-1, pg/mL | Base (779) | 59.0 [59.0, 59.0] | 59.0 [59.0, 59.0] | 59.0 [59.0, 59.0] | 0.230 | 0.467 |
| Post (713) | 59.0 [59.0, 111] | 60.4 [59.0, 133] | 59.0 [59.0, 124] | 0.549 | 0.762 | |
| Delta (656) | 0 [0, 53.2] | 2.96 [0, 75.7] | 0 [0, 61.5] | 0.827 | 0.507 | |
| L-FABP, ng/mL | Base (843) | 7.1 [1.75, 23.7] | 13.5 [5.1, 32.6] | 9.8 [3.4, 26.2] | <.001 | 0.017 |
| Post (714) | 31.3 [15, 53.7] | 46.3 [15.8, 98.5] | 28.1 [14, 69.3] | 0.020 | 0.985 | |
| Delta (714) | 18.9 [9.3, 34.3] | 19.3 [6.8, 53.5] | 13.15 [4.8, 34.8] | 0.862 | 0.025 | |
| Resistance, mmHg/mL/min | 1 hr (808) | 0.37 [0.26, 0.51] | 0.27 [0.2, 0.37] | 0.25 [0.19, 0.33] | <.001 | <.001 |
| 2 hr (744) | 0.34 [0.24, 0.44] | 0.25 [0.19, 0.32] | 0.24 [0.17, 0.31] | <.001 | <.001 | |
| 4 hr (726) | 0.32 [0.22, 0.41] | 0.23 [0.18, 0.29] | 0.22 [0.16, 0.28] | <.001 | <.001 | |
| 6 hr (660) | 0.31 [0.2, 0.39] | 0.22 [0.17, 0.28] | 0.22 [0.16, 0.28] | <.001 | <.001 | |
| 8 hr (549) | 0.32 [0.21, 0.38] | 0.23 [0.16, 0.28] | 0.21 [0.16, 0.28] | <.001 | <.001 | |
| Delta 2-1 h (738) | −0.03 [−0.06, 0] | −0.02 [−0.05, 0] | −0.02 [−0.04, 0] | 0.179 | 0.003 | |
| Delta 4-1 h (720) | −0.05 [−0.11, −0.02] | −0.04 [−0.09, −0.01] | −0.03 [−0.07, −0.01] | 0.132 | 0.003 | |
| Delta 6-1 h (654) | −0.07 [−0.12, −0.03] | −0.05 [−0.11, −0.01] | −0.04 [−0.09, −0.01] | 0.058 | 0.001 | |
| Delta 8-1 h (542) | −0.07 [−0.13, −0.03] | −0.05 [−0.12, −0.02] | −0.05 [−0.1, −0.02] | 0.136 | 0.013 | |
| Flow, mL/min | 1 hr (807) | 74 [54, 106] | 98 [76.5, 124] | 106 [84, 134] | <.001 | <.001 |
| 2 hr (744) | 82 [65, 105] | 103 [84, 125] | 108 [89, 128] | <.001 | <.001 | |
| 4 hr (726) | 87 [67.5, 116] | 109 [92, 130] | 114 [96, 133] | <.001 | <.001 | |
| 6 hr (660) | 90 [69, 118] | 113 [94, 131] | 116.5 [100, 133] | <.001 | <.001 | |
| 8 hr (547) | 93 [68, 117] | 113 [98, 132] | 119 [103, 136] | <.001 | <.001 | |
| Delta 2-1 h (737) | 4 [−2, 12] | 4.5 [−3, 17] | 3 [−6, 11] | 0.528 | 0.306 | |
| Delta 4-1 h (719) | 8 [0, 22] | 9 [−2.5, 24] | 6 [−5, 21] | 0.829 | 0.124 | |
| Delta 6-1 h (653) | 12 [1, 26] | 15 [−1, 32] | 11 [−5, 28] | 0.610 | 0.406 | |
| Delta 8-1 h (540) | 15 [3, 30] | 18 [0, 36] | 16 [[−1.5, 36] | 0.434 | 0.942 |
Values are medians [interquartile range]. DGF, delayed graft function; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein. Values in parenthesis in represent number of observations used in analysis. P-values obtained by Wilcoxon rank sum tests comparing discarded kidneys with transplanted kidneys that either did or did not develop DGF as indicated. Bonferroni-adjusted alpha is 0.025.
Figure 4.
Association of 6-month eGFR and DGF with deciles of renal resistance at 1 hour and proportion of discarded kidneys.
Upper panel: Smoothed curve depicting relationship of 6-month estimated glomerular filtration rate (eGFR) to renal resistance, estimated from a generalized additive model with multivariable adjustments; categorical adjustments are fixed at the most common value, and continuous adjustments are fixed at the cohort average. Middle panel: Smoothed curve depicting relationship of delayed graft function (DGF) to renal resistance, estimated from a generalized additive model with multivariable adjustments as above. Lower panel: histogram of renal resistance values, categorized by discard status.
Additional Secondary Outcomes and Sensitivity Analysis
PNF was reported for 15 (2%) recipients. At 6 months post-transplant, there were 52 (8%) overall graft losses with 33 (5%) death-censored graft failures and 27 (4%) recipient deaths. Neither perfusate NGAL nor L-FABP nor resistance at any time-point were independently associated with PNF; however, each 10 ml/min increase in 2-hour perfusate flow was associated with PNF with an adjusted relative risk of 0.84 (0.72, 0.97). Neither perfusate NGAL nor L-FABP nor either pump parameter at any time-point were independently associated with 6-month overall graft loss or with recipient mortality at 6 months. Similarly, there were no significant associations for perfusate NGAL, L-FABP or pump parameters with 6-month death-censored graft failure.
A sensitivity analysis for 6-month eGFR, in which the 52 overall graft losses were excluded, produced slightly attenuated but otherwise similar results compared with the primary analysis. The fully adjusted linear coefficients for post-perfusate NGAL and L-FABP and for 1-hour perfusate flow were −1.52 (−2.92, −0.11), −1.37 (−2.5, −0.24) and 0.73 (0.26, 1.19), respectively.
Discussion
This is the largest prospective, multicenter cohort study of perfusate biomarkers and pump parameters that reports associations with graft outcomes. Our data indicate that kidney injury biomarkers are released into perfusate and increase in concentration over time. The perfusate biomarkers and pump parameters were only modestly correlated with one another, but there was high correlation of these parameters between left and right kidneys from the same donor. One-hour flow was associated with DGF, but we did not detect an independent association between the injury biomarkers and DGF. However, perfusate NGAL and L-FABP measured near the end of HMP as well as pump parameters (resistance and flow) were modestly associated with 6-month eGFR. We also demonstrated that discarded kidneys have substantially less favorable pump parameters than transplanted kidneys, suggesting that transplant centers are actively utilizing these assessments as criteria for organ acceptance/refusal.
Following our recent systematic review (12), additional studies have evaluated several biochemical analytes in perfusate. In a subset of 111 kidneys from the Eurotransplant trial, Nagelschmidt et al. showed that levels of total GST, alpha-GST and lipid peroxidation products (LPOP) at the end of HMP were higher in kidneys that developed DGF (24). However, only LPOP remained significantly associated with DGF after multivariable adjustment, and no biomarkers were associated with later outcomes. Hoogland et al. measured 4-hour perfusate samples from 335 transplanted DCD kidneys and noted independent associations with primary non-function for LDH and IL-18 (25). Total GST, heart-type fatty acid-binding protein, redox-active iron, and NGAL were not associated with primary non-function, and no measured biomarkers were associated with 1-year graft failure. Snoeijs et al. used 1-hour perfusate samples from 18 kidneys to demonstrate that alpha1-antritrypsin was up-regulated in kidneys that developed DGF (26). Guy et al. performed 1D proton-nuclear magnetic resonance (1H-NMR) spectroscopy on 45-minute and 4-hour perfusate samples from 26 kidneys to show that 28 different metabolites vary in concentration throughout HMP, while certain metabolites (leucine, inosine, gluconate and glucose) predicted DGF with areas under the curve ranging between 0.732 and 0.895 (27). Overall, no perfusate biomarker has yet emerged as a useful diagnostic tool to predict graft outcomes although the field remains dynamic with many biomarkers being investigated.
Our study advances existing knowledge about biomarkers of acute injury and pump parameters for perfused kidneys. We evaluated the potential utility for measuring perfusate NGAL, IL-18, KIM-1 and L-FABP relative to DGF as well as 6-month allograft function. We assessed some of the best-studied biomarkers of ischemia-reperfusion injury which have shown promise when measured in serum or urine in patients with acute kidney injury. NGAL and LFABP are approved as biomarkers of kidney injury in Europe and available for clinical use in Japan (28, 29). The development of point-of-care measurement tools for these biomarkers expands their potential for use in clinical practice, even in settings such as allograft evaluation which require rapid decision-making. However, the modest associations that we observed for perfusate NGAL and L-FABP toward the end of HMP with subsequent allograft function indicate that, at the individual patient level in the current cohort, these biomarkers have poor prognostic utility.
Although our study provides supportive data about the value of HMP parameters relative to allograft function, consistent with two recent European studies (16, 30), our data also indicate that transplant professionals may be relying too much on discrete cut-points to make organ acceptance/refusal decisions. Notably, 90% of resistance measurements from our cohort (including kidneys that were ultimately discarded) ranged between 0.1 and 0.6 mmHg/mL/min. Other investigators have considered a resistance cutoff of >0.3 mmHg/mL/min as “the earliest significant predictor of 1-year allograft outcome” (31). Given the limitations of current evidence, however, we believe that specific numerical thresholds for pump resistance and/or flow are not justifiable and would lead to inappropriately higher kidney discard rates where many viable kidneys may be lost. Just as the transplant community has begun to consider donor risk on a more continuous scale (i.e., KDRI, which incorporates several more risk factors compared with the dichotomous ECD designation), we need to consider the most effective way to incorporate HMP physical parameters like renal resistance and perfusate flow into scores like the KDRI to communicate risk in a more continuous and probabilistic manner.
Important study strengths are the generalizability of this multicenter cohort, data collected in real-world practice and the relatively large sample size. Study limitations stem from the observational design. Specifically, each OPO followed its own policy in determining which kidneys would undergo HMP. Similarly, the “post” value for perfusate biomarkers was assessed at a variable time-point (dependent on when the kidney left the control of the OPO en route to the recipient), and many factors could have influenced pump duration. In this cohort, kidneys with DGF were pumped longer. However, the absolute difference in pump time was small (median of 11 vs. 10 hours for non-DGF kidneys). We focused on biomarkers of ischemia-reperfusion injury in the perfusate, but other metabolites and biomarkers involved in other pathways could also be helpful in this setting. Detailed and standardized central pathology interpretation of kidney histology at the time of procurement would be very informative but is not available within the OPTN/UNOS database. Lastly, despite rigorous adjustment for donor, transplant and recipient variables, residual confounding may persist.
Renewed interest in HMP could help expand the kidney donor pool because of its apparent therapeutic effect but also by allowing for more accurate kidney quality assessment. As demonstrated in the current study, early perfusate flow was independently associated with DGF, while perfusate flow throughout HMP, renal resistance beginning at 4 hours, and specific perfusion biomarkers toward the end of HMP independently associated with allograft function at 6 months. However, the meagerness of associations indicates that these pump parameters and perfusate biomarkers have insufficient prognostic utility at the individual kidney-recipient level. The larger impact of other donor kidney characteristics, logistical concerns, and the modest effects of these HMP-derived measurements on 6-month eGFR argue against relying heavily on the pump parameters and biomarkers for organ discard decisions. These parameters may only add value in situations where clinicians have equipoise about discard. Given the ever-widening gap between organ supply and demand, the use of perfusion biomarkers and pump parameters relative to kidney allocation acceptance/refusal decisions warrants further critical inquiry. Regarding future prospective research, the transplant community should evaluate other commercially available HMP devices (along with newer technology/devices as they become available) and employ standardized kidney procurement, HMP protocols and perfusate biomarker assessment to further elucidate the predictive ability of novel combinations with regard to longer-term allograft outcomes. Continued research in this area is clearly needed in hopes of increasing access to larger pools of kidneys while simultaneously improving kidney allograft function to reduce the need for re-transplantation.
Supplementary Material
Acknowledgments
Dr. Parikh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Parikh also affirms that he has listed everyone who made substantial contributions to this work. We appreciate the assistance of Dr. Eoin Cotter at the Royal College of Surgeons in Ireland and Dr. Peter Doran at the University College Dublin Clinical Research Center (UCD CRC) Biomarker Laboratory for performing NGAL assays. We are tremendously grateful for the study participation of partners at four organ procurement organizations: Gift of Life Philadelphia (Rick Hasz, Sharon West, Vicky Reilly), the New York Organ Donor Network (Harvey Lerner, Anthony Guidice, Allison Hoffman), the Michigan Organ and Tissue Donation Program (Burton Mattice, Susan Shay), and the New Jersey Sharing Network (William Reitsma, Cindy Godfrey, Alene Steward, Joel Padilla Benitez). This work was supported by 1) the National Institutes of Health grant R01DK-93770, grant K24DK090203, 2) a Roche Organ Transplantation Research Foundation Award to Dr. Parikh, 3) a career development award from the American Heart Association to Dr. Hall, and 4) the Health Resources and Services Administration contract 234-2005-37011C. NGAL assays were donated by Abbott Diagnostics and measured at the UCD CRC Biomarker Laboratory in Dublin, Ireland. L-FABP assays were donated by Sekisui Medical Co. Ltd. The companies did not participate in design, analysis, or interpretation of study results.
Abbreviations
- 1H-NMR
1D proton-nuclear magnetic resonance
- CV
coefficient of variation
- DCD
donation after circulatory determination of death
- DGF
delayed graft function
- ECD
expanded criteria donor
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- GST
glutathione S-transferase
- HMP
hypothermic machine perfusion
- HRSA
Health Resources and Services Administration
- IL-18
interleukin-18
- KDPI
kidney donor profile index
- KDRI
kidney donor risk index
- KIM-1
kidney injury molecule-1
- L-FABP
liver-type fatty acid-binding protein
- LPOP
lipid peroxidation products
- NGAL
neutrophil gelatinase-associated lipocalin
- OPO
organ procurement organization
- OPTN
Organ Procurement and Transplantation Network
- UCD CRC
University College Dublin Clinical Research Center
- UNOS
United Network for Organ Sharing
Footnotes
Disclaimer
The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
Table S1: Donor characteristics for pumped versus cold-stored kidneys.
Table S2: Spearman rank correlations between biomarkers of transplanted kidneys and between kidneys from the same donor.
Figure S1: Post-perfusate biomarker concentrations by pump duration.
References
- 1.Belzer FO, Kountz SL. Preservation and transplantation of human cadaver kidneys: a two-year experience. Annals of Surgery. 1970;172(3):394–404. doi: 10.1097/00000658-197009000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):973–86. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 3.Cannon RM, Brock GN, Garrison RN, Smith JW, Marvin MR, Franklin GA. To pump or not to pump: a comparison of machine perfusion vs cold storage for deceased donor kidney transplantation. Journal of the American College of Surgeons. 2013;216(4):625–33. doi: 10.1016/j.jamcollsurg.2012.12.025. discussion 33–4. [DOI] [PubMed] [Google Scholar]
- 4.O'Callaghan JM, Morgan RD, Knight SR, Morris PJ. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. The British journal of surgery. 2013;100(8):991–1001. doi: 10.1002/bjs.9169. [DOI] [PubMed] [Google Scholar]
- 5.Gill J, Dong J, Eng M, Landsberg D, Gill JS. Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time. Transplantation. 2014;97(6):668–74. doi: 10.1097/01.TP.0000438637.29214.10. [DOI] [PubMed] [Google Scholar]
- 6.Moers C, Pirenne J, Paul A, Ploeg RJ. Machine perfusion or cold storage in deceased-donor kidney transplantation. The New England journal of medicine. 2012;366(8):770–1. doi: 10.1056/NEJMc1111038. [DOI] [PubMed] [Google Scholar]
- 7.Watson CJ, Wells AC, Roberts RJ, Akoh JA, Friend PJ, Akyol M, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10(9):1991–9. doi: 10.1111/j.1600-6143.2010.03165.x. [DOI] [PubMed] [Google Scholar]
- 8.Jochmans I, Moers C, Smits JM, Leuvenink HGD, Treckmann J, Paul A, et al. Machine Perfusion Versus Cold Storage for the Preservation of Kidneys Donated After Cardiac Death A Multicenter, Randomized, Controlled Trial. Annals of Surgery. 2010;252(5):756–62. doi: 10.1097/SLA.0b013e3181ffc256. [DOI] [PubMed] [Google Scholar]
- 9.Jochmans I, Pirenne J. Graft quality assessment in kidney transplantation: not an exact science yet! Curr Opin Organ Transplant. 2011;16(2):174–9. doi: 10.1097/MOT.0b013e3283446b31. [DOI] [PubMed] [Google Scholar]
- 10.Dare AJ, Pettigrew GJ, Saeb-Parsy K. Preoperative assessment of the deceased-donor kidney: from macroscopic appearance to molecular biomarkers. Transplantation. 2014;97(8):797–807. doi: 10.1097/01.TP.0000441361.34103.53. [DOI] [PubMed] [Google Scholar]
- 11.Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess. 2009;13(38):iii–iv. xi–xiv, 1–156. doi: 10.3310/hta13380. [DOI] [PubMed] [Google Scholar]
- 12.Bhangoo RS, Hall IE, Reese PP, Parikh CR. Deceased-donor kidney perfusate and urine biomarkers for kidney allograft outcomes: a systematic review. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(8):3305–14. doi: 10.1093/ndt/gfr806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall IE, Bhangoo RS, Reese PP, Doshi MD, Weng FL, Hong K, et al. Glutathione S-transferase iso-enzymes in perfusate from pumped kidneys are associated with delayed graft function. Am J Transplant. 2014;14(4):886–96. doi: 10.1111/ajt.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozaki K, Sakurai E, Kubota K, Iwamoto H, Hama K, Narumi Y, et al. Prediction of kidney nonfunction after transplantation with machine perfusion preservation. Transplantation Proceedings. 2000;32(2):275–6. doi: 10.1016/s0041-1345(99)00955-0. [DOI] [PubMed] [Google Scholar]
- 15.Talbot D, Shenton BK, Buckley PE, Gok MA. Experiences learned in the successful establishment of a nonheart beating donor program for renal transplantation. J Urol. 2003;170(4 Pt 1):1088–92. doi: 10.1097/01.ju.0000086774.12582.0f. [DOI] [PubMed] [Google Scholar]
- 16.Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am J Transplant. 2011;11(10):2214–20. doi: 10.1111/j.1600-6143.2011.03685.x. [DOI] [PubMed] [Google Scholar]
- 17.Cho YW, Bunnapradist S, Cho ES, Stadtler M, Simmons V, Locke J, et al. Can machine perfusion decrease the likelihood of discard among biopsied kidneys? Transplant Proc United States. 2008;40:1029–31. doi: 10.1016/j.transproceed.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Sung RS, Christensen LL, Leichtman AB, Greenstein SM, Distant DA, Wynn JJ, et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant. 2008;8(4):783–92. doi: 10.1111/j.1600-6143.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 19.van Smaalen TC, Hoogland ER, van Heurn LW. Machine perfusion viability testing. Curr Opin Organ Transplant. 2013;18(2):168–73. doi: 10.1097/MOT.0b013e32835e2a1b. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–6. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 22.A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI) 2012 Available from: http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf.
- 23.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 24.Nagelschmidt M, Minor T, Gallinat A, Moers C, Jochmans I, Pirenne J, et al. Lipid peroxidation products in machine perfusion of older donor kidneys. The Journal of surgical research. 2013;180(2):337–42. doi: 10.1016/j.jss.2012.04.071. [DOI] [PubMed] [Google Scholar]
- 25.Hoogland ER, de Vries EE, Christiaans MH, Winkens B, Snoeijs MG, van Heurn LW. The Value of Machine Perfusion Biomarker Concentration in DCD Kidney Transplantations. Transplantation. 2013;95(4):603–10. doi: 10.1097/TP.0b013e31827908e6. [DOI] [PubMed] [Google Scholar]
- 26.Snoeijs MG, Pulinx B, van Dieijen-Visser MP, Buurman WA, van Heurn LW, Wodzig WK. Characterization of the perfusate proteome of human donor kidneys. Annals of clinical biochemistry. 2013;50(Pt 2):140–6. doi: 10.1258/acb.2012.011144. [DOI] [PubMed] [Google Scholar]
- 27.Guy AJ, Nath J, Cobbold M, Ludwig C, Tennant DA, Inston NG, et al. Metabolomic analysis of perfusate during hypothermic machine perfusion of human cadaveric kidneys. Transplantation. 2015;99(4):754–9. doi: 10.1097/TP.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 28.Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, et al. A rapid urine test for early detection of kidney injury. Kidney international. 2009;76(1):108–14. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh CR. A point-of-care device for acute kidney injury: a fantastic, futuristic, or frivolous 'measure'? Kidney international. 2009;76(1):8–10. doi: 10.1038/ki.2009.125. [DOI] [PubMed] [Google Scholar]
- 30.de Vries EE, Hoogland ER, Winkens B, Snoeijs MG, van Heurn LW. Renovascular resistance of machine-perfused DCD kidneys is associated with primary nonfunction. Am J Transplant. 2011;11(12):2685–91. doi: 10.1111/j.1600-6143.2011.03755.x. [DOI] [PubMed] [Google Scholar]
- 31.Yushkov YY, Stern J, Ying A, Icitovic N, Dikman SH, Sheth M, et al. Identifying risk factors in renal allografts before transplant: machine-measured renal resistance and posttransplant allograft survival. Progress in transplantation. 2012;22(2):175–82. doi: 10.7182/pit2012968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.