Abstract
Background
Increased levels of impulsivity are associated with increased illicit drug use and alcoholism. Previous research in our lab has shown that increased levels of delay discounting (a decision-making form of impulsivity) are related to appetitive processes governing alcohol self-administration as opposed to purely consummatory processes. Specifically, the high seeking/high drinking alcohol preferring P rats showed increased delay discounting compared to nonselected Long Evans rats (LE) whereas the high drinking/moderate seeking HAD2 rats did not (Beckwith & Czachowski, 2014). The P rats also displayed a perseverative pattern of behavior such that during operant alcohol self-administration they exhibited greater resistance to extinction.
Methods
One explanation for the previous findings is that P rats have a deficit in response inhibition. The current study followed up on this possibility by utilizing a countermanding paradigm [stop signal reaction time (SSRT) task] followed by operant self-administration of alcohol across increasing fixed ratio requirements (FR; 1, 2, 5, 10 & 15 responses). In separate animals, 24hr access 2-bottle choice (10% EtOH vs. water) drinking was assessed.
Results
In the SSRT task, P rats exhibited an increased SSRT compared to both LE and HAD2 rats indicating a decrease in behavioral inhibition in the P rats. Also, P rats showed increased operant self-administration across all FRs and the greatest increase in responding with increasing FR requirements. Conversely, the HAD2 and LE had shorter SSRT, and lower levels of operant alcohol self-administration. However, for 2 bottle choice drinking HAD2s and P rats consumed more EtOH as well as had a greater preference for EtOH compared to LE.
Conclusions
These data extend previous findings showing the P rats to have increased delay discounting (decision-making impulsivity) and suggest that P rats also have a lack of behavioral inhibition (motor impulsivity). This supports the notion that P rats are a highly impulsive as well as “high seeking” model of alcoholism, and that the HAD2s elevated levels of alcohol consumption are not mediated via appetitive processes or impulsivity.
Keywords: Alcohol Self-Administration, Rat, Impulsivity, Selected Line
Introduction
Impulsivity can be conceptualized as an inability to stop a behavior that has adverse consequences as well as exhibiting behaviors that are poorly controlled, lacking foresight, or inappropriate to the situation. With a lack of behavioral control as a cornerstone, impulsivity has considerable definitional overlap with addiction. DSM criteria, past and present, have included using substances despite adverse consequences, using in inappropriate situations, and an inability to stop or cut down on usage (American Psychiatric Association, 1994, 2013). However, the relationship between impulsivity and addiction is more than definitional overlap. Numerous studies have found that individuals who abuse drugs and alcohol have higher levels of impulsivity compared to control subjects, and it is hypothesized that elevated levels of impulsivity may cause and/or be caused by drug abuse (for review: Perry & Carroll, 2008).
One caveat with regard to the relationship between impulsivity and addiction is that “impulsivity” is not a unitary construct. Different self-report measures of impulsivity do not intercorrelate strongly (Cyders & Coskunpinar, 2011). Also behavioral measures of impulsivity are pharmacologically dissociable (Evenden, 1999), and while there is overlap, different neural structures are implicated in these different forms of impulsivity (for review see: Dalley, Everitt, & Robbins, 2011). Similar to our previous body of work that showed that ethanol-seeking and ethanol consumption are moderated by different neural structures (e.g., Czachowski, 2005; Czachowski et al., 2012) and drug treatments (e.g., Verplaetse et al., 2012; Henderson-Redmond & Czachowski, 2014), the present study is part of an ongoing series of experiments assessing the different behavioral impairments encompassed by “impulsivity” that may be risk factors for increased appetitive and/or consummatory behaviors. We hypothesize that excessive behavior that is poorly controlled will be related to appetitive processes while internalizing-like processes such as passive avoidance and anxiety will be related to consummatory processes.
Recently our lab found that increased levels of delay discounting track specifically with appetitive processes governing alcohol self-administration (Beckwith & Czachowski, 2014). The high alcohol-drinking (HAD2) rats, who exhibit a high level of alcohol consumption (>1.25g/kg in less than 20 minutes; Czachowski & Sampson, 2002) but only moderate levels of alcohol seeking comparable to unselected Long Evans (LE) rats (Czachowski & Samson, 1999, 2002; Beckwith & Czachowski, 2014), did not differ in delay discounting as compared to LE rats. However, alcohol preferring P rats who display elevated levels of alcohol seeking, as much as 4 fold greater than the HAD2s (Czachowski & Samson 2002), possessed greater levels of delay discounting compared to both LEs and HAD2s. The P rats also displayed increased resistance to extinction of operant responding for alcohol. This pattern of findings suggests that the P rats may have impaired response inhibition as well as increased delay discounting.
One way to determine if a response inhibition deficit exists is to use a stop signal reaction time (SSRT) task. This paradigm is a simple countermanding task wherein a subject has to stop an already initiated response before it is completed. Whether or not the behavior is inhibited depends on if a “go” or “stop” process finishes first (Logan, 1994). By biasing this “race” between these two processes in favor of one or the other via altering the starting time of the stop process, one is able to obtain a single, quantitative measure of the time it takes to stop a behavior, or SSRT. Shorter SSRTs correspond to greater behavioral control and less impulsivity because, with all other variables being equal, shorter SSRTs should result in more inappropriate responses being successfully inhibited.
Since our previous findings indicated that P rats showed increased delay discounting (Beckwith and Czachowski, 2014), the present study investigated possible behavioral inhibition and motor impulsivity deficits and whether or not these deficits were specifically related to appetitive as opposed to consummatory processes governing alcohol self-administration. To this end, P rats were compared to HAD2s and LEs on a SSRT task. Subjects then underwent a simple fixed ratio self-administration procedure with increasing fixed ratio requirements across days. It was hypothesized that lower levels of response inhibition (i.e., higher levels of motor impulsivity) would be present in only the P rats as measured in the SSRT task. Also it was predicted that in the fixed ratio self-administration sessions the P rats would “defend” their intake of alcohol by increasing their responding while the HAD2s and LEs would not.
Materials and Methods
Subjects
The subjects were HAD2s (65th generation) and P rats (77th generation) from the Indiana University School of Medicine and LE rats (Harlan, Indianapolis, IN; n=12/strain). All animals were male and age matched at approximately 50 days old resulting in average weights (±SEM) of 182±4, 272±7, and 278±5 grams for the HAD2s, P rats, and LE respectively at the start of experiments. Animals had ad libitum access to food, were singly housed in plastic shoebox cages, and maintained on 12 hour light/dark cycles. Sessions were conducted 5 days a week during the light cycle. For the SSRT task, animals were water restricted receiving access to 0.035ml of water per gram of body weight up to a maximum of 15ml one hour after the session for a period of 30 minutes. For the operant alcohol self-administration sessions, water restriction was only in place to initiate lever pressing (2 sessions). Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (2011) and had Institutional Animal Care and Use Committee approval.
Apparatus
All sessions were conducted in modular chambers with electrical inputs and outputs controlled by an IBM compatible PC (Med-Associates, St. Albans, VT; 30×30×24.5cm). All chambers had a stainless steel bar floor, house light, and were enclosed in sound attenuating boxes with exhaust fans for ventilation and masking external noise. Chambers were equipped with a nosepoke recess with an internal stimulus light and photocell to record beam breaks centered on the front wall 2cm above the floor. A 4500Hz tone generator was located 18cm above the nosepoke. On both sides of the nosepoke recess were retractable levers with stimulus lights 4cm above each lever. A retractable graduated cylinder tube with a rubber stopper, stainless steel spout with double ball bearings, and a lickometer was located opposite the nosepoke.
SSRT Task
The SSRT task was based on the procedure of Eagle and Robbins (2003) and incorporated elements from Feola et al. (2000) (i.e., titrating reinforcer magnitude based on GoRT). In brief, subjects completed a chained response consisting of a nosepoke followed by a lever press on each trial (go response; GoRSP). On a subset of trials (20%) a stop signal (SS) was presented after the animal nosepoked indicating that the GoRSP needed to be inhibited to receive reinforcement and avoid punishment (5 second timeout). A delay between the nosepoke and the SS (stop signal delay; SSD) was used on the majority of the “stop trials.” The SSD varied pseudo-randomly in order to differentially bias the race between the stop and go process. The percent inhibition as a function of the SSD was then used to estimate the SSRT.
Training was conducted in daily 20 minute sessions and began by providing subjects with 200 “free licks” at the sipper tube which contained 2% sucrose (w/v; in tap water). After receiving the licks, the sipper tube was retracted and then presented non-contingently for 15 seconds on a VT15 second schedule for 10 minutes. In the following sessions, subjects were handshaped to nosepoke on a FR1 schedule for 10 (1st session) and 5 seconds (subsequent sessions) of access to the sipper tube. After subjects acquired nosepoking, the lever to the left of the nosepoke was extended. Nosepoking now resulted in the stimulus light above the extended lever turning on and the availability of the reinforcer for depressing the lever. Initially, 10 seconds of access was provided for completing the chained response while animals were handshaped to press the lever after nosepoking. The access time was then decreased to 5 seconds and the maximum number of trials was set to 100. Once subjects completed the chained response at least 75 times in 20 minutes, the access duration was titrated based upon GoRT and a limited hold on the GoRSP were introduced. Failure to respond before the limited hold expired resulted in a 5 second timeout. During timeouts, all lights were extinguished and there were no programed consequences on any response manipulandum.
The sipper access was titrated using a simple quasi-hyperbolic equation for each trial wherein the maximum sipper access duration was divided by the GoRT times a coefficient (F) plus a constant (1). In this fashion longer GoRTs were reinforced with shorter sipper access times and vice versa. This procedure was used to reinforce animals for responding as quickly as possible and negatively punish animals for “waiting” to see if a SS would be presented. The equation used is displayed below:
RFT is the access time in seconds, and F is the coefficient. The values of F were optimized during pilot studies and are displayed in Table S1 along with a full layout of the training. This method allows for a total possible range of reinforcer magnitudes between 4 seconds and asymptotically approaching zero. In the final stage of the paradigm, the sipper access on each trial ranged between 1.39 and 3.2 seconds with a mean of 2.38 seconds (SD=.41).
Throughout training F was increased across sessions along with decreases in the limited hold. Specifically, every three sessions where the animal completed at least 70 trials in 20 minutes the parameters were changed. F started at .25 and increased to 1.25. This increase resulted in sipper access being decreased to a greater degree for longer GoRTs. The limited hold began at the 75th percentile GoRT or 3 seconds (whichever was greater) slowly changed to 1.75 seconds. Then the limited hold was set individually for each subject based on their median GoRT from go trials only (mGoRT). For 2 sessions, the limited hold was set at their average mGoRT over the past 3 sessions plus an additional .7 seconds. The additional time then decreased to .6 for two sessions and then to .5.
After the limited hold reached mGoRT+.5 seconds and subjects completed 70% of trials, the SS was introduced. The SS consisted of an 80dB tone being played with the stimulus light above the extended lever extinguishing and the light above the retracted lever turning on. On stop trials, the SS was presented without any delay after the subject nosepoked. If the animal completed the GoRSP it was punished with a 5 second timeout. Access to the sipper tube for successful stop trials was based upon their last completed go trial. Stop trials accounted for 20% of trials and were assigned via block randomization such that 4 of every 20 consecutive trials were stop trials. If a subject completed the GoRSP before the SS, the trial was recoded as a go trial and in the next block there was one additional stop trial. Once subjects achieved 70% accuracy on both go and stop trials, at least 5 additional sessions were conducted. The additional sessions ensured that the final stage of the SSRT task began on the 2nd day of the week in which 4 new delays (SSD) were implemented in addition to stop trials without any SSD. The 4 SSDs were set relative to the mGoRT of each subject by subtracting .05, .1, .2, or .3 seconds from a three day average of the mGoRT in each session. The SSD for each stop trial was selected randomly. This phase continued for 9 sessions, and data represent averages of these 9 sessions where at least 70% go trial accuracy occurred.
The inhibition function was derived by calculating the probability of inhibiting the GoRSP at each SSD from subjects who completed at least 2 sessions with at least 70% go trial accuracy. These probabilities were then corrected for omissions using the method of Tannock et al. (1989): p(inhibition)corrected= [p(inhibition)observed − p(omission)] /[1−p(omission)]. SSRT was estimated by rank ordering the GoRTs and taking the nth GoRT [n= (1−p(inhibition)corrected) number of GoRTs in the distribution] and subtracting the SSD from the nth GoRT. This was done for every SSD delay where a minimum of at least 6 stop trials occurred. Each animal’s estimates were then averaged to yield a single estimate per subject. A second estimate of SSRT was also calculated using only the mGoRT-.1 and mGoRT-.2 SSDs. These SSDs were selected because the mean probability of inhibition collapsed across strain (mGoRT-.1=.59 & mGoRT-.2 SSD=.46) was within .1 of .5, collectively they spanned the 50% inhibition mark, and there were no pairwise differences between any strain at either of these 2 SSD.
Alcohol Self-Administration
Of the subjects who completed the SSRT task, 6 were selected randomly per strain. These animals completed daily, 20 minute, operant alcohol self-administration sessions. This procedure began with the lever to the right of the nosepoke (previously “unused” lever) extended and the lever to the left of the nosepoke (GoRSP lever in the SSRT) retracted. Similarly the stimulus light over the right lever was illuminated and the left was extinguished. Subjects were initially water restricted and were reinforced on an FR1 schedule with 20 second presentations of the sipper tube filled with 10% sucrose solution (w/v). The fixed ratio requirement was increased to an FR2, and after animals acquired responding (≥10 responses) they had ad lib access to water for the rest of the experiment. Over the next three weeks, subjects underwent a modified sucrose fading procedure (Samson, 1986) in which the concentration of sucrose was decreased as ethanol was increased until it reached 10% (v/v). During the third week, the left lever was extended into the chamber as an inactive lever with no programed consequences. All animals then responded for 10% EtOH for one week at an FR2. Over the next two weeks the FR increased in ascending order every 2 days from an FR1, FR5, FR10, & FR15 with the exception that on the first day of both weeks the schedule was an FR2.
Two-Bottle Choice
A separate group of HAD2s (60th & 62nd generation; n=11), P rats (74th & 75th generation, n=6), and LE (Harlan Indianapolis, IN; n=14) with similar experimental experience (see Beckwith & Czachowski, 2014) were assessed for free-choice drinking of both water and 10% ethanol. Animal, water, and ethanol bottle weights were obtained daily during the light cycle for 14 days. Bottle sides were alternated daily and two “leak cages” were utilized to account for spillage from the bottles. Daily body weights taken at the beginning and end of every 24hr period were averaged and then used to calculate g/kg intake for a given period. Ethanol preference was determined by dividing the volume of ethanol consumed by the total fluid volume consumed. Days in which there was a clear perturbation of a subject’s intake measure for any reason (e.g., excessive leakage) were replaced with the average of the period before and the period after.
Statistical Analysis
Animals were excluded for failing to learn the SSRT task or having an inverted inhibition function. This resulted in 7 animals being excluded and final n’s of 11, 6, & 12 for LEs, HAD2s, and P rats respectively. Mixed factorial analysis of variances (ANOVA) were used to examine the corrected inhibition functions with strain as the between subjects factor and SSD-mGoRT as the within subjects factor. Inhibition functions were also analyzed as a continuous variable. Linear regressions inside each strain were conducted with SSD, mGoRT-SSD, mGoRT-SSD-SSRT, and (mGoRT-SSD-SSRT)/GoRT standard deviation (SD) used as predictors, and differences in slope were assessed via Bonferonni corrected 95% confidence intervals (CIs). GoRTs were analyzed with a series of linear regressions. Across failed stop and go trials, trial type and strain were used as predictors with LEs serving as the comparator strain where appropriate. Inside of failed stop trials, SSD and SSRT were used as predictors in addition to strain. All other variables from the SSRT task were analyzed with univariate ANOVAs to examine strain differences. Variables characterizing general SSRT performance such as mGoRT, GoRT variance, and the mean number of trials completed were calculated for each session and then averaged across sessions.
Non-linear regression was used to analyze the alcohol self-administration data by fitting an exponential demand equation (Hursh & Silberberg, 2008):
Q is the number of reinforcer presentations. Both Q0 and α are free parameters describing the intensity (consumption when there is no cost) and elasticity (rate of change in consumption with increases in price) of demand respectively. k is a constant defined by the range of the dependent variable in logarithmic units (~1.24), and C is the cost of a reinforcer (i.e., FR).
Mixed ANOVAs were also used to examine the alcohol self-administration and home cage drinking with FR and/or day as the within subjects factor where appropriate. All main effects were followed up with Fishers least significant difference tests, and interactions were examined with Student t tests utilizing Bonferroni corrected alphas All variables were screened for normality and unequal variance using the Shapiro-Wilk and Levene’s test, and base 10 logarithmic transformations were used when necessary. Data were sorted with Microsoft Excel, and statistical tests were carried out in SPSS (22) and Graphpad Prism (4).
Results
SSRT Task
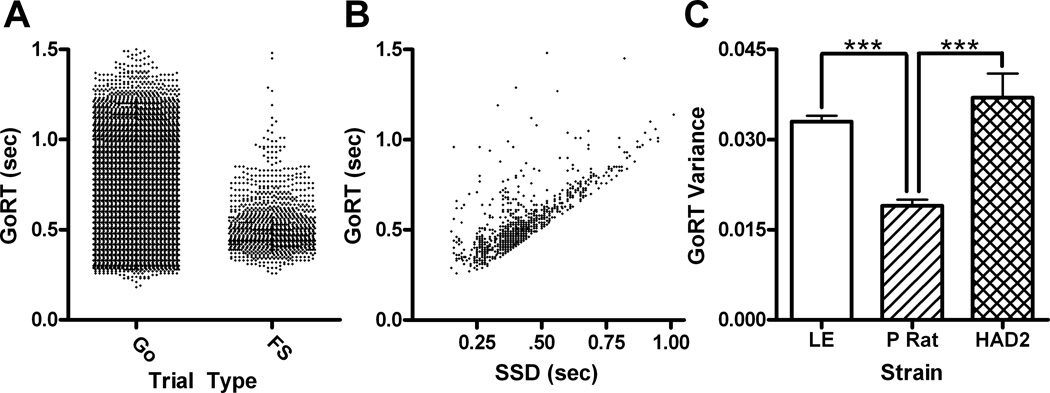
GoRTs were first examined with linear regression analyses to ensure they conformed to the race model as a manipulation check. GoRTs on stop trials in which the animal failed to inhibit a response were shorter. Also inside of failed stop trials GoRT was positively related to SSD-mGoRT and SSD (i.e., they became longer with increases in SSD). Both of these results conform to predictions based on the race model (Logan, 1994). Moreover these effects were present when strain was included in the model. Standard and unstandardized regression coefficients are presented in Tables 1 and 2, and GoRTs are graphed in Fig. 1a–b. The variance in individual GoRTs within each session was significantly different between the strains, F(2,24)=20.7, p<.001; P rats had less variance in their GoRT compared to both LE (p<.001) and HAD2s (p<.001) who weren’t different from each other (p=.22; Fig 1c). The average mGoRT across sessions had unequal variance between strains and were log transformed. The transformed values differed between strain, F(2,24)=13.4, p<.001, with the P rats having shorter average mGoRTs that both the LE (p<.001) and the HAD2s (p<.001) who were not different (p<.001). The untransformed values are presented in Table 3.
Table 1.
Multiple regression on GoRTs (sec) from both go trials and failed stop trials.
| Model Statistics | Predictor Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Step | R | F | p | Predictor | β | b | p |
| 1 | .362 | 1119.5 | <.001 | P Rat | −.295 | −.110 | <.001 |
| HAD2 | .104 | .051 | <.001 | ||||
| 2 | .368 | 772.7 | <.001 | P Rat | −.294 | −.110 | <.001 |
| HAD2 | .104 | .051 | <.001 | ||||
| Failed Stop Trial | −.063 | −.047 | <.001 | ||||
Table 2.
Multiple regression on GoRTs (sec) from failed stop trials.
| Model Statistics | Predictor Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Step | R | F | p | Predictor | β | b | p |
| 1 | .328 | 57.6 | <.001 | P Rat | −.261 | −.075 | <.001 |
| HAD2 | .103 | .041 | .005 | ||||
| 2 | .675 | 265.0 | <.001 | P Rat | −.016 | −.005 | .590 |
| HAD2 | −.034 | −.013 | .246 | ||||
| SSD | .680 | .791 | <.001 | ||||
| 3 | .678 | 202.7 | <.001 | P Rat | −.039 | −.011 | .203 |
| HAD2 | .006 | .002 | .850 | ||||
| SSD | .687 | .801 | <.001 | ||||
| SSRT | .093 | .334 | .003 | ||||
Fig. 1.
Go reaction times (GoRT) from the SSRT task. A) GoRT collapsed across strain and plotted based on trial type (Go versus failed stop; FS). B) GoRT on failed stop trials plotted as a function of SSD. C) Mean (±SEM) variance in GoRT per session graphed as a function of strain. *** P<.001 using Fischer’s LSD test.
Table 3.
Secondary variables from SSRT task.
| Strain | Trials | Go Trial Accuracy |
Trial Initiation Latency (sec) |
Median GoRT (sec) |
Sipper Access (sec) |
Limited Hold (sec) |
Omissions |
|---|---|---|---|---|---|---|---|
| LE | 79.6±3.6‡‡‡99.3±0.5## | .79±.01‡‡‡ | 4.8±.6‡‡ | .62±.04‡‡‡ | 2.23±.06‡‡‡ | 1.2. ±04‡‡‡ | 16.9±1.3‡‡ |
| P Rat | 79.5±7.8 | .89±.01### | 3.2±.2 | .48±.01### | 2.46±.02### | 1.0±.01### | 10.3±1.5 |
| HAD | .83±.02 | 3.3±.3& | .65±.04 | 2.18±.05 | 1.2±.04 | 14.5±2.7 |
All values are mean ±SEM.
P vs HAD2; & LE vs. HAD;
LE vs. P with 1, 2, & 3 symbols corresponding to p<.05, .01,
.001 on Fishers LSD test.
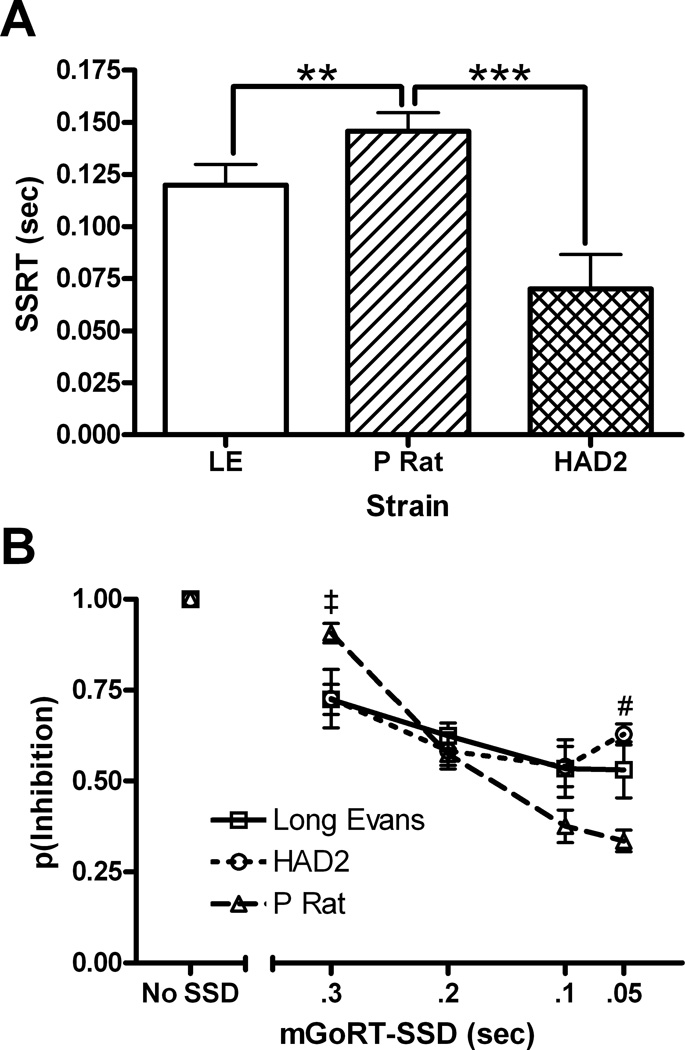
SSRT differed between strain, F(2,24)=11.2, p<.001; P rats exhibited longer SSRT than both LE (p<.01) and HAD2s (p<.001), but HAD2s and LEs were not different (p=.08; Fig. 2a). For the second means of calculating SSRT using only the .1 and .2 mGoRT-SSD SSDs, a univariate ANOVA found SSRT differed by strain, F(2,24)=7.2, p=.003. Fischer’s LSD post hoc tests revealed that the P rat had longer SSRTs than either the HAD2s (p=.001) or the LE (p=.02), but the HAD2s and LEs were not different (p=.21). The correlation between SSRT estimates derived using all the SSD versus only the .1 and .2mGoRT-SSD SSDs was very strong, r(25)=.856, p<.001. A mixed factorial ANOVA on the inhibition function (Fig. 2b) revealed no effect of strain, F(2,24)=2.0, p=.16, a main effect of SSD-mGoRT, F(4,96)=73.3, p<.001, and an interaction of both factors, F(8,96)=5.5, p<.001. When no SSD was used, all animals were able to inhibit their GoRSP. As the SS was presented closer to mGoRT, the p(inhibition) decreased. At .3 mGoRT-SSD, P rats had greater p(inhibition) than LE, and at .05 mGoRT-SSD P rats had a smaller p(inhibition) than HAD2s (Fig. 2b). Bonferroni corrected paired samples t-tests inside each strain showed all strains had decreased inhibition at the .2, .1, and .05 mGoRT-SSD compared to when no SSD was used; the LE also showed a significant difference at .3. However, only the P rats had decreased inhibition between the .3 versus .2 and .1 SSDs as well as between .2 versus .1 and .05 SSDs. All other within subjects pairwise comparisons were not significant.
Fig. 2.
Graphic depiction of the primary findings from the SSRT paradigm displaying impaired behavioral inhibition in the P rats. A) Mean (±SEM) SSRT estimate for each strain. B) Mean (±SEM) p(inhibition) graphed as a function of mGoRT-SSD. *, **, and *** correspond to p<.05, p<.01, and p<.001 using Fischer’s LSD test; #, ‡, and & indicate differences between P vs. HAD2, P vs. LE, and LE vs. HAD2 respectively using Student t tests after Bonferroni correction for multiple comparisons.
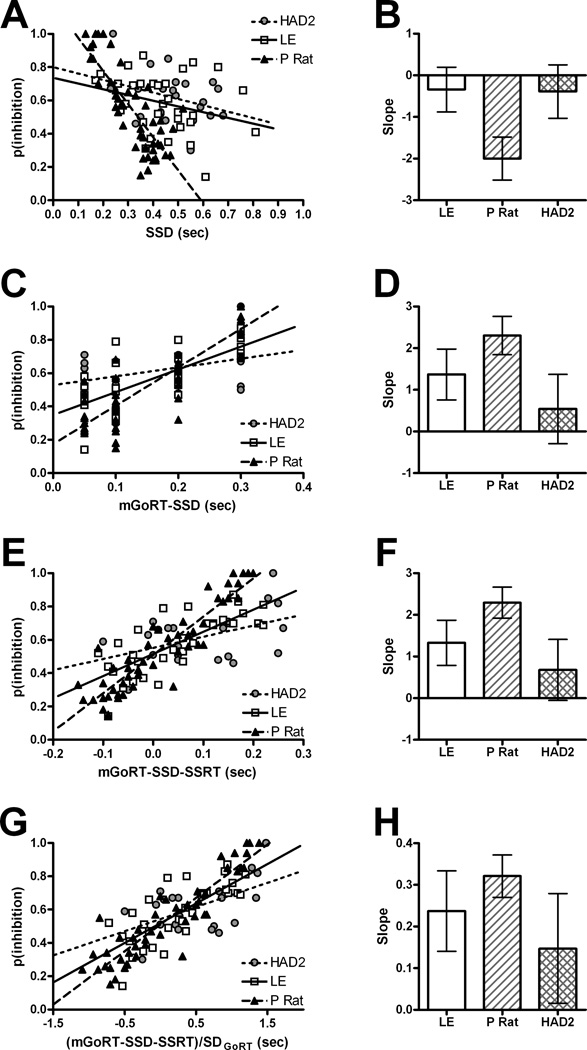
To aid in understanding the crossover interaction in the inhibition function, linear regression analyses were conducted on the p(inhibition)corrected. The linear regression analysis of the inhibition functions revealed that once differences in GoRT central tendency, variance, and SSRT were accounted for together there were no longer any differences between the strains for the slope of their regression lines. When SSD was used as a predictor (Fig. 3a–b), the slope CIs for the LE (−.88, .2) and HAD2s overlapped (−1.03, .25) but not the P rats’ (−2.52, −1.49). For mGoRT-SSD, which accounts only for GoRT central tendency (Fig. 3c–d), the LE CIs (.76, 1.98) overlapped with both the HAD2s (−.29, 1.37) and P Rats (1.84, 2.76) which did not overlap each other. When mGoRT-SSD-SSRT (Fig. 3e–f) was used as a predictor, only the LE (.78, 1.87) and HAD2s (−.05, 1.41) CIs overlapped (P rat: 1.92, 2.67). Finally when (mGoRT-SSD-SSRT)/SDGoRT (Fig. 3g–h) was used as a predictor all of the CIs overlapped (LE: .14, .33; HAD2 .02, .28; P rat: .27, .37). As this last analysis alone produced no differences between strains, only once differences in mGoRT, SSRT, and GoRT variance were accounted for did the inhibition functions become aligned. This finding suggests that all three are important for explaining differences in inhibition that exist between the three strains (Logan, 1994) and provides a rationale for the presence of the crossover interaction seen when only mGoRT is taken into account (Fig 2b).
Fig. 3.
Linear regression results treating inhibition functions as a continuous variable. A, C, E, & G display the scatter plots with regression lines for each predictor broken down by strain, and B, D, F, & H show the corresponding slopes of the regression lines and their Bonferroni corrected 95% confidence intervals (CI). A–B) p(inhibition) based on SSD. C–D) p(inhibition) by mGoRT-SSD E–F) p(inhibition) plotted mGoRT-SSD-SSRT G–H) p(inhibition) graphed as a function of mGoRT-SSD-SSRT divided by GoRT standard deviation (SDGoRT) to control for differences in GoRT variance.
Secondary variables from the SSRT task are presented in Table 3. The average number of total trials completed per session differed between strain, F(2,24)=11.1, p<.001; P rats completed more trials than LE (p<.001) and HAD2s (p<.01) who did not differ from each other (p=.99). Go trial accuracy also differed between strain, F(2,24)=12.0, p<.001 with P rats being the most accurate (p<.001; p<.01). For the median trial initiation latency per session, F(2,24)=5.9, p<.001, LE took longer to initiate trials compared to P rats (p<.01) and HAD2s (p<.05), P rats and HAD2s did not differ (p=.82). The mean sipper access differed between strains, F(2,24)=14.1, p<.001, with the P rats earning more sipper access per trial on average than both the LE (p<.001) and the HAD2 (p<.001; LE vs. HAD2 p=.45)
Operant Alcohol Self-Administration
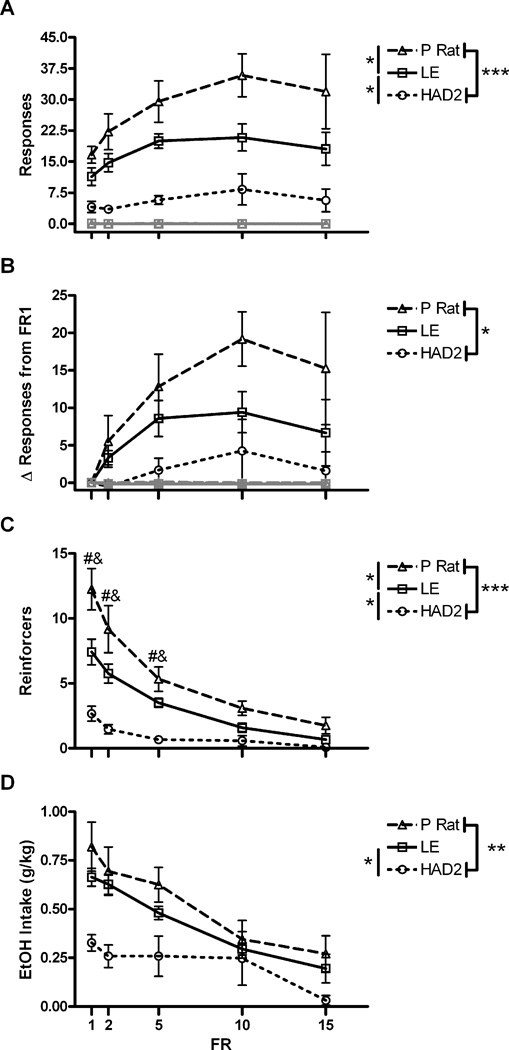
For active responses, there was a main effect of strain, F(2,15)=13.4, p<.001, a main effect of FR, F(4,60)=8.0, p<.001, but no interaction, F(8,60)=1.4, p=.21. P rats completed the most active responses (p<.05 vs. LE; p<.001 vs. HAD2s), and LE responded more than HAD2s (p<.05) (Fig 4a). By contrast there were no differences in inactive lever presses [strain: F(2,15)=0.6, p=.56; FR: F(4,60)=0.8,p=.53; FR by strain: F(8,60)=1.1,p=.38]. Examination of the change in active responses from the baseline FR 1 showed an effect of strain, F(2,15)=4.3, p<.05, FR, F(3,45)=4.5, p<.01, but no interaction, F(6,45)=.649, p=.69. The P rats showed a greater increase in responding compared to the HAD2s (p<.05), and the LE did not differ significantly between the P rats (p=.13) nor the HAD2s (p=.20; Fig. 4b). Inactive responses showed no significant differences [strain: F(2,15)=1.1, p=.35; FR: F(3,45)=1, p=.40; FR by strain: F(6,45)=1, p=.44]. Examination of the number of reinforcers earned exhibited effects of strain, F(2,15)=16.3, p<.001, FR, F(4,60)=73.8, p<.001, and an interaction, F(8,60)=9.7, p<.001. The interaction was driven by the LE and P rats earning more reinforcers at low but not high FR requirements (Fig. 4c). Alcohol intake (g/kg) differed between strain, F(2,15)=6.7, p<.01, and FR, F(4,60)=26.4, p<.001, and a trend toward an interaction of the two, F(8,60)=2.1, p=.052. Both the P rats (p<.01) and the LEs (p<.05) had higher alcohol intakes than the HAD2s (Fig. 4d).
Fig. 4.
Operant alcohol self-administration as a function of FR and strain. A) Mean (±SEM) responding on both the active (black) and inactive (grey) levers. B) Mean (±SEM) change in responding from responding at an FR1 on the active (black) and inactive (grey) levers. C) Mean (±SEM) number of reinforcer presentations earned. D) Mean (±SEM) intake of 10% EtOH. *, **, and *** correspond to p<.05, p<.01, and p<.001 using Fischer’s LSD test; #, ‡, and & indicate differences between P vs. HAD2, P vs. LE, and LE vs. HAD2 respectively using Student t tests after Bonferroni correction for multiple comparisons.
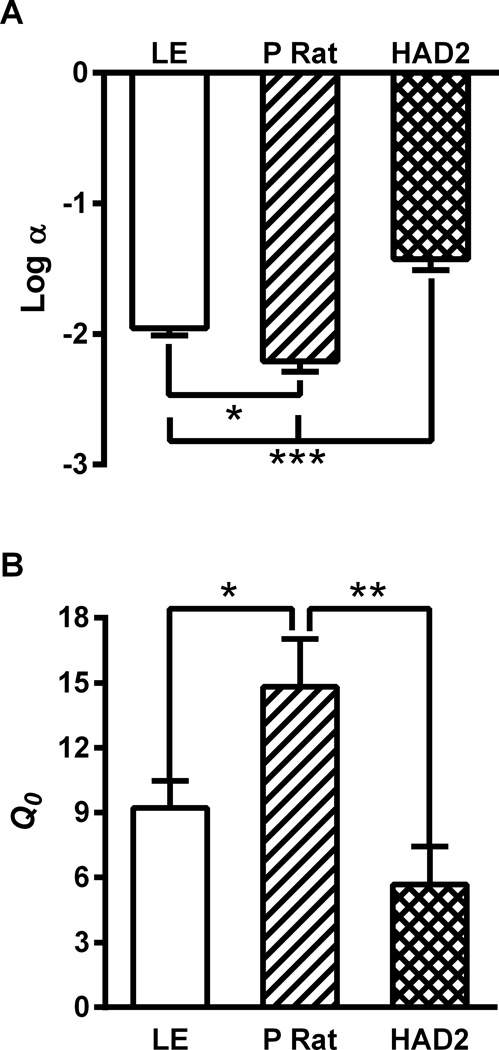
When fitting the exponential demand curve, 3 HAD2s were excluded as they did not yield enough non-zero data points to successfully fit a curve. The elasticity of demand, α (Fig 5a), was not normally distributed in either the HAD2s or the LE, displayed unequal variance between the strains, and was log transformed. The log transformed values differed by strain, F(2,12)=22.1, p<.001, with the P rats showing decreased elasticity compared to the LE (p<.05) and the HAD2s (p<.001). The LE also showed decreased elasticity compared to the HAD2s (p<.001). The intensity of demand, Q0 (Fig 5b), differed between strain, F(2,12)=5.4, p<.05. The P rats showed increased intensity of demand compared to both the LE (p<.05) and the HAD2s (p<.01) who were not different from each other (p=.26).
Fig. 5.
Free parameters from the exponential demand equation used to quantify the operant alcohol self-administration. A) Mean (±SEM) α values representing the elasticity of demand, the rate of change in the number of reinforcers earned with increases in cost (FR). B) The intensity of demand, reinforcers that would be earned at no cost, described by mean (±SEM) Q0 values. *, **, and *** correspond to p<.05, p<.01, and p<.001 using Fischer’s LSD test.
Two-Bottle Choice
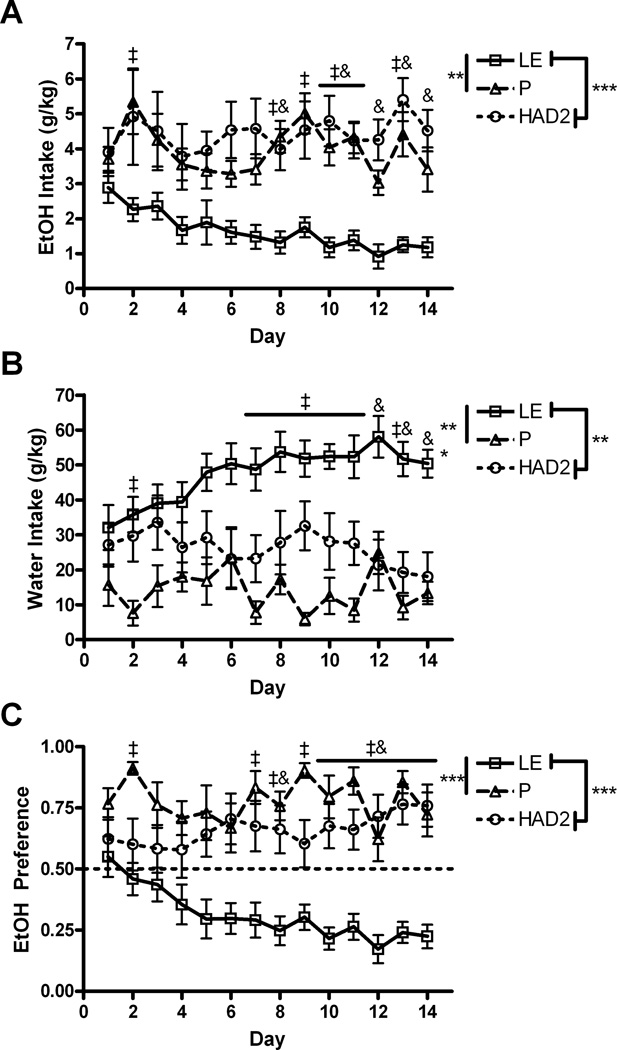
For intake of EtOH in g/kg, there was a main effect of strain, F(2,28)=11.4, p<.001, day, F(13,364)=2.6, p<.01, and an interaction of the two, F(26,364)=1.8, p<.05. Overall the Ps and HAD2s had higher intakes than the LEs especially after the 8th day (Fig. 6a). Water intake in g/kg showed a similar pattern with a main effect of strain, F(2,28)=79.0, p<.001, day, F(13,364)=1.8, p<.05, and an interaction of the two, F(26,364)=3.2, p<.001. However LE exhibited greater intake of water that increased across days (Fig. 6b). Ethanol preference showed main effects of strain, F(2,28)=12.7, p=<.001, day, F(13,364)=2.1, p<.05, and an interaction, F(26,364)=4.2, p<.001. Both the Ps and HAD2s were not different from each other and displayed greater ethanol preference than the LE. This difference became larger across days (Fig 6c).
Fig. 6.
24hr access two-bottle choice drinking of water and 10% EtOH. A) Mean (±SEM) EtOH intake in g/kg in each 24hr period. B) Mean (±SEM) water intake in g/kg. C) Mean (±SEM) EtOH preference score for each 24hr period. *, **, and *** correspond to p<.05, p<.01, and p<.001 using Fischer’s LSD test; #, ‡, and & indicate differences between P vs. HAD2, P vs. LE, and LE vs. HAD2 respectively using Student t tests after Bonferroni correction for multiple comparisons.
Discussion
Our recent work showed increased delay discounting and heightened appetitive drive for alcohol in P rats relative to both HAD2 rats and nonselected LE rats (Beckwith & Czachowski, 2014) and suggested that P rats may also exhibit a behavioral inhibition deficit. Based on this we tested P, LE, and HAD2 rats in a SSRT task followed by operant alcohol self-administration with an increasing response requirement. Our hypotheses that the P rats would show increased behavioral disinhibition and operant self-administration were supported. The SSRT estimate in the P rats was longer than both the LE and the HAD2s, and the P rats also subsequently responded more in an operant task and earned more reinforcers than the LE and HAD2s.
In the SSRT task, GoRTs were shorter on failed stop trials compared to go trials. Moreover on failed stop trials, GoRT was positively related to the length of the SSD. Both of these findings are consistent with predictions made by the race model (Logan, 1994). This theory characterizes countermanding behavior as a race between a go and stop process with the behavioral output of the animal determined by which process finishes first. As such, only the faster go process events (measured in GoRT) should escape inhibition by finishing before the stop process, and as SSD increases slower go process events would be allowed to escape inhibition as the go process essentially has a head start on the stop process. The confirmation of these predictions suggests that the current data can be adequately explained via the race model.
Consequently, the longer SSRT estimates of the P rats indicate that their stop processes are less efficacious and allow more go process events to escape inhibition, corresponding to a lack of behavioral inhibition. The shorter SSRTs of the LE and HAD2s allow fewer responses to escape and represent a tighter degree of behavioral control. Decreased behavioral inhibition in the P rats is also supported by the inhibition functions. At long SSD delays, the P rats showed a decreased p(inhibition), but at the shortest SSD they exhibited a greater p(inhibition). However, the inhibition functions are affected by the GoRT variance (Logan, 1994). The amount of inhibition is determined by how often, or what percentage of, go process events finish before the stop process. When the finishing times of the go process are more “spread out”, the ratio of the change in SSD to the distance (in time) between the finishing times of the go process is smaller. Consequently, a change in SSD no longer causes as large of a change in the proportion of go process events finishing before the stop process. The greater variance in GoRTs in both the LE and HAD2s compared to the P rats may result in their inhibition functions being “flatter” allowing P rats to display both greater and less inhibition despite having increased SSRT. This interpretation is supported by the fact that the linear regression analysis of the inhibition functions revealed that once differences in GoRT central tendency, variance, and SSRT were accounted for together there were no longer any differences between the strains for the slope of their regression lines.
It is possible that the additional ~.25 seconds of reinforcer access that the P rats received on average influenced their behavior. However, studies examining the effect of reward on SSRT have found that rewarding only the go trials increases SSRT (Padmala & Pessoa, 2010), while rewarding only stop trials or both stop and go trials actually decreases SSRT and improves inhibitory control (Boehler et al., 2012; Rosell-Negre et al., 2014). This literature suggests that if the additional reward that the P rats received affected their behavior, then it would likely be in the opposite direction of the observed differences in SSRT because both stop and go trials were rewarded to a greater degree in the P rats.
Importantly, the determination of behavioral inhibition or “motor impulsivity” was conducted in animals that were ethanol naïve. In the subsequent operant self-administration paradigm, P rats responded the most, earned the most reinforcers, and had the greatest increase in responding across increases in FR using an ethanol reinforcer. Moreover the P rats showed an increased intensity of demand and decreased elasticity of demand, indicating that they are consuming more when cost is negligible and that they show smaller decreases in consumption with increases in cost. Conversely, the HAD2s exhibited the lowest levels of responding, earned the least number of reinforcers, had the smallest increase in responding with increasing FRs, the lowest intensity of demand, and highest elasticity. This pattern reflects the differences in appetitive drive that have been seen previously in a variety of operant paradigms that use ethanol as a reinforcer (Beckwith & Czachowski, 2014; Files et al., 1998; Czachowski & Samson, 2002) and is consistent with the decreased GoRTs seen in the SSRT task that used a sucrose reinforcer. The low ethanol intake of the HAD2s during operant self-administration is likely due to the work requirement. As confirmed using the two bottle choice procedure, when exposed to “free” ethanol the Ps and HAD2s intake in g/kg and preference was equally high and far exceeded the LE intake and preference. Additionally this group has previously shown the HAD2s to drink as much (Samson et al., 1998) if not more than the P rats (Czachowski & Samson, 2002) under free access conditions.
The finding that elevated motor impulsivity is related to excessive drug seeking is consistent with other preclinical studies. Increased levels of premature responding in the 5 choice serial reaction time task (5-CSRTT) were found to be related to increased compulsive seeking defined as greater continued responding for cocaine despite contingent footshocks (Belin et al., 2008) and a greater increase in the resumption of responding after suppression of responding via footshocks (Economidou et al., 2009). Moreover, greater levels of drug primed reinstatement of MDMA seeking (Bird & Schenk, 2012) and increased cocaine self-administration in both limited and extended access operant sessions (Dalley et al., 2007) were reported in highly impulsive responders in the 5-CSRTT.
Looking at the clinical literature, increased SSRT is heavily implicated in addiction. Case-control comparison studies have consistently shown individuals with alcohol and substance use problems have increased SSRT [Monterosso et al., 2005; Joos et al., 2013; Lawrence et al., 2009; Fillmore & Rush 2002; but see Courtney et al., 2012; Moreno et al., 2012 (only binge drinkers studied)]. Moreover, increased SSRT is also seen in the unaffected siblings of drug dependent individuals (Ersche et al., 2012), and in both youths (Dougherty et al, 2015) as well as adults (Acheson et al., 2011) with a family history of alcoholism. Additionally SSRT predicts increases in alcohol related problems (Fernie et al., 2013), even independently of family history status (Nigg et al., 2006).
The current findings are in line with a body of prior research on the P rats and HAD2s that suggests they may be two behaviorally different models of alcoholism. For instance, Salimov et al. (1999), using a principle component analysis, found the P rats’ behavior was explained by a factor with positive relationships with free access alcohol drinking, active avoidance in a slip funnel test, and increased exploration of novel arms on a cross maze test. While the HAD2s’ behavior was also associated with high levels of free access alcohol drinking, they were characterized by passive avoidance and an increased latency to explore a novel environment. Salimov ultimately characterized the P rats as having "alcohol drive with novelty seeking and persistence" while the HAD rats have "alcohol drive with timidity and meekness" (Salimov, 1999). Similarly, HAD2s, but not P rats, have been seen to possess an avoidance (but not appetitive) learning deficit that may be due to increased anxiety (Blankenship et al., 1998; 2000). This view is supported by findings that the HAD2s avoidance learning deficit is decreased with moderate levels of alcohol, presumably via its anxiolytic effects (Rorick et al., 2003), and the HAD2s have increased heart rate reactivity in a Pavlovian fear conditioning paradigm (Rorick et al., 2004). When also considering recently demonstrated differences in decision making (Beckwith & Czachowski, 2014) and the present findings of action cancelation impulsivity, greater number of overall trials completed (Table 3), and greater responding for ethanol and persistence in the face of response requirement increases, the P rats appear to be prone to emit behavior and by contrast the HADs are perhaps more anxious and/or controlled.
In summary, the findings that P rats, but not the HAD2s, display increased motor impulsivity and operant responding for alcohol add to a body of research delineating these two strains as distinct models of alcoholism. The P rats are a reinforcer-driven, high seeking, high drinking model characterized by multiple forms of poor impulse control that precede any exposure to ethanol. The HAD2s, on the other hand, are a high drinking line but do not display increased seeking or impulse control problems. These distinct risk factors and the differential patterns of excessive appetitive and consummatory behaviors suggest that different neuroanatomical and neurochemical factors underlie the behavioral impairments. It is possible that by further studying both animal models that were genetically selected based on the same behavioral phenotype of free-choice ethanol preference, important genetic and phenotypic predisposing factors for craving and binge drinking can be identified and targeted for pharmacological or behavioral intervention.
Supplementary Material
Acknowledgments
All sources of support, including pharmaceutical and industry support, that require acknowledgement
This work was supported by P60AA007611 and T32AA07462 from the National Institute on Alcohol Abuse and Alcoholism. We also gratefully acknowledge Michael DeLory’s contribution to running the behavioral sessions.
Footnotes
The authors report no conflicts of interest.
Contributor Information
Steven Wesley Beckwith, Department of Psychology, Indiana University Purdue University Indianapolis, Indianapolis, Indiana.
Cristine Lynn Czachowski, Department of Psychology, Indiana University Purdue University Indianapolis, Indianapolis, Indiana.
References
- Acheson A, Richard DM, Mathias CW, Dougherty DM. Adults with a family history of alcohol related problems are more impulsive on measures of response initiation and response inhibition. Drug Alcohol Depend. 2011;117(2–3):198–203. doi: 10.1016/j.drugalcdep.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: 2013. [Google Scholar]
- Beckwith SW, Czachowski CL. Increased delay discounting tracks with a high ethanol-seeking phenotype and subsequent ethanol seeking but not consumption. Alcohol Clin Exp Res. 2014;38(10):2607–2614. doi: 10.1111/acer.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine taking. Science. 2008;5881;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J, Schenk S. Contribution of impulsivity and novelty-seeking to the acquisition and maintenance of MDMA self-administration. Addict Biol. 2012;18:654–664. doi: 10.1111/j.1369-1600.2012.00477.x. [DOI] [PubMed] [Google Scholar]
- Blankenship MR, Finn PR, Steinmetz JE. A characterization of approach and avoidance learning in alcohol-preferring and alcohol-nonpreferring rats. Alcohol Clin Exp Res. 1998;22(6):1227–1233. [PubMed] [Google Scholar]
- Blankenship MR, Finn PR, Steinmetz JE. A characterization of approach and avoidance learning in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res. 2000;24(12):1778–1784. [PubMed] [Google Scholar]
- Boehler CN, Hopf JM, Stoppel CM, Krebs RM. Motivating inhibition - reward prospect speeds up response cancellation. Cognition. 2012;125:498–503. doi: 10.1016/j.cognition.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Arellano R, Barkley-Levenson E, Gálvan A, Poldrack RA, MacKillop J, Jentsch JD, Ray LA. The relationship between measures of impulsivity and alcohol misuse: An integrative structural equation modeling approach. Alcohol Clin Exp Res. 2012;36(6):923–931. doi: 10.1111/j.1530-0277.2011.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity. Clin Psychol Rev. 2011;31:965–982. doi: 10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Czachowski CL. Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol- and sucrose-seeking and intake. Alcohol Clin Exp Res. 2005;29(7):1146–1155. doi: 10.1097/01.alc.0000171944.50381.86. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, DeLory MJ, Pope JD. Behavioral and neurotransmitter specific roles for the ventral tegmental area in reinforcer-seeking and intake. Alcohol Clin Exp Res. 2012;36(10):1659–1668. doi: 10.1111/j.1530-0277.2012.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcohol Clin Exp Res. 2002;26(11):1653–1661. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Breakpoint determination and ethanol self-administration using an across-session progressive ratio procedure in the rat. Alcohol Clin Exp Res. 1999;23(10):1580–1586. [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald EH, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BH, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;5816;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Mathias CW, Ryan SR, Bray BC, Charles NE, Acheson A. Behavioral Impulsivity and risk-taking trajectories across early adolescence in youths with and without family histories of alcohol and other drug use disorders. Alcohol Clin Exp Res. 2015;39(8):1501–1509. doi: 10.1111/acer.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: Effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117(6):1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Feola TW, de Wit H, Richards JB. Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci. 2000;114(4):838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, Field M. Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction. 2013;108:1916–1923. doi: 10.1111/add.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Denning CE, Marvin S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. II. Operant self-administration in a continuous-access situation. Alcohol Clin Exp Res. 1998;22(9):2147–2158. [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Henderson-Redmond AN, Czachowski CL. Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology. 2014;231(22):4309–4321. doi: 10.1007/s00213-014-3571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic Demand and Essential Value. Psychological Review. 2008;115(1):186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Joos L, Schmall L, Goudriaan AE, Fransen E, Van den Brink W, Sabbe BG, Dom G. Age of onset and neuropsychological functioning in alcohol dependent inpatients. Alcohol Clin Exp Res. 2013;37(3):407–416. doi: 10.1111/j.1530-0277.2012.01949.x. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakin BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology (Berl) 2009;207:163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan G. On the ability to inhibit thought and action: A users guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory process in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- McMillen BA, Means LW, Matthews JN. Comparison of the alcohol-preferring P rat to the wistar rat in behavioral tests of impulsivity and anxiety. Physiol Behav. 1997;63(3):371–375. doi: 10.1016/s0031-9384(97)00442-3. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associate with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Moreno M, Estevez AF, Zaldivar F, Montes JMG, Gutiérrez-Ferre VE, Esteban L, Sánchez-Santed F, Flores P. Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug Alcohol Depend. 2012;124:355–362. doi: 10.1016/j.drugalcdep.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Moschak TM, Stang KA, Mitchell SH. Mice bred for severity of acute alcohol withdrawal respond differently in a go/no-go task. Alcohol Clin Exp Res. 2013;37(9):1483–1490. doi: 10.1111/acer.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Interactions between cognition and motivation during response inhibition. Neuropsychologia. 2010;48:558–565. doi: 10.1016/j.neuropsychologia.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Oliver Y, Giuliano C, Economidou D, Goodlett CR, Robbins TW, Dalley JW, Everitt BJ. Alcohol-preferring rats show goal oriented behavior to food incentives but are neither sign-trackers nor impulsive. PloS one. 2015;10(6):e0131016. doi: 10.1371/journal.pone.0131016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carrol ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Robinson ESJ, Eagle DM, Economidou D, Theobald DEH, Mar AC, Murphy ER, Robbins TW, Dalley JW. Behavioural characterization of high impulsivity on the 5-choice serial reaction time task: Specific deficits in ‘waiting’ versus ‘stopping’. Behav Brain Res. 2009;196:310–316. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Rorick LM, Finn PR, Steinmetz JE. Moderate doses of ethanol partially reverse avoidance learning deficits in high-alcohol-drinking rats. Pharmacol Biochem Behav. 2003;75:89–102. doi: 10.1016/s0091-3057(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Rorick LM, Finn PR, Steinmetz JE. Heart rate reactivity in HAD and LAD rats during Pavlovian fear conditioning. Integr Psychol Behav Sci. 2004;39(1):24–41. doi: 10.1007/BF02734254. [DOI] [PubMed] [Google Scholar]
- Rosell-Negre P, Bustamante JC, Fuentes-Claramonte P, Costumero V, Benabarre S, Barros-Loscertales A. Reward anticipation enhances brain activation during response inhibition. Cog Affect Behav Neurosci. 2014;14:621–634. doi: 10.3758/s13415-014-0292-9. [DOI] [PubMed] [Google Scholar]
- Salimov RM. Different behavioral patterns related to alcohol use in rodents: A factor analysis. Alcohol. 1999;17(2):157–162. doi: 10.1016/s0741-8329(98)00049-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning CD, Marvin S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. I. Ethanol initiation and limited access operant self-administration. Alcohol Clin Exp Res. 1998;22(9):2133–2146. [PubMed] [Google Scholar]
- Steinmetz JE, Blankenship MR, Green JT, Smith GB, Finn PR. Evaluation of behavioral disinhibition in P/NP and HAD1/LAD1 rats. Prog Neuro-Psychopharmacol & Biol Psychiat. 2000;24:1025–1039. doi: 10.1016/s0278-5846(00)00122-6. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17(5):473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Moschak TM, Mitchell SH. Behavioral disinhibition in mice bred for high drinking in the dark (HDID) and HS controls increases following ethanol. Drug Alcohol Depend. 2014;136:149–152. doi: 10.1016/j.drugalcdep.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effects of prazosin, an α1-adrenergic receptor antagonist, on the seeking and intake of ethanol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36(5):881–886. doi: 10.1111/j.1530-0277.2011.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res. 2007;31(11):1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.