Abstract
The use of HIV protease inhibitors (PIs) has extended the duration and quality of life for HIV-positive individuals. However there is increasing concern that this antiviral therapy may promote premature cardiovascular disease by impairing endothelial cell (EC) function. In the present study, we investigated the effect of HIV PIs on EC function and determined if the enzyme heme oxygenase (HO-1) influences the biological action of these drugs. We found that three distinct PIs, including ritonavir, atazanavir, and lopinavir, stimulated the expression of HO-1 protein and mRNA. The induction of HO-1 was associated with an increase in NF-E2-related factor-2 (Nrf2) activity and reactive oxygen species (ROS). PIs also stimulated HO-1 promoter activity and this was prevented by mutating the antioxidant responsive element or by overexpressing dominant-negative Nrf2. In addition, the PI-mediated induction of HO-1 was abolished by N-acetyl-L-cysteine and rotenone. Furthermore, PIs blocked EC proliferation and migration and stimulated the expression of intercellular adhesion molecule-1 and the adhesion of monocytes on ECs. Inhibition of HO-1 activity or expression potentiated the anti-proliferative and inflammatory actions of PIs which was reversed by bilirubin but not carbon monoxide. Alternatively, adenovirus-mediated overexpression of HO-1 attenuated the growth-inhibitory and inflammatory effect of PIs. In contrast, blocking HO-1 activity failed to modify the anti-migratory effect of the PIs. Thus, induction of HO-1 via the ROS–Nrf2 pathway in human ECs counteracts the anti-proliferative and inflammatory actions of PIs by generating bilirubin. Therapeutic approaches targeting HO-1 may provide a novel approach in preventing EC dysfunction and vascular disease in HIV-infected patients undergoing antiretroviral therapy.
Keywords: HIV protease inhibitors, Endothelial cells, Heme oxygenase-1, Bilirubin, Carbon monoxide
1. Introduction
The use of HIV type 1 protease inhibitors (PIs) as part of combined antiretroviral therapy has dramatically reduced HIV-related mortality and morbidity [1]. However, there is increasing concern that chronic use of PIs results in significant metabolic disorders, including systemic insulin resistance, hyperlipidemia, and tissue lipodystrophy [2]. In addition, long-term use of PIs is associated with the development of atherosclerosis and other cardiovascular diseases. Large cohort studies have implicated PIs with increased risk for acute myocardial infarctions and coronary syndromes [3–7]. Prolonged exposure to antiretroviral therapy also augments mortality and hospitalization for cardiovascular complications [8–10]. Furthermore, several clinical studies report a clear link between PIs and the development of subclinical atherosclerotic lesions and thrombotic events [11–13]. However, the overall risk for cardiovascular disease may vary depending on the drugs used and duration of treatment.
While the metabolic derangements provoked by PIs may contribute to the accelerated development of atherosclerosis in HIV-positive patients, direct actions on endothelial cells may also predispose patients to vascular disease. The endothelium serves as a key regulator of vascular homeostasis influencing vascular tone and permeability, thrombosis, fibrinolysis, and inflammation. Significantly, alterations in endothelial cell function precede the development of atherosclerosis and contribute to lesion development and subsequent clinical complications [14]. Ample evidence indicates that PIs directly interfere with endothelial function. PIs impair endothelium-dependent vasodilation in patients with HIV-infections and endothelium-dependent relaxation in animals [15–17]. These impaired endothelial responses are paralleled by increases in reactive oxygen species (ROS) along with diminished endothelial nitric oxide synthase expression and nitric oxide synthesis [16,18]. Moreover, PIs stimulate mitochondrial dysfunction, premature senescence, and permeability in cultured endothelial cells [19–21]. Although numerous adverse effects of PIs on endothelial function have been identified, effective therapeutic approaches mitigating the negative functional impact of PIs on endothelial cells have yet to be realized.
Heme oxygenase-1 (HO-1) is a highly inducible enzyme that catalyzes the metabolism of heme to carbon monoxide (CO), biliverdin, and free iron, with biliverdin being rapidly metabolized to bilirubin by biliverdin reductase [22,23]. Although initial interest in HO-1 focused on its heme degrading capacity, recent work indicates that HO-1 protects against vascular disease, including atherosclerosis [24]. While multiple mechanisms mediate the vasoprotective actions of HO-1, the ability of this enzyme to preserve endothelial cell function is of critical importance. HO-1 is induced by various forms of cellular stress and its expression in endothelial cells provides an important defense against cellular injury [25–27]. In addition, HO-1 promotes the proliferation and migration of endothelial cells, and prevents inflammatory responses in these cells through the generation of carbon monoxide and/or bilirubin [28–31]. Several studies have also demonstrated that HO-1 is able to correct endothelial dysfunction in various pathological states [32,33]. However, the ability of HO-1 to modify PI-induced endothelial cell malfunction is not known.
In the present study, we investigated the effect of three different PIs on HO-1 gene expression in human endothelial cells. In addition, we examined the effect of PIs on the proliferation, migration, and inflammation of endothelial cells. Finally, we also determined if HO-1 modulates the biological actions of PIs on endothelial cells.
2. Materials and methods
2.1. Reagents
M199 medium, bovine calf serum, lipofectamine, penicillin, streptomycin, Trizol, 5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA), and epidermal growth factor were from Life Technologies Corporation (Carlsbad, CA). Gelatin, Nonidet P40, dithiothreitol, bromophenol blue, SDS, NaCl, EDTA, DMSO, glycerol, mercaptoethanol, ethidium bromide, Triton X-100, carbon monoxide-releasing molecule-2 (CORM2), rotenone, hydrocortisone, heparin, trypan blue, agarose, trypsin, Tris, HEPES, trichloroacetic acid, and N-acetyl-L-cysteine (NAC) were from Sigma-Aldrich (St. Louis, MO). LY294002 was from Calbiochem (La Jolla, CA). Inactivated CORM2 (iCORM2) was prepared by leaving CORM2 solutions at room temperature for 2 days and then purging the solutions of any residual CO with nitrogen. Phenylmethylsulfonyl fluoride (PMSF), aprotinin, leupeptin, and pepstatin A were from Roche Applied Sciences (Indianapolis, IN). Endothelial cell growth factor was from Becton Dickinson (Bedford, MA). Tin protoporphyrin-IX (SnPP) and bilirubin were from Frontier Scientific (Logan, UT, USA). A polyclonal antibody against HO-1 was from Assay Designs (Ann Arbor, MI, USA) while antibodies against glycogen synthase kinase-3β (GSK3β) and phospho (Ser-9)-GSK3β (GSK3β-P) were from Cell Signaling Technologies (Danvers, MA). Antibodies against NF-E2-related factor-2 (Nrf2), intercellular adhesion molecule-1 (ICAM-1), and β-actin were from Santa Cruz (Santa Cruz, CA). α-[32P]dCTP (3000 Ci/mmol) and [3H]thymidine (90 Ci/mmol) was from Amersham Life Sciences (Arlington Heights, IL). The PIs, ritonavir (RTV), atazanavir (ATV), and lopinavir (LPV) were obtained through the NIH AIDS Research and Reference Reagent Program.
2.2. Cell culture
Human umbilical vein endothelial cells (HUVEC), human aortic endothelial cells (HAEC), and human dermal microvascular endothelial cells (HDMEC) were purchased from Lonza Incorporated (Allendale, NJ). HUVEC and HAEC were serially cultured on gelatin-coated plates in M199 medium supplemented with 20% bovine calf serum, 2 mM L-glutamine, 50 μg/ml endothelial cell growth factor, 90 μg/ml heparin, and 100 U/ml of penicillin and streptomycin, while HDMEC were serially propagated on gelatin-coated plates in EGM-2 medium (Lonza Incorporated, Allendale, NJ) supplemented with 10% serum, 5 ng/ml epidermal growth factor, 1 mg/ml hydrocortisone, and 100 U/ml of penicillin and streptomycin [26,31,34]. The human monocytic cell line U937 (American Type Culture Collection, Manassas, VA) was grown in suspension in RPMI-1640 (Invitrogen, Carlsbad, CA) containing 2 mm L-glutamine, 1 mM sodium pyruvate, 4.5 g/L glucose, 10% fetal bovine serum, and 100 U/ml penicillin and streptomycin. All cells were incubated in an atmosphere of 95% air and 5% CO2 at 37 °C.
2.3. Western blotting
Cells were scrapped in lysis buffer (125 mM Tris [pH 6.8], 12.5% glycerol, 2% SDS, and bromophenol blue), boiled for 5 min, and proteins (20–50 μg) resolved by SDS-polyacrylamide gel electrophoresis. Following transfer to nitrocellulose membranes, blots were blocked with PBS containing Triton X-100 (0.25%) and nonfat milk (5%), and then incubated for one hour with antibodies against HO-1 (1:1,500), Nrf2 (1:200), ICAM-1 (1:500), or β-actin (1:1000). Membranes were then washed, incubated with horseradish peroxidase-conjugated secondary antibodies, and developed with commercial chemoluminescence reagents (Amersham, Arlington Heights, IL). Blots were then stripped of antibodies at 50 °C for 30 min using a stripping solution (10% SDS, and 100 mM mercaptoethanol in 62.5 mM Tris buffer, pH 6.8) before reprobing with complementary antibodies. Protein expression was quantified by densitometry and normalized with respect to β-actin or GSK3β.
2.4. Northern blotting
Total RNA was isolated from endothelial cells with Trizol, loaded onto 1.2% agarose gels and fractionated by electrophoresis. RNA was blot transferred to Gene Screen Plus membranes and prehybridized at 68 °C for 4 h in rapid hybridization buffer (Amersham, Arlington Heights, IL). Membranes were then incubated overnight at 68 °C in hybridization buffer containing [32P]DNA probes (1 × 108 cpm) for HO-1 or 18S mRNA [31,22]. DNA probes were generated by RT-PCR and labeled with α-[32P]dCTP using a random primer kit (Amersham, Arlington Heights, IL) as previously described [31,35]. Following hybridization, membranes were washed, exposed to X-ray film at −70 °C, and mRNA expression quantified by densitometry and normalized with respect to 18S rRNA.
2.5. HO-1 promoter analysis
Transcriptional activity was measured using a dual-luciferase reporter assay using HO-1 promoter/firefly luciferase constructs that were generously supplied by Dr. Jawed Alam (Ochsner Clinic Foundation, New Orleans, LA). These constructs consisted of the wild type enhancer (E1) that contains three antioxidant responsive elements (ARE) core sequences coupled to a minimum HO-1 promoter as well as the mutant enhancer (M739) that has mutations in its three ARE sequences. In some experiments, a plasmid expressing dominant-negative Nrf2 (dnNrf2) was employed. Transfection efficiency was controlled by introducing a plasmid encoding Renilla luciferase (Promega, Madison, WI) into cells. Cells were transfected with plasmids using lipofectamine (Invitrogen Corporation, Carlsbad, CA), incubated for 48 h, and then exposed to PIs for 8 h. Firefly luciferase activity was determined using a Glomax luminometer (Promega, Madison, WI) and normalized with respect to Renilla luciferase activity, and this ratio was expressed as fold induction over control cells.
2.6. Small interference RNA and HO-1 gene transfer
HO-1 was silenced by transfecting cells with HO-1 small interference RNA (siRNA) (100 nM) or a non-targeting (NT) siRNA (100 nM) that was purchased from Dharmacon (Lafayette, CO), as we previously described [31,35]. For HO-1 gene transfer, endothelial cells were infected with a replication-deficient adenovirus expressing HO-1 (AdHO-1) or green fluorescent protein (AdGFP) at a multiplicity of infection of 20 [31].
2.7. Nrf2 activation
Cells were incubated in lysis buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.5 mM PMSF, 10 μg/ml aprotonin, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 0.5 mM dithiothreitol, and 0.4% Nonidet P-40) for 10 min and then centrifuged at 14,000g for 3 min. Nuclear pellets were suspended in extraction buffer (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1.0 mM EDTA, 1 mM dithiothreitol, 10 μg/ml aprotonin, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 10% glycerol), and centrifuged at 14,000g for 5 min. The supernatant containing nuclear protein was collected and Nrf2 activity monitored by measuring the binding of Nrf2 to the ARE using an ELISA-based TransAM Nrf2 kit (Active Motif, Carlsbad, CA). Nuclear extracts (5 μg) were incubated with ARE consensus site oligonucleotides (5′-GTCACAGTGACTCAGCAGAATCTG-3′) immobilized to 96-well plates. Bound protein was detected using an antibody specific to DNA-bound Nrf2, visualized by colorimetric reaction catalyzed by a horseradish peroxidase-conjugated secondary antibody, and absorbance measured at 405 nm.
2.8. Measurement of intracellular ROS
Intracellular ROS production was assessed using the cell-permeable probe CM-H2DCFDA. Cells were grown to confluence and treated with PIs in the presence and absence of NAC or rote-none. Following treatment, cells were incubated with the dye (10 μM) for 30 min at 37 °C. The cells were then washed with PBS and fluorescence quantified by microplate fluorimetry with excitation at 485 nm and emission at 530 nm, as previously described [36]. Mean values from each treatment were expressed as a fraction relative to untreated control cells.
2.9. Cell proliferation and DNA synthesis
Cells were seeded (5 × 104 cells) onto six-well plates in serum-containing media and grown overnight. After 24 h, the cells were incubated with fresh culture media in the absence or presence of PIs. Cell number determinations were made by dissociating cells with trypsin (0.05%):EDTA (0.53 mM) and counting cells in a Beckman Z1 Coulter Counter (Beckman Coulter, Fullerton, CA, USA). Endothelial cell proliferation was also monitored by measuring DNA synthesis, as previously described [36]. Cells were incubated with [3H]thymidine for 4 h, washed three times with ice-cold PBS, and fixed with 10% trichloroacetic acid for 30 min at 4 °C. DNA was then extracted with 0.2% SDS/0.2 N NaOH and radioactivity quantified by liquid scintillation spectroscopy.
2.10. Cell viability
Cell viability was monitored by measuring the uptake of membrane impermeable stain trypan blue. Cells were treated with trypsin (0.25%), collected, diluted (1:4) with trypan blue, and examined by microscopy. Viability was determined by the percentage of cells that excluded the dye, as previously reported [26,37].
2.11. Cell migration
Cell migration was determined by using a scratch wound assay. Confluent cell monolayers were scraped with a pipette tip and injured monolayers incubated in the presence and absence of PIs. Cell monolayers were photographed immediately and 20 h after scratch injury, and the degree of wound closure determined by planimetry, as we previously described [37].
2.12. Monocyte adhesion
U937 cells were labeled with [3H]thymidine (1 μCi/ml for 24 h) and layered onto endothelial cell monolayers. Following a one hour incubation, non-adherent monocytes were removed by PBS washing and radioactivity associated with adherent monocytes quantified by liquid scintillation spectrometry [31].
2.13. Statistical analyses
Results are expressed as mean±SEM. Statistical analyses were performed with the use of a Student’s two-tailed t-test and an analysis of variance with the Tukey post hoc test when more than two treatment regimens were compared. P values <0.05 were considered statistically significant.
3. Results
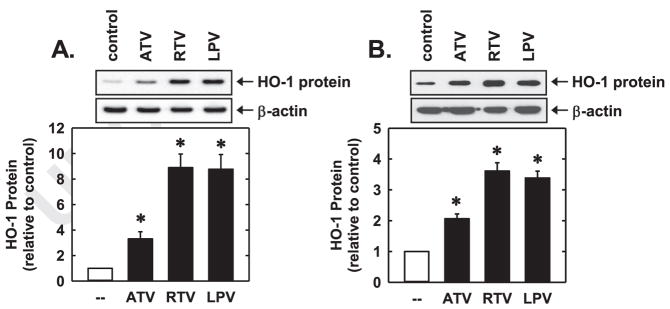
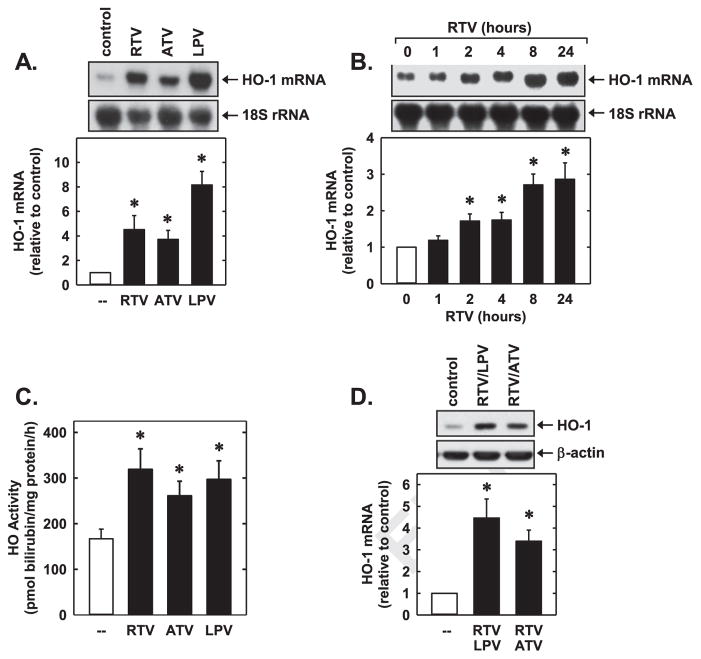
Treatment of HUVEC with RTV, ATV, or LPV for 24 h resulted in a concentration-dependent increase in HO-1 protein (Fig. 1A–C). A significant induction of HO-1 protein was observed with 5 μM of RTV, while higher concentrations of ATV or LPV (10 μM) were required to elevate HO-1 expression. The induction of HO-1 protein by all three PIs was time-dependent and was first observed at 4 h, and levels of HO-1 progressively increased during 24 h of exposure (Fig. 1D–F). An increase in HO-1 protein expression was also noted following the exposure of HAEC or HDMEC to ATV, RTV, or LPV (Fig. 2A and B). Furthermore, the PIs stimulated the expression of HO-1 mRNA (Fig. 3A). A significant increase in HO-1 transcripts was first detected 2 h following the administration of RTV and peaked between 8 and 24 h (Fig. 3B). In addition, the PIs evoked a marked increase in HO activity (Fig. 3C). Moreover, the incubation of HUVEC with a low concentration of RTV (2 μM) with either LPV or ATV (10 μM), which represents plasma concentrations attained using RTV-boosted PI therapy [38–40], results in the robust induction of HO-1 (Fig. 3D).
Fig. 1.
PIs stimulated HO-1 protein expression in HUVEC (A–C) Concentration-dependent increase in HO-1 protein expression 24 h following administration of RTV, ATV, or LPV. (D–F) Time-dependent increase in HO-1 protein expression following administration of RTV (20 μM), ATV (20 μM), or LPV (20 μM). HO-1 protein was quantified by scanning laser densitometry and normalized with respect to β-actin, and expressed relative to that of control, untreated cells (open bars). Results are means±SEM (n=3–4). *Statistically significant effect of PIs.
Fig. 2.
PIs stimulated HO-1 protein expression in HAEC and HDMEC. (A) Increase in HO-1 protein expression following the administration of ATV (50 μM), RTV (50 μM), or LPV (50 μM) to HAEC for 24 h. (B) Increase in HO-1 protein expression following the administration of ATV (50 μM), RTV (50 μM), or LPV (50 μM) to HDMEC for 24 h. HO-1 protein was quantified by scanning laser densitometry and normalized with respect to β-actin, and expressed relative to that of control, untreated cells (open bars). Results are means±SEM (n=3). *Statistically significant effect of PIs.
Fig. 3.
PIs stimulated HO-1 expression and activity in HUVEC. (A) Induction of HO-1 mRNA expression following the administration of RTV (50 μM), ATV (50 μM), or LPV for (50 μM) for 24 h. (B) Time-dependent increase in HO-1 mRNA expression following the administration of RTV (50 μM). (C) Induction of HO activity following the administration of RTV (50 μM), ATV (50 μM), or LPV (50 μM) for 24 h. (D) Effect of RTV-boosted PI treatment on HO-1 protein expression. Cells were treated with RTV (2 μM) and LPV (10 μM) or ATV (10 μM) for 24 h. HO-1 mRNA or protein was quantified by scanning laser densitometry and normalized with respect to 18S rRNA or β-actin, respectively, and expressed relative to that of control, untreated cells (open bars). Results are means±SEM (n=3–5). *Statistically significant effect of PIs.
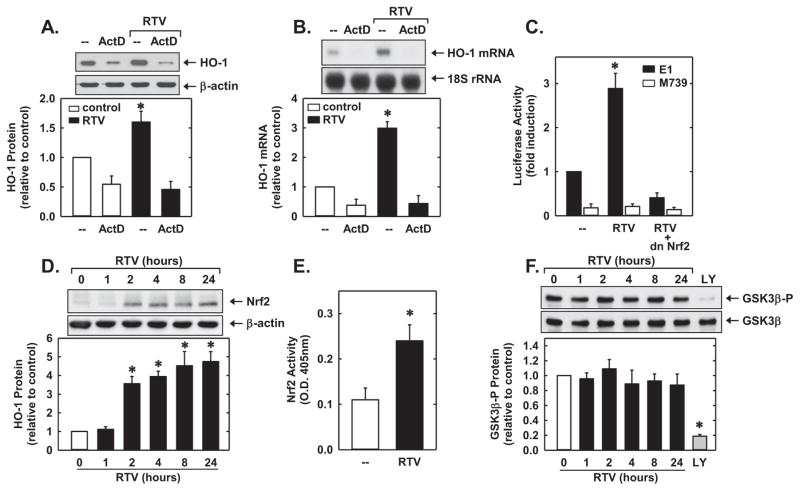
Incubation of HUVEC with the transcriptional inhibitor actinomycin D attenuated basal and abolished PI-induced HO-1 protein and mRNA expression (Fig. 4A and B), suggesting that PI-mediated HO-1 expression required de novo RNA synthesis. To further examine the molecular mechanism by which PIs stimulate HO-1 expression, HUVEC were transfected with an HO-1 promoter construct and reporter activity monitored. Treatment of the HU-VEC with RTV stimulated an increase in HO-1 promoter activity that was abolished by mutating the ARE sequences in the promoter (Fig. 4C). Mutation of the ARE sequences also reduced basal promoter activity. Since the transcription factor Nrf2 plays a major role in ARE-mediated gene activation [24], we investigated whether Nrf2 contributed to the activation of HO-1 by PIs. Transfection of the HUVEC with a dnNrf2 mutant that had its activation domain deleted inhibited basal HO-1 promoter activity and the RTV-mediated elevation in promoter activity (Fig. 4C). In addition, RTV evoked a significant increase in Nrf2 protein beginning 2 h after exposure to the PI that persisted for 24 h (Fig. 4D). RTV also stimulated the activation of Nrf2, as reflected by the increase in binding of nuclear Nrf2 to the ARE (Fig. 4E). Since it was recently reported that the GSK3β-mediated phosphorylation of Nrf2 targets the protein for degradation by the E3 ligase, β-transducin repeats-containing protein (β-TrCP) [41,42], we examined if PIs could stimulate Nrf2 expression by repressing the activity of GSK3β. As GSK3β activity is inhibited through phosphorylation of serine-9, we determined if PIs influence the phosphorylation status of this serine residue in GSK3β. In contrast, treatment of HUVEC with RTV failed to stimulate GSKβ phosphorylation (Fig. 4F). However, pharmacological inhibition of the phosphatidylinositol-3 kinase–Akt pathway, a negative regulator of GSK3β [43], with LY294002 resulted in a marked increase in GSK3β activity as reflected by a significant decrease in GSK3β phosphorylation, demonstrating that changes in GSK3β activity could be detected by the assay.
Fig. 4.
PIs stimulated HO-1 gene expression via the Nrf2/ARE complex in HUVEC. (A, B) PI-mediated HO-1 protein and mRNA expression required de novo RNA synthesis. Effect of actinomycin D (ActD; 0.10 μg/ml) on RTV (50 μM for 8 h)-mediated increases in HO-1 protein and mRNA. (C) PIs stimulated HO-1 promoter activity. Cells were transfected with a HO-1 promoter construct (E1) or a mutated HO-1 promoter construct (M739) and a Renilla luciferase construct, treated with RTV (50 μM for 8 h), and then analyzed for luciferase activity. In some instances, a dominant-negative Nrf2 (dnNrf2) construct was co-transfected into the cells. (D) Time-course of Nrf2 protein expression after administration of RTV (50 μM) in native non-transfected endothelial cells. (E) PIs stimulated Nrf2 activity in native non-transfected endothelial cells. Cells were treated with RTV (50 μM for 8 h) and nuclear extracts analyzed for Nrf2 binding by ELISA. O.D., optical density. (F) Effect of PIs on GSK3β activity. Native non-transfected cells were treated with RTV (50 μM) for various times (0–24 h) or with the phosphatidylinositol-3-kinase inhibitor LY294002 (LY; 10 μM) for 4 h. Protein or mRNA was quantified by scanning laser densitometry and normalized with respect to β-actin (or GSK3β) or 18S rRNA, respectively, and expressed relative to that of control, untreated cells (open bars). Results are means±SEM (n=3–5). *Statistically significant effect of RTV and LY.
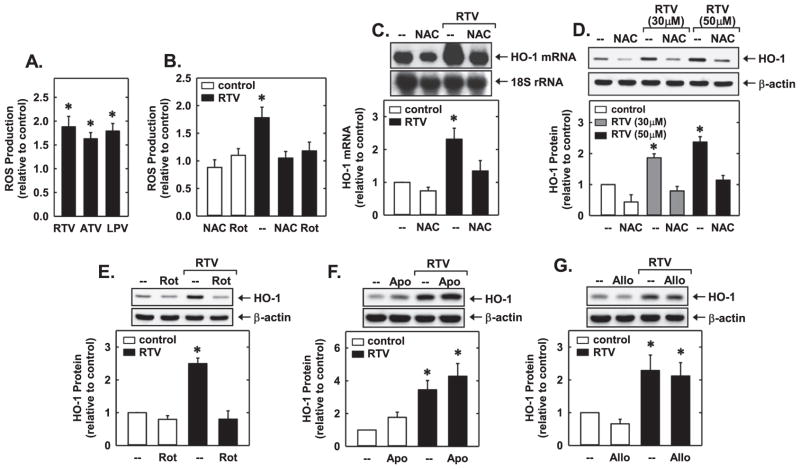
Since oxidative stress has been implicated in the activation of Nrf2 [44], the contribution of ROS in the induction of HO-1 was investigated. Incubation of HUVEC with the three PIs elicited a significant rise in ROS production (Fig. 5A). However, pretreatment of HUVEC with the antioxidant NAC or the mitochondrial electron transport chain inhibitor, rotenone, prevented the generation of ROS by RTV (Fig. 5B). In addition, NAC and rotenone blocked the RTV-mediated increase in HO-1 mRNA and/or protein (Fig. 5C and D). In contrast, neither the NADPH oxidase inhibitor, apocynin, nor the xanthine oxidase inhibitor, allopurinol, had any effect on the induction of HO-1 (Fig. 5E and F).
Fig. 5.
PI-induced HO-1 expression was dependent on oxidative stress. (A) ROS production in human endothelial cells treated with RTV (50 μM), ATV (50 μM), or LPV (50 μM) for 8 h. (B) Effect of NAC (10 mM) or rotenone (Rot; 5 μM) on RTV (50 μM for 8 h)-mediated ROS production. (C) Effect of NAC (10 mM) on RTV (50 μM for 24 h)-mediated HO-1 mRNA expression. (D) Effect of NAC (10 mM) on RTV (30 μM or 50 μM for 24 h)-mediated HO-1 protein expression. (E) Effect of Rot (5 μM) on RTV (50 μM for 24 h)-mediated HO-1 protein expression. (F) Effect of apocynin (Apo;300 μM) on RTV (50 μM for 24 h)-mediated HO-1 protein expression. (G) Effect of allopurinol (Allo; 100 μM) on RTV (50 μM for 24 h)-mediated HO-1 protein expression. HO-1 mRNA or protein was quantified by scanning laser densitometry and normalized with respect to 18S rRNA or β-actin, respectively, and expressed relative to that of control, untreated cells (open bars). Results are means±SEM (n=3–6). *Statistically significant effect of PIs.
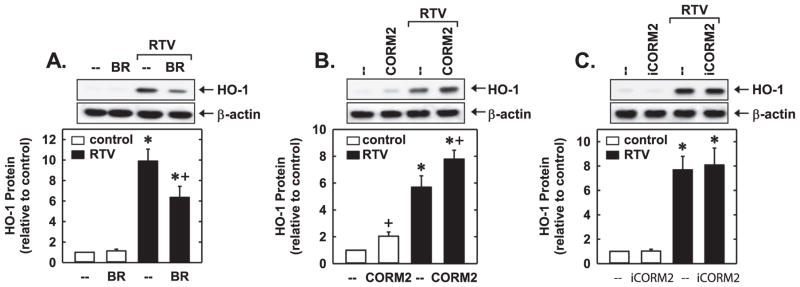
Interestingly, the products of the HO-1 reaction modulate the ability of PIs to stimulate HO-1 expression. Treatment of HUVEC with bilirubin had no effect on baseline HO-1 protein levels but clearly attenuated the induction of HO-1 by RTV (Fig. 6A). Conversely, CORM2 stimulated basal expression of HO-1 and enhanced the RTV-mediated induction of HO-1 protein (Fig. 6B), while iCORM2 had no effect on HO-1 expression (Fig. 6C). Thus, both bilirubin and CO regulate the expression of HO-1 by PIs.
Fig. 6.
Regulation of PI-induced HO-1 protein expression by bilirubin and carbon monoxide. (A–C) Effect of bilirubin (BR; 10 μM), CORM2 (10 μM), or iCORM2 (10 μM) on RTV (50 μM for 24 h)-mediated HO-1 protein expression. Results are means±SEM (n=3–4). *Statistically significant effect of RTV. †Statistically significant effect of BR or CORM2.
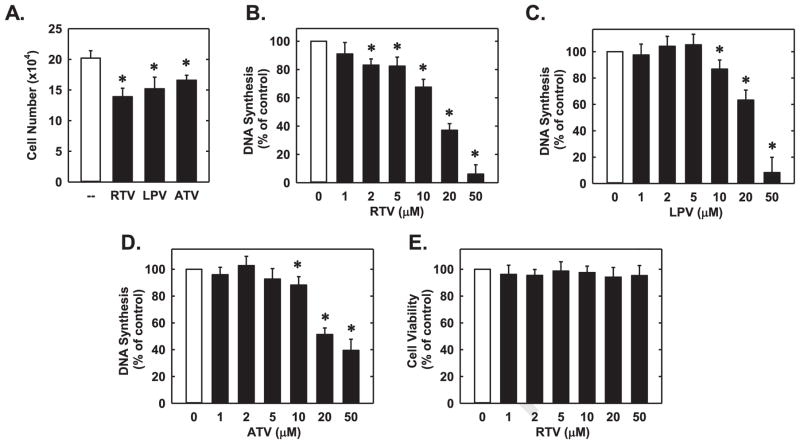
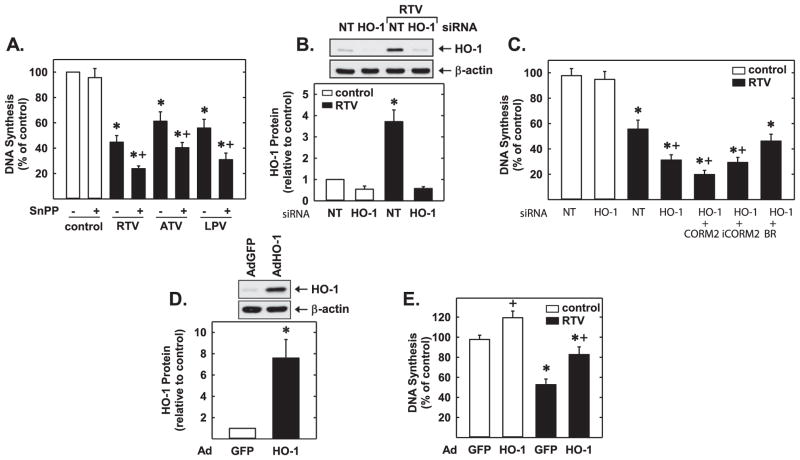
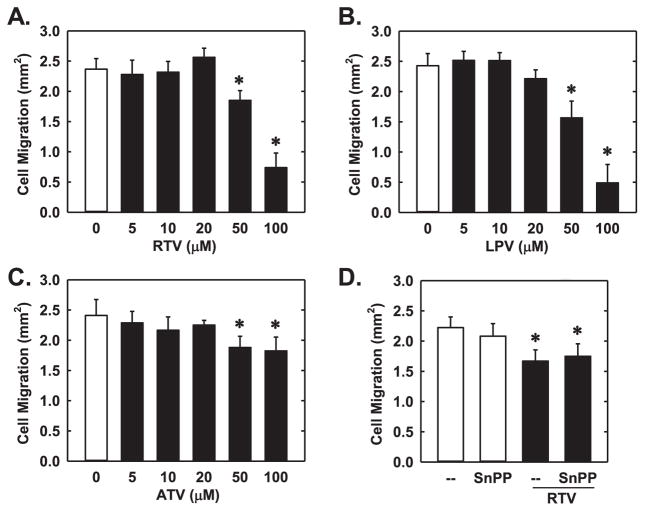
Next, the functional significance of the induction of HO-1 by PIs was investigated. Treatment of HUVEC with RTV, ATV, or LPV inhibited the proliferation of these cells (Fig. 7A). In addition, all three PIs markedly suppressed endothelial cell DNA synthesis in a dose-dependent manner without affecting cell viability (Fig. 7B–E). Interestingly, administration of the HO-1 inhibitor, SnPP, markedly increased the anti-proliferative action of all three PIs (Fig. 8A). Alone, SnPP had no effect on endothelial cell DNA synthesis. Transfection of HUVEC with HO-1 siRNA inhibited basal and RTV-induced HO-1 expression, confirming the efficacy of the HO-1 knockdown approach (Fig. 8B). Moreover, silencing HO-1 expression potentiated the anti-proliferative effects of RTV (Fig. 8C). In the absence of PIs, HO-1 knockdown did not affect endothelial cell DNA synthesis. Next, we determined which of the HO-1 products is able to restore growth in HO-1-silenced endothelial cells. While the exogenous administration of bilirubin could substitute for the loss of HO-1 and reverse the inhibitory effect of RTV on DNA synthesis, CORM2 failed to restore DNA replication in RTV-treated endothelial cells (Fig. 8C). In fact, a further decrease in DNA synthesis was noted after the application of CORM2, whereas iCORM2 had no effect on DNA synthesis. The proliferative effect of HO-1 in endothelial cells was also corroborated by overexpression of HO-1 in these cells. Infection of HUVEC with AdHO-1 resulted in a pronounced increase in HO-1 protein (Fig. 8D), and this was associated with an increase in baseline DNA synthesis and an attenuation in the reduction of DNA synthesis by RTV (Fig. 8E). In addition, all three PIs significantly retarded the migration of HUVEC in a concentration-dependent fashion (Fig. 9A–C). However, pharmacological inhibition of HO-1 activity did not modify the anti-migratory action of the PIs (Fig. 9D).
Fig. 7.
PIs blocked the proliferation of HUVEC. (A) RTV (20 μM), LPV (20 μM), or ATV (20 μM) inhibited endothelial cell proliferation. (B–D) RTV, LPV, or ATV inhibited HUVEC DNA synthesis in a concentration-dependent manner. (E) RTV had no effect on HUVEC survival. Results are means±SEM (n=5). *Statistically significant effect of PIs.
Fig. 8.
PI-mediated inhibition of HUVEC proliferation was attenuated by HO-1. (A) HO-1 inhibition potentiated PI-mediated reductions in HUVEC DNA synthesis. Cells were treated with RTV, LPV or ATV (20 μM) in the presence or absence of the HO-1 inhibitor SnPP (10 μM). (B) HO-1 protein expression in cells transfected with HO-1 siRNA (0.1 μM) or NT siRNA (0.1 μM) and exposed to RTV (20 μM). (C) HO-1 silencing potentiated PI-mediated inhibition of HUVEC DNA synthesis. Cells were transfected with HO-1 siRNA (0.1 μM) or non-targeting (NT) siRNA (0.1 μM) and then exposed to RTV (20 μM) in the presence and absence of CORM2 (10 μM), iCORM2 (10 μM), or bilirubin (BR; 10 μM). (D) Infection of HUVEC with AdHO-1 for two days stimulates HO-1 protein expression relative to cells infected with the control AdGFP. (E) HO-1 overexpression inhibits PI-mediated growth inhibition. Cells were infected with AdHO-1 or AdGFP and two days later treated with RTV (20 μM) for 24 h. HO-1 protein was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells (open bars). Results are means±SEM (n=3–6). *Statistically significant effect of PI. †Statistically significant effect of HO-1 inhibition or silencing, or HO-1 overexpression.
Fig. 9.
PIs blocked the migration of HUVEC. (A–C) RTV, LPV, or ATV inhibited endothelial cell migration in a concentration-dependent manner. (D) HO-1 inhibition had no effect on PI-mediated inhibition of cell migration. Cells were treated with RTV (50 μM) in the presence or absence of the HO-1 inhibitor SnPP (10 μM). Results are means±SEM (n=6). *Statistically significant effect of PIs.
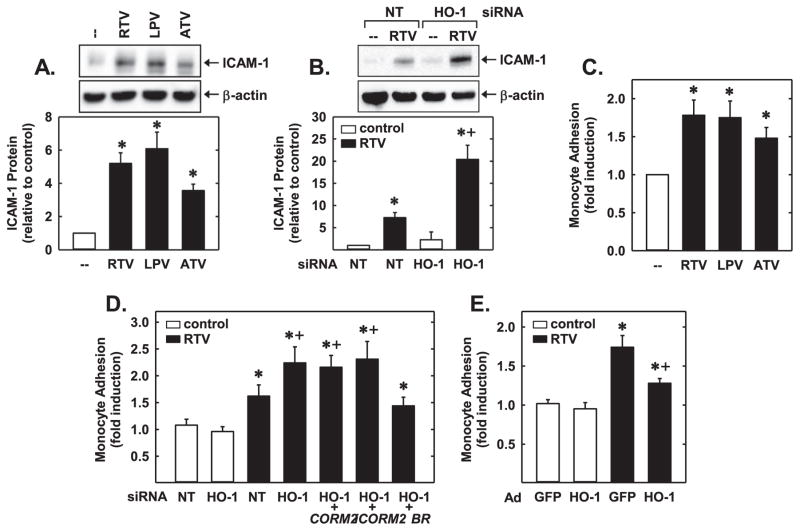
Finally, we investigated the capacity of PIs to stimulate inflammatory responses in endothelial cells. Treatment of HUVEC with RTV, LPV, or ATV induced a prominent increase in ICAM-1 protein expression (Fig. 10A). Knockdown of HO-1 further increased the expression of ICAM-1 by RTV (Fig. 10B). The induction of ICAM-1 by PIs was accompanied by a pronounced increase in monocyte adhesion (Fig. 10C). Furthermore, HO-1 silencing increased the ability of RTV to stimulate monocyte adhesion but failed to modify monocyte binding in the absence of RTV (Fig. 10D). Moreover, addition of bilirubin, but neither CORM2 nor iCORM2, could reverse the increase in monocyte adhesion observed in HO-1-silenced cells treated with RTV. Lastly, infection of HUVEC with AdHO-1 had no effect on basal monocyte adhesion, but AdHO-1 markedly reduced the adhesion of monocytes by RTV (Fig. 10E).
Fig. 10.
PIs stimulated inflammatory responses in HUVEC were attenuated by HO-1. (A) PI exposure (RTV 20 μM, LPV 20 μM or ATV 20 μM) for 24 h stimulated ICAM-1 protein expression. (B) HO-1 silencing potentiated PI-mediated ICAM-1 expression. Cells transfected with HO-1 siRNA (0.1 μM) or non-targeting (NT) siRNA (0.1 μM) and then exposed to RTV (20 μM) for 24 h. (C) PI exposure (RTV 20 μM, LPV 20 μM or ATV 20 μM) for 24 h stimulated monocyte adhesion. (D) HO-1 silencing potentiated PI-mediated monocyte adhesion. Cells transfected with HO-1 siRNA (0.1 μM) or non-targeting (NT) siRNA (0.1 μM) and then exposed to RTV (20 μM) for 24 h in the presence or absence of CORM2 (10 μM), iCORM2 (10 μM), or bilirubin (BR; 10 μM). (E) HO-1 overexpression inhibits PI-mediated monocyte adhesion. Cells were infected with AdHO-1 or AdGFP and two days later treated with RTV (20 μM) for 24 h. ICAM-1 protein was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells (open bars). Results are means±SEM (n=3–5). *Statistically significant effect of PIs. †Statistically significant effect of HO-1 silencing or HO-1 overexpression.
4. Discussion
In the present study, we identified HIV PIs as novel inducers of HO-1 gene expression in human vascular endothelium. The induction of HO-1 is observed with three different PIs, requires the production of ROS, and is mediated by the Nrf2-ARE complex. We also found that PIs block the proliferation and migration of endothelial cells and stimulates inflammation in these cells. In addition, we showed that the induction of HO-1 counteracts the anti-proliferative and inflammatory actions of PIs. Thus, therapeutic strategies directed at HO-1 represent a promising approach in preserving endothelial function and limiting cardiovascular complications in HIV-positive patients.
Although PIs were designed to counteract a viral enzyme, a growing list of off-target molecules has been identified [45]. In the current study, we show that three US Food and Drug Agency-approved PIs are capable of stimulating HO-1 gene expression, suggesting that the induction of this enzyme represents a generalized response to this class of drugs. The induction of HO-1 by PIs is widespread and is observed in endothelial cells derived from the arterial and venous circulation as well as the macro and micro-circulation. Significantly, the ability of PIs to stimulate HO-1 expression is observed at or near plasma concentrations of HIV-positive patients undergoing single or combined RTV-boosted PI antiretroviral therapy [38–40,46,47]. Moreover, the concentrations of PIs within endothelial cells may be significantly greater than circulating levels, as substantial intracellular accumulation of PIs has been reported [48]. In addition, hemodynamic forces generated by blood flow can increase the sensitivity of endothelial cells to pharmacological inducers of HO-1 [49], providing a potential mechanism whereby the potency of PIs is augmented in vivo.
The upregulation of HO-1 by PIs is dependent on de novo RNA synthesis and is likely due to the transcriptional activation of the gene since luciferase reporter assays demonstrate that PIs directly stimulates HO-1 promoter activity. The induction of HO-1 gene transcription is dependent on the presence of AREs since mutation of this responsive element nullifies the stimulation of promoter activity by PIs. While several transcription factors bind to AREs, we previously found that Nrf2 plays an essential role in ARE-dependent HO-1 gene expression in endothelial cells [26,31,35,50]. Consistent with this earlier work, we found that PIs stimulate Nrf2 protein expression and nuclear Nrf2 binding to the AREs. Moreover, transfection of endothelial cells with a dominant-negative Nrf2 construct abolishes the activation of HO-1 promoter activity in response to PIs. Thus, mobilization of Nrf2 plays a fundamental role in mediating HO-1 gene transcription by PIs in endothelial cells.
The ability of PIs to stimulate HO-1 expression is dependent on oxidative stress. Incubation of endothelial cells with PIs elicits an increase in ROS production and prevention of this oxidative responsive by the antioxidant NAC blocks the induction of HO-1 by PIs. Although the precise intracellular source of PI-induced ROS production remains to be fully delineated, previous work indicates a mitochondrial origin [19]. Consistent with this notion, we found that rotenone, a mitochondrial electron transport chain inhibitor, blocks PI-mediated ROS formation and HO-1 expression, implicating this organelle with the induction of HO-1. In contrast, pharmacological inhibition of NADPH or xanthine oxidase fails to alter the induction of HO-1 by PIs, indicating that these ROS-generating enzymes are not involved in the induction of HO-1. The mechanism by which PI-derived oxidative stress activates Nrf2 likely involves the oxidation of specific cysteine residues in Kelch-like erythroid cell-derived protein-1 (Keap1) which has been implicated in the release and/or inhibition of Keap1-dependent ubiquitination and degradation of Nrf2 [51]. Although GSK3β has been shown to regulate Nrf2 levels by phosphorylating and targeting Nrf2 for proteasomal degradation via β-TrCP [41,42], RTV has no effect on GSK3β activity suggesting that this pathway is unlikely to contribute to the increase in Nrf2 noted in our study. Our results in endothelial cells are at odds with recent work showing that RTV and other PIs stimulate GSK3β activity in vascular smooth muscle cells and adipocytes, reflecting possible cell-specific actions of PIs [52,53]. Finally, PIs may also promote Nrf2 expression and activity by preventing the destruction of the transcription factor by the proteasome [54,55].
Our finding that PIs stimulate HO-1 expression via oxidative stress is consistent with studies in other cells. In particular, nelfinavir was shown to stimulate HO-1 expression in mouse adipocytes in a ROS-dependent manner, as the induction of HO-1 is suppressed by NAC and the superoxide dismutase mimetic MnTBAP [56]. The latter observation suggests that superoxide mediates the induction of HO-1 by PIs in adipocytes. Interestingly, RTV also induces HO-1 gene expression in human colon carcinoma cells [57]. In this case, the induction of HO-1 is dependent on the activation of p38 mitogen-activated protein kinase and is associated with the activation of the redox-sensitive transcription factor, activator protein-1. Thus, cells may utilize distinct redox-dependent signaling pathways to stimulate HO-1 expression in response to PIs.
Intriguingly, the HO-1 products modulate the induction of HO-1 by PIs and may provide a feedback regulatory mechanism to control HO-1 levels. However, this regulatory control is complex as the HO-1 products exert divergent effects on HO-1 expression. Bilirubin, like NAC, inhibits the PI-mediated expression of HO-1, likely reflecting the anti-oxidant properties of the pigment [58]. In contrast, CO augments both basal and PI-evoked increases in HO-1 expression, most probably due to the activation of Nrf2 [59–61].
In the present study, we demonstrate that PIs inhibit endothelial cell proliferation and DNA synthesis. A significant inhibition of endothelial cell DNA synthesis is detected at very low concentrations of RTV (2 μM) while higher concentrations (10 μM) of LPV and ATV are needed to block DNA synthesis. Moreover, we show that PIs block the migration of endothelial cells; however, this is only observed at high concentrations (50–100 μM). The anti-proliferative and anti-migratory action of PIs is observed in the absence of cell death, which is consistent with some, but not all studies [19,62,63]. Discrepancies between these studies may reflect variances in cell culture conditions, treatment regimen (dose, duration, and choice of PI), and/or vascular source of endothelial cells. The ability of PIs to suppress endothelial cell growth and migration may hinder the restoration of endothelial integrity and function of injured blood vessels and adversely affect the vasomotor, coagulative, and thrombotic properties of the vessel wall. In addition, inhibition of endothelial cell proliferation and migration by PIs may mediate their anti-angiogenic effect and contribute to the known antineoplastic actions of these agents [63,64].
We also found that PIs induce inflammatory responses in endothelial cells. Treatment of endothelial cells with RTV, LPV, or ATV stimulates the expression of ICAM-1 and increases the adhesion of monocytes to endothelial cell monolayers. These findings are in-line with previous work showing that PIs elevate adhesion receptor expression, the secretion of inflammatory cytokines, and monocyte adhesion in HAEC or human coronary endothelial cells [65,66]. Given the central role that inflammation plays in the initiation and progression of atherosclerosis, the inflammatory responses evoked by PIs may contribute to the atherogenic potential of these drugs.
Significantly, the induction of HO-1 in endothelial cells operates in an adaptive fashion to constrain the anti-proliferative and inflammatory effects of PIs. We found that inhibition of HO-1 activity and/or expression augments the anti-proliferative and inflammatory actions of RTV, while overexpression of HO-1 attenuates these responses. These findings are in accord with earlier studies showing that HO-1 enhances endothelial cell proliferation and retards endothelial inflammation [28–30]. In contrast, the induction of HO-1 does not modulate the anti-migratory action of PIs. Although HO-1 stimulates endothelial cell migration [29], inhibition of HO-1 activity has no effect on the ability of RTV to repress cell migration. In this instance, levels of HO-1 may be not be sufficient to regulate cell locomotion since we previously reported that HO-1 reaction products are more effective regulators of vascular cell growth than migration [67]. Significantly, the exogenous administration of bilirubin but not CO can substitute for HO-1 and restore the proliferative and anti-inflammatory responses of PI-challenged endothelial cells. These results are in agreement with previous work demonstrating that bilirubin exerts anti-inflammatory and mitogenic effects in endothelial cells [68,69]. Notably, the CO-releasing molecule CORM2 not only fails to reverse the inflammatory actions of HO-1 depletion, it leads to a further decline in growth in PI-treated endothelial cells. This later finding is consistent with recent studies showing that CO inhibits the proliferation of HUVEC [70,71]. Thus, HO-1-derived bilirubin is responsible for counteracting PI-induced endothelial cell growth arrest and inflammation.
Current strategies for preventing PI-induced endothelial dysfunction and cardiovascular disease are limited and largely restricted to the use of statins [20,66,72]. However, HO-1 represents an attractive therapeutic target in averting PI-associated endothelial dysfunction. Significantly, HO-1 restores endothelial function in diabetes and atherosclerosis which are major cardiometabolic disorders associated with chronic PI administration [32,33]. In addition, the present study found that HO-1 counteracts the deleterious effects of PIs on endothelial cell growth and inflammation. Thus, pharmacological approaches directed at HO-1 provides an appealing strategy in ameliorating endothelial dysfunction and the cardiovascular complications arising from the prolonged use of PIs. Numerous inducers of HO-1 have been identified, including its substrate heme which is approved for the treatment of acute porphyria [23,24,73]. Furthermore, as an alternative option, bilirubin may be directly administered for therapeutic effect [32,33].
In conclusion, the present study demonstrates that PIs induce HO-1 gene expression in endothelial cells via the activation of the ROS–Nrf2 signaling pathway. In addition, it found that PIs block endothelial cell proliferation and migration, and increases the expression of ICAM-1 and the adhesion of monocytes to vascular endothelium. Moreover, it showed that the induction of HO-1 antagonizes the anti-proliferative and inflammatory actions of PIs by generating bilirubin. HO-1 represents a promising therapeutic target in preventing PI-associated endothelial dysfunction and cardiovascular disease in HIV-positive patients.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award number R01HL59976.
Abbreviations
- PI
protease inhibitors
- ROS
reactive oxygen species
- HO-1
heme oxygenase-1
- CO
carbon monoxide
- CORM2
carbon monoxide releasing molecule-2
- iCORM2
inactivated carbon monoxide releasing molecule-2
- CM-H2DCFDA
5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate acetyl ester
- NAC
N-acetyl-L-cysteine
- PMSF
phenylmethylsulfonyl fluoride
- SnPP
tin protoporphyrin-IX
- Nrf2
NF-E2-related factor-2
- β-TrCP
β-transducin repeats-containing protein
- GSK3β
glycogen synthase kinase-3β
- ICAM-1
intercellular adhesion molecule-1
- RTV
ritonavir
- ATV
atazanavir
- LPV
lopinavir
- HUVEC
human umbilical vein endothelial cells
- HAEC
human aortic endothelial cells
- HDMEC
human dermal microvascular endothelial cells
- dnNrf2
dominant-negative Nrf2
- ARE
antioxidant responsive element
- siRNA
small interference RNA
- NT
non-targeting
- AdHO-1
replication-deficient adenovirus expressing HO-1
- AdGFP
replication-deficient adenovirus expressing green fluorescent protein
- Keap-1
Kelch-like erythroid cell-derived protein-1
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV out-patient study investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Flint OP, Noor MA, Hruz PW, Hyelmon PB, Yarasheski K, Kotler DP, Parker RA, Bellamine A. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications. Toxicol Pathol. 2009;37:65–77. doi: 10.1177/0192623308327119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d’Arminio Monforte A, Pradier C, Morfeldt L, Mateau S, Law M, El-Sadr W, De Wit S, Sabin CA, Phillips AN, Lundgren JD. Cardiovascular disease risk factors in HIV patients–association with antiviral therapy: results from the DAD study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 4.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte AD, El-Sadr W, Thebaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD. Class of antiviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, Greenberg AE, Janssen RS HIV outpatient study (HOPS) investigators. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 6.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D Clinical Epidemiology Group from the French Hospital Database. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 7.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–253. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 8.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. Clin Endocrinol. 2009;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein D, Hurley LB, Quesenberry CP, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV infection? J AIDS. 2002;30:471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Lifeson AR, Kranz EM, Grambsch PL, Macalino GE, Crum-Cianflone NF, Ganesan A, Okulicz JF, Eaton A, Powers JH, Eberly LE, Agan BK. Clinical, demographic and laboratory parameters at HAART initiation associated with decreased post-HAART survival in a US military perspective HIV cohort. AIDS Res Ther. 2012;9:4. doi: 10.1186/1742-6405-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggi P, Lillo A, Perilli F, Maserati R, Chirianni A PREVALEAT Group. Colour-Doppler ultrasonography of carotid vessels in patients treated with antiretroviral therapy: a comparative study. AIDS. 2004;18:1023–1028. doi: 10.1097/00002030-200404300-00010. [DOI] [PubMed] [Google Scholar]
- 12.de Saint Martin L, Vandhuick O, Guillo P, Bellein V, Bressollette L, Roudaut N, Amaral A, Pasquier E. Premature atherosclerosis in HIV positive patients and cumulated time of exposure to antiretroviral therapy (SHIVA study) Atherosclerosis. 2006;185:361–367. doi: 10.1016/j.atherosclerosis.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 13.Lijfering WM, Ten Kate MK, Sprenger HG, van der Meer J. Absolute risk of venous and arterial thrombosis in HIV-infected patients and effects of combination antiviral therapy. J Thromb Haemost. 2006;4:1928–1930. doi: 10.1111/j.1538-7836.2006.02047.x. [DOI] [PubMed] [Google Scholar]
- 14.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 15.Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipid changes and endothelial dysfunction. Circulation. 2001;104:257–261. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 16.Conklin BS, Fu W, Lin PH, Lumsden AB, Yao Q, Chen C. HIV protease inhibitor ritonavir decreases endothelium-dependent vasorelaxation and increases superoxide in porcine arteries. Cardiovasc Res. 2004:168–175. doi: 10.1016/j.cardiores.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Chai H, Lin PH, Yao Q, Chen C. Roles and mechanisms of human immunodeficiency virus protease inhibitor ritonavir and other anti-human immunodeficiency virus drugs in endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. Am J Pathol. 2009;174:771–781. doi: 10.2353/ajpath.2009.080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai H, Yang H, Yan S, Li M, Lumsden AB, Yao Q, Chen C. Effects of 5 HIV protease inhibitors on vasomotor function and superoxide anion production in porcine coronary arteries. J Acquir Immune Defic Syndr. 2005;40:12–19. doi: 10.1097/01.qai.0000172368.05327.7b. [DOI] [PubMed] [Google Scholar]
- 19.Jiang B, Hebert VY, Li Y, Mathis JM, Alexander JS, Dugas TR. HIV antiviral drug combination induces endothelial mitochondrial dysfunction and reactive species production but not apoptosis HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive species production but not apoptosis. Toxicol Appl Pharmacol. 2007;224:60–71. doi: 10.1016/j.taap.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Lefevre C, Auclair M, Boccara F, Bastard JP, Capeau J, Vigouroux C, Caron-Debarle M. Premature senescence of vascular cells is induced by HIV protease inhibitors: implication of prolamin A and reversion by statin. Arterioscler Thromb Vasc Biol. 2010;30:2611–2620. doi: 10.1161/ATVBAHA.110.213603. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Lu XH, Yan S, Chai H, Yao Q. HIV protease inhibitor ritonavir increases endothelial monolayer permeability. Biochem Biophys Res Commun. 2005;335:874–882. doi: 10.1016/j.bbrc.2005.07.155. [DOI] [PubMed] [Google Scholar]
- 22.Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durante W. Targeting heme oxygenase-1 in vascular disease. Curr Drug Targets. 2010;11:1504–1516. doi: 10.2174/1389450111009011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durante W. Protective role of heme oxygenase-1 against inflammation in atherosclerosis. Front Biosci. 2011;16:2372–2388. doi: 10.2741/3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y, Liu XM, Peyton KJ, Wang H, Johnson FK, Johnson RA, Durante W. Hypochlorous acid-induced heme oxygenase-1 gene expression promotes human endothelial cell survival. Am J Physiol Cell Physiol. 2009;297:C907–C915. doi: 10.1152/ajpcell.00536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yachie A, Niida Y, Wada T, Igarishi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Volti G, Wang J, Traganos F, Kappas A, Abraham NG. Differential effect of heme oxygenase-1 in endothelial and smooth muscle cell cycle progression. Biochem Biophys Res Commun. 2002;296:1077–1082. doi: 10.1016/s0006-291x(02)02054-5. [DOI] [PubMed] [Google Scholar]
- 29.Deshane J, Chen S, Caballero S, Grot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MB, Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role for vegf-induced heme oxygenase-1 in vivo: Induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 31.Lin CC, Liu XM, Peyton KJ, Wang H, Yang WC, Lin SJ, Durante W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol. 2008;28:739–745. doi: 10.1161/ATVBAHA.107.160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, Itabe H, Kodama T, Maruyama Y. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Wang L, Tian XY, Liu L, Wong WT, Zhang Y, Han QB, Ho HM, Wang N, Wong SL, Chen ZY, Yu J, Ng CF, Yao X, Huang Y. Unconjugated bilirubin mediates heme oxygenase-1-induced vascular benefits in diabetic mice. Diabetes. 2015;64:1564–1575. doi: 10.2337/db14-1391. [DOI] [PubMed] [Google Scholar]
- 34.Peyton KJ, Liu XM, Durante W. Prolonged cyclic strain inhibits human endothelial cell growth. Front Biosci. 2016;8:205–212. doi: 10.2741/e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu XM, Peyton KJ, Durante W. Physiological cyclic strain promotes endothelial cell survival via the induction of heme oxygenase-1. Am J Physiol Heart Circ Physiol. 2013;302:H1634–H1643. doi: 10.1152/ajpheart.00872.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durante W, Peyton KJ, Schafer AI. Platelet-derived growth factor stimulates heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2666–2672. doi: 10.1161/01.atv.19.11.2666. [DOI] [PubMed] [Google Scholar]
- 37.Peyton KJ, Liu XM, Yu Y, Yates B, Durante W. Activation of AMP-activated protein kinase inhibits the proliferation of human endothelial cells. J Pharmacol Exp Ther. 2012;342:827–834. doi: 10.1124/jpet.112.194712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noor MA, Flint OP, Maa JF, Parker RA. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS. 2006;20:1813–1821. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 39.Taburet AM, Raguin G, Le Tiec C, Droz C, Barrail A, Vincent I, Morand-Joubert L, Chêne G, Clavel F, Girard PM. Interactions between amprenavir and the lopinavir–ritonavir combination in heavily pretreated patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 2004;75:310–323. doi: 10.1016/j.clpt.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Taburet AM, Piketty C, Chazallon C, Vincent I, Gérard L, Calvez V, Clavel F, Aboulker JP, Girard PM. Interactions between atazanavir–ritonavir, and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:2091–2096. doi: 10.1128/AAC.48.6.2091-2096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobon-Velasco JC, Devijver H, Garcia-Mayoral MF, Van Leuven F, Hayes JD, Bertho G, Cuadrado A. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol Cell Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowdrhry S, Zhang Y, McHahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase, B. Nature. 1995;1985(378):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 44.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 45.Ly Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV/AIDS. 2015;7:95–104. doi: 10.2147/HIV.S79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaedicke S, Firat-Geier E, Constantiniu O, Lucchiari-Hartz M, Freudenberg M, Galanos C, Niedermann G. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumor cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 2002;62:6901–6908. [PubMed] [Google Scholar]
- 47.Deeks SG, Smith M, Holodiniy M, Kahan JO. HIV protease inhibitors. A review for clinicians. J Am Med Assoc. 1997;277:145–153. [PubMed] [Google Scholar]
- 48.D’Avolio A, Carcieri C, Cusato J, Simiele M, Calcagno A, Allegra S, Sciandra S, Trentini L, Di Perri G, Bonora S. Intracellular accumulation of atazanavir/ritonavir according to plasma concentrations and OATP1B1m ABCB1m abd PXR genetic polymorphisms. J Antimicrob Chemother. 2014;69:3061–3066. doi: 10.1093/jac/dku234. [DOI] [PubMed] [Google Scholar]
- 49.Ali F, Zakkar M, Karu K, Liddington EA, Hamdulay SS, Boyle JJ, Zloh M, Bauer A, Haskard DO, Evans PC, Mason JC. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem. 2009;284:1882–1892. doi: 10.1074/jbc.M109.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu XM, Peyton KJ, Shebib AR, Wang H, Durante W. Compound C stimulates heme oxygenase-1 gene expression via the Nrf2-ARE pathway to preserve human endothelial cell survival. Biochem Pharmacol. 2011;82:371–379. doi: 10.1016/j.bcp.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang DD, Hannink M. Distinct cysteine residues in keap1 are required for keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventative agents and oxidative stress. Mol Cell Biol. 2003;23:137–151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gary-Bobo G, Houssaini A, Amsellem V, Rideau D, Pacaud P, Perrin A, Bregeon J, Marcos E, Dubois-Rande JL, Sitbon O, Savale L, Adnot S. Effects of HIV protease inhibitors on progression of monocrotaline- and hypoxia-induced pulmonary hypertension. Circulation. 2010;122:1937–1947. doi: 10.1161/CIRCULATIONAHA.110.973750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kachko I, Maissel A, Mazor L, Ben-Romano R, Watson RT, Hou JC, Pessin JE, Bashan N, Rudich A. Postreceptoral adipocyte insulin resistance induced by nelfinavir is caused by insensitivity of PKB/Akt to phosphatidylinositol-3,4,5-triphosphate. Endocrinology. 2009;150:2618–2626. doi: 10.1210/en.2008-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang JS, Distler O, Cooper DA, Jamil H, Deckelbaum RJ, Ginsberg HN, Sturley SL. HIV protease inhibitors protect apolipoprotein B from degradation by the proteasome: a potential mechanism for protease inhibitor-induced hyperlipidemia. Nat Med. 2001;7:1327–1331. doi: 10.1038/nm1201-1327. [DOI] [PubMed] [Google Scholar]
- 55.Pajonk F, Himmelsbach J, Riess K, Sommer A, McBride WH. The human immunodeficiency virus (HIV)-protease inhibitor saquinavir inhibits proteasomal function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002;62:5230–5235. [PubMed] [Google Scholar]
- 56.Ben-Romano R, Rudich A, Etzion S, Potashnik R, Kagan E, Greenbaum U, Bashan N. Nelfinavir induces adipocyte insulin resistance through the induction of oxidative stress: differential protective effect of antioxidant agents. Antiviral Ther. 2006;11:1051–1060. [PubMed] [Google Scholar]
- 57.Muhl H, Paulukat J, Hofler S, Hellmuth M, Franzen R, Pfeilschifter J. The HIV protease inhibitor ritonavir synergizes with butyrate for induction of apoptotic cell death and mediates the expression of heme oxygenase-1 in DLD-1 colon carcinoma cells. Br J Pharmacol. 2004;143:890–898. doi: 10.1038/sj.bjp.0706023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stocker R, Yamamoto Y, McDonagh AF, Glazer An, Ames BN. Bilirubin is an antioxidant of possible biological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 59.Lee BS, Heo J, Kim YM, Shim SM, Pae HO, Kim YM, Chung HT. Carbon Monoxide Mediates Heme Oxygenase 1 Induction via Nrf2 Activation in Hepatoma Cells. doi: 10.1016/j.bbrc.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 60.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chi PL, Lin CC, Chen YW, Hsiao LD, Yang CM. CO induces Nrf2-dependent heme oxygenase-1 transcription by cooperating with Sp1 and c-Jun in rat brain astrocytes. Mol Neurobiol. 2015;52:277–292. doi: 10.1007/s12035-014-8869-4. [DOI] [PubMed] [Google Scholar]
- 62.Zhong DS, Lu XH, Conklin BS, Lin PH, Lumsden AB, Yao Q, Chen C. HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:1560–1566. doi: 10.1161/01.atv.0000034707.40046.02. [DOI] [PubMed] [Google Scholar]
- 63.Sgadari C, Barillari G, Toschi E, Carlei D, Bacigalupo I, Baccarini S, Palladino C, Leone P, Bugarini R, Malavasi L, Cafaro A, Falchi M, Valdembri D, Rezza G, Bussolino F, Monini P, Ensoli B. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8:225–232. doi: 10.1038/nm0302-225. [DOI] [PubMed] [Google Scholar]
- 64.Pyrko P, Kardosh A, Wang W, Xiong W, Schonthal AH, Chen TC. HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma cell death by triggering endoplasmic reticulum stress. Cancer Res. 2007;67:10920–10928. doi: 10.1158/0008-5472.CAN-07-0796. [DOI] [PubMed] [Google Scholar]
- 65.Mondal D, Pradhan L, Ali M, Agarwal KC. HAART drugs induce oxidative stress in human endothelial cells and increase endothelial recruitment of mononuclear cells: exacerbation by inflammatory cytokines and amelioration by antioxidants. Cardiovasc Toxicol. 2004;4:287–302. doi: 10.1385/ct:4:3:287. [DOI] [PubMed] [Google Scholar]
- 66.Auclair M, Afonso P, Capel E, Caron-Debarle M, Capeau J. Impact of darunavir, atazanavir, and lopinavir boosted with ritonavir on cultured human endothelial cells: beneficial effect of pravastatin. Antiviral Ther. 2014;19:773–782. doi: 10.3851/IMP2752. [DOI] [PubMed] [Google Scholar]
- 67.Peyton KJ, Shebib AR, Azam MA, Liu XM, Tulis DA, Durante W. Bilirubin inhibits neointima formation and vascular smooth muscle cell proliferation and migration. Front Pharmacol. 2012;3:48. doi: 10.3389/fphar.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 69.Ikeda Y, Hamano H, Satoh A, Horinouchi Y, Izawa-Ishizawa Y, Kihiri Y, Ishizawa K, Aihara KI. Bilirubin exerts pro-angiogenic property through Akt-eNOS-dependent pathway. Hypertens Res. 2016 doi: 10.1038/hr.2015.74. in press. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Wang H, Yang B, Yang J, Ruan X, Yang Y, Wakeland EK, Li Q, Fang X. Influence of carbon monoxide on growth and apoptosis of human umbilical artery smooth muscle cells and vein endothelial cells. Int J Biol Sci. 2012;8:1431–1446. doi: 10.7150/ijbs.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmad S, Hewitt PW, Fujisawa T, Sissaoui S, Cai M, Gueron G, Al-Ani B, Cudmore M, Ahmed SF, Wong MK, Weigel B, Otterbein LE, Vitek L, Ramma W, Wang K, Ahmed A. Carbon monoxide inhibits sprouting angiogenesis and vascular endothelial growth factor receptor-2 phosphorylation. Thromb Haemost. 2015;113:329–337. doi: 10.1160/TH14-01-0002. [DOI] [PubMed] [Google Scholar]
- 72.Hurlimann D, Chenevard R, Ruschitzka F, Flepp M, Enseleit F, Bechir M, Kobza R, Muntwyler J, Ledergerber B, Luscher TF, Noll G, Weber R. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomized double blind crossover trial. Heart. 2006;92:110–112. doi: 10.1136/hrt.2004.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrandiz ML, Devesa I. Inducers of heme oxygenase-1. Curr Pharm Des. 2008;14:473–486. doi: 10.2174/138161208783597399. [DOI] [PubMed] [Google Scholar]