Abstract
The global incidence of obesity and its comorbidities continues to rise along with a demand for novel therapeutic interventions. Brown adipose tissue (BAT) is attracting attention as a therapeutic target because of its presence in adult humans and high capacity to dissipate energy as heat, and thus burn excess calories, when stimulated. Another potential avenue for therapeutic intervention is to induce, within white adipose tissue (WAT), the formation of brown-like adipocytes called brite (brown-like-in-white) or beige adipocytes. However, understanding how to harness the potential of these thermogenic cells requires a deep understanding of their developmental origins and regulation. Recent cell labeling and lineage tracing experiments are beginning to shed light on this emerging area of adipocyte biology. Here, we review adipocyte development giving particular attention to thermogenic adipocytes.
Keywords: brown adipocyte, brite adipocyte, beige adipocyte, white adipocyte, UCP1, thermogenesis, adipose tissue development, obesity and lipodystrophy
New frontiers in adipose tissue biology
Great progress is being made towards understanding the role of adipose tissue in metabolic health largely driven by the obesity pandemic. It is now clear that in addition to its primary role in energy storage white adipose tissue (WAT) is a central controller of glucose and lipid homeostasis that communicates locally and with distant tissues through complex metabolite and protein based signals. It is also clear that different WAT depots and even adipocytes within the same depot can have quite different functional properties. One example is the higher risk for metabolic disease associated with excess visceral fat compared to subcutaneous fat. A more recent addition to the broad discussion of adipose tissue in health and disease is the role of brown adipose tissue (BAT). Interest in brown fat has been reinvigorated by recent descriptions of adult human BAT, its positive correlation with metabolic health, and prospects of harnessing properties of energy expenditure in brown fat as a therapeutic approach for obesity. Understanding the mechanisms linking these diverse types of adipose tissue to metabolic health is critical to fighting metabolic disease, diabetes, cardiovascular disease and even some cancers.
Central to understanding adipose tissue diversity is its developmental origins, which remains one of the least understood aspects of adipose tissue biology. However, it is an area that has seen tremendous interest and progress in the last few years. Defining adipocyte origins could: i. help explain the variable fat distribution patterns seen in the human population, particularly in obese or lipodystrophic individuals; ii. provide clues to the metabolic differences between some fat depots; iii. reveal the identity of adipocyte precursor cells and the mechanisms that promote or block their expansion; and iv. offer insight into strategies to engineer the development of specific types of adipocytes (such as brown adipocytes) for cell-based therapies aimed at fighting fat with fat. Below we discuss the different classes of adipocytes and current opinions and controversies regarding their origins. We aim to provide a framework for future investigations in this important, expanding and exciting area of research.
Types of Adipocytes
Brown Adipocytes
Many features of brown adipocytes distinguish them from energy-storing white adipocytes including more abundant mitochondria, the presence of many small lipid droplets, and high expression of uncoupling protein 1 (UCP1) [Figure 1A]. UCP1 is an inner mitochondrial membrane protein that when active uncouples the mitochondrial respiratory chain from ATP production to dissipate heat—a process called non-shivering thermogenesis [1]. In un-stimulated brown adipocytes, UCP1 is thought to be inactive due in part to the inhibitory action of purine nucleotides (ATP or GDP) [2, 3]. In response to cold stress, adrenergic stimuli like noradrenaline activate G-protein coupled β-adrenergic receptors. This stimulates cyclic-AMP/PKA signaling to increase UCP1 expression and trigger lipolysis, which liberates fatty acids that both bind and activate UCP1 and provide fuel for thermogenesis [2–5]. Active BAT also imports glucose and fatty acids to provide additional fuel for sustained thermogenesis [6, 7]. Exactly how BAT coordinates fuel selection and usage under different physiological states remains incompletely understood. Whether BAT has critical functions beyond thermal regulation is also not clear although BAT can secrete adipokines such as IL6, FGF21 and chemerin [8, 9]. Although these adipokines are not BAT specific it suggests that BAT might have additional endocrine functions.
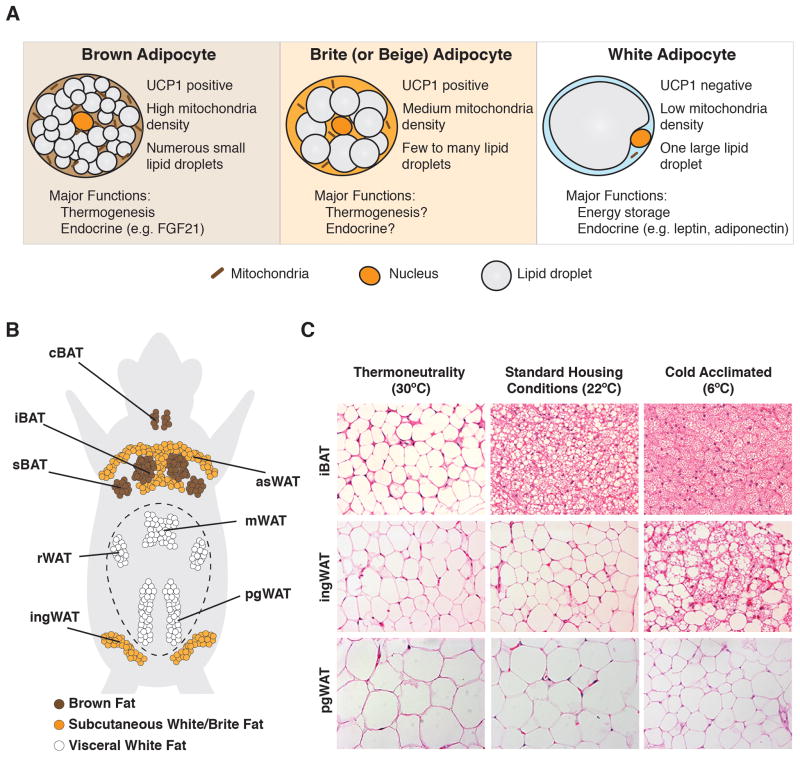
Figure 1. The Anatomy of adipose tissue and its plasticity in response to temperature.
(A) General morphological and functional differences between brown, brite/beige and white adipocytes. (B) Anatomical distribution of major adipose tissue depots. iBAT: interscapular BAT; sBAT: subscapular BAT; cBAT: cervical BAT: asWAT: anterior subcutaneous WAT; ingWAT: inguinal WAT; mWAT: mesenteric WAT; rWAT: retroperitoneal WAT; pgWAT: perigonadal WAT. The peritoneum is represented by a dotted line (C) Brown and scWAT remodeling in 13-week old male C57Bl/6 mice is temperature sensitive. In mice living in their thermoneutral zone (~30°C for 4 weeks), brown adipocytes appear characteristically like white adipocytes, as shown here by H&E staining. At standard mouse housing temperatures (~22°C), BAT assumes its familiar “active” appearance. At significantly colder temperatures (progressively decreasing form 22 to 6°C in a period of 4 weeks), scWAT remodels, and appears characteristically more like BAT in mice living in standard housing conditions. Perigonadal visceral WAT is largely resistant to temperature-induced remodeling.
In humans and mice, brown adipocytes concentrate in discrete depots strategically clustered in regions with high blood flow. The largest depots in mice are in the inter-scapular, sub-scapular and cervical regions [Figure 1B] [10–12]. Smaller depots are present at the hilum of the kidney and around the aorta [10, 13]. Post-mortem analysis of adult humans originally revealed human BAT deposits around the carotid artery, aorta, and subscapular region [Box 1]. Surprisingly however, the existence of adult human BAT was largely ignored until recently when 18FDG PET-CT analysis of glucose uptake was used to image human BAT deposits [14–25]. These studies collectively revealed the widespread existence of BAT in humans and an inverse correlation between BAT amount and BMI (body mass index). This ignited interest in the prospect of therapeutically increasing BAT activity to fight obesity. The extent to which BAT amount varies in the population remains unclear but should be clarified as new tracer methods are developed [26].
Box 1. Do humans have brown or brite fat?
Until recently it was widely assumed that adult humans lack significant brown fat deposits. This view changed in 2007 when the Cannon and Nedergaard lab reported that some adult human fat exhibits BAT characteristics [20]. Several labs later confirmed these findings [21–24, 84], thereby inspiring the idea that activating thermogenic fat in humans could be a strategy to fight obesity [14–19]. In fact, administration of a β3-adrenergic agonist to humans increases BAT glucose uptake and increases resting metabolic rate by 13% [85]. A question currently under debate is whether humans have classic brown or brite/beige fat. Importantly, because humans typically live in thermoneutral conditions, the distinction between “brown” and “brite” may be difficult to make [See also Figure 1C]. Thus, understanding both brown and brite adipocyte biology in mice (the only genetic model currently available) is of equal interest at present.
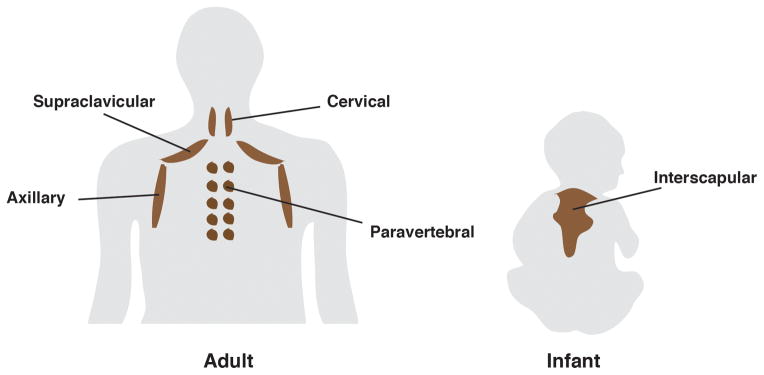
Human infants have a large interscapular BAT depot that shares both molecular and functional features with brown adipocytes in rodents indicating that newborn humans have classic brown fat [86]. In humans, interscapular BAT disappears with age. In adults, thermogenic adipocytes concentrate in smaller cervical, supraclavicular, paravertebral depots [Figure I]. Some groups conclude that adult human BAT depots are composed of brite/beige adipocytes, based on marker genes that express selectively in murine beige/brite adipocytes [71, 87, 88]. Other groups propose that thermogenic adipocytes isolated from the deep neck and supraclavicular region most closely resemble classic murine brown fat but that some brite-like adipocytes might also be present [89–91]. Collectively, these studies suggest heterogeneity in the prevalence of human brown and brite adipocytes that varies with anatomical location and other inter-subject variations such as gender, age, and genetics. Importantly, studies of human BAT origin often rely on gene expression signatures using markers identified in mice. However, whether these markers are informative in humans is unclear and only a few—Zic1 (brown), Cd137, Epsti1, Tbx1, Tmem26 (brite/beige) and Tcf21 (white)—have been carefully validated [10]. Because murine brown fat appears similar to white/brite fat in mice living in their thermoneutral zone [See Figure 1C] it may be informative to revisit gene expression comparisons between human and murine fat using samples isolated from mice living without thermal stress.
Figure I.
The distribution of brown adipose tissues in human adults (left) and infants (right)
BAT can expand both by increasing cell number (hyperplasia) and cell size (hypertrophy). Hypertrophic growth is largely mediated by changes in intracellular lipid content. For example, brown adipocytes in mice living without thermal stress (i.e. in their thermoneutral zone) are less metabolically active and therefore accumulate lipids in a single large unilocular lipid droplet. Under these conditions, brown adipocytes appear characteristically more similar to that of a classic white adipocyte [Figure 1C]. In contrast, the brown adipocytes in mice living at standard mouse facility temperatures (e.g. ~22°C), a temperature in which mice are cold-stressed, have the familiar multi-locular lipid droplet morphology [Figure 1C]. This morphology is exaggerated at more extreme cold temperatures [Figure 1C]. In response to high caloric (fat) diets or increasing age, brown adipocytes also become characteristically more like white adipocytes, increasing their intracellular lipid content and overall size [27, 28]. Although cold exposure reduces brown adipocyte size by triggering lipolysis and β-oxidation, prolonged cold exposure also increases total BAT mass by triggering the proliferation and differentiation of brown adipocyte precursors, thereby increasing the number of brown adipocytes [29–31].
White Adipocytes
White adipocytes, the most abundant type of adipocyte, contain a single large lipid droplet and mostly concentrate in discrete depots [Figure 1B]. White adipocytes primarily function to store fuel and release adipokines such as leptin and adiponectin that regulate systemic energy homeostasis. WAT expansion (e.g. in obesity) also protects organs like muscle and liver from lipotoxicity [32]. The major WAT depots are generally distinguished anatomically as being subcutaneous or visceral, with excess of the latter being linked to metabolic disease while excess of the former being protective [33, 34]. Why subcutaneous and visceral fats have seemingly opposing metabolic properties is incompletely understood. The largest subcutaneous (sc) depots in mice are the anterior, interscapular, and posterior inguinal scWATs. The largest visceral depots are the perigonadal, mesenteric, and retroperitoneal WATs. Most studies focus on the inguinal scWAT and perigonadal visceral WAT in male mice as representative depots and thus the distinctions between subcutaneous and visceral are often oversimplified. WAT can also expand by increasing in size or number though the mechanism varies depending upon the depot and stimulus [35].
Brite/Beige Adipocytes
Considerable attention has recently shifted to understanding a potential third class of adipocyte known as a “brite” (brown-like-in-white) or “beige” adipocyte [Figure 1A] [36, 37]. As the brite nomenclature implies, these are brown-like adipocytes (i.e. multilocular and UCP1+) that appear within certain white fat depots (those in the inguinal scWAT being the most studied) when the body experiences cold stress. When un-stimulated, brite adipocytes appear characteristically similar to white adipocytes [Figure 1C]. Although it is widely argued that brite adipocytes in sWAT are completely different from the white adipocytes they reside with, it has been suggested that the entire inguinal scWAT depot may be a brite adipocyte organ [38, 39].
The formation of brite adipocytes (or remodeling of scWAT) in response to cold is called the “browning” of WAT. Browning is also associated with cancer cachexia and severe burns [40–42]. It is generally accepted that brite adipocytes are distinct from classic brown adipocytes, but that their primary function is also to generate heat to help maintain body temperature. This is supported by studies showing that individual active brown and brite adipocytes express similar levels of UCP1 mRNA [43]. However, in cold acclimated mice the total amount of UCP1 in whole inguinal WAT is still much lower than that of whole BAT indicating that the bulk of thermogenesis still occurs in BAT [44]. Multiple reports also indicate that the presence of brite adipocytes positively affects whole body glucose regulation, which has important implications in treating type 2 diabetes [45]
Although the model that WAT browning evolved to maintain body temperature seems intuitive, an alternative possibility is that browning occurs secondary to a change in the intracellular metabolic environment. For example, browning associates with physiological states characterized by increased systemic energy demand (e.g. cold stress; exercise). As high sympathetic activity increases lipolysis in WAT to meet those systemic demands, intracellular fatty acid availability also rises imposing a significant metabolic stress on the small number of mitochondria in white adipocytes [46], which could trigger UCP1-mediated uncoupling. This could also explain why browning is observed in cancer cachexia and burning. Brite cells might also produce specific adipokines although this requires further investigations [Box 2].
Box 2. Temperature matters for metabolic studies.
Therapeutics that can enhance thermogenesis may be useful in fighting obesity. Several agents and genetic models have been proposed to improve metabolism by inducing thermogenesis [45]. However, several criteria need to be considered when interpreting these results [38]. First, non-shivering thermogenesis and UCP1 expression (brown and brite) is linked to ambient temperature and most studies perform experiments at 22°C, well below the thermoneutral zone for mice, which is between 29–31°C [92]. At 22°C, mice are cold stressed and have an approximately 50% higher metabolic rate and consume more food than at thermoneutrality [92]. To accurately assess the effect of a thermogenic agent, metabolic experiments should be performed at thermoneutral temperatures to ensure that any non-shivering thermogenesis and improved metabolic performance is due only to the treatment. Moreover, thermogenic agents may indirectly cause cold stress and browning, for example by affecting insulation via the alteration of fur density or dermal adipocyte layering [38].
Case in point: FGF21 is a cytokine secreted by brown and white fat and liver that profoundly affects metabolism by significantly improving glucose homeostasis. In various studies FGF21 induces browning, leading to the model that increased thermogenesis is at least partially causing its beneficial effects [93–95]. However, when mice living at thermoneutrality or genetically ucp1-deficient are treated with FGF21, the same beneficial metabolic effects are observed in the absence of WAT browning or UCP1 induction [47, 96], suggesting that neither brite adipocytes nor non-shivering thermogenesis are required for the beneficial effects of FGF21. However, these results may depend on the conditions used (e.g. lean versus obese mice) [97]. Importantly, the influences of temperature are also beginning to be recognized in other fields [98–100] suggesting that housing mice at current standard laboratory conditions may actually compromise the ability to model certain human diseases.
The distinction between brown, white, and brite adipocytes is based largely on depot morphology and gene expression analysis of whole depots isolated from cold-stressed rodents (e.g. housed at 22°C). However, using whole depots rather than purified adipocytes may mask the gene expression changes most relevant to the metabolic state of each cell type because mature adipocytes comprise only a fraction of the total cells in fat depots. Moreover, when mice are housed under thermoneutral conditions (e.g. 30°C) in which thermal stress is eliminated, classic brown adipocytes appear unilocular and have a more WAT-like gene expression signature [Figure 1C][27, 47] further blurring the adipocyte color spectrum. As temperature progressively decreases, classic BAT depots are activated first (e.g. at 22°C), followed by the appearance of brite adipocytes in the scWAT at colder temperatures, suggesting that both BAT and scWAT have similar remodeling capacity and that they respond in a hierarchical manner in response to cold [38]. Perigonadal visceral WAT (at least in C57Bl6 mice) is largely resistant to browning under these conditions [Figure 1C]. The ability of rodent brown adipocytes to switch between unilocular and multilocular states is relevant to the study of human thermogenic adipocytes because it is under debate as to whether humans possess brown or brite adipocytes [Box 1]. For example, comparisons are often made between human brown fat and the brite/beige adipocytes in scWAT; however, the BAT of mice living at thermoneutral conditions, which is more similar to scWAT, may be a better comparison.
Tissue Resident Adipocyte Progenitors
All defined adipose tissue depots appear to have a pool of adipocyte progenitor cells that reside in the stromal vascular fraction and can be enriched for by fluorescence activated cell sorting (FACS) [48–51]. Several groups have developed slightly varied cell surface marker profiles useful for isolating adipocyte progenitor cells. They often include the positive selection markers PDGFRα, CD34, Sca1, CD29 and CD24, and negative selection markers CD31, Ter119 and CD45. Pref1 (Preadipocyte factor 1) also expresses in adipocyte precursors and is a trans-membrane protein suggesting it might also be useful for isolating adipocyte progenitors [52]. Similar protocols can also be used to isolate human adipogenic precursor cells for study and for cell-based therapies. However, with the exception of CD24, these markers label a large fraction of the total SVF population and it remains unclear whether they capture the true adipocyte progenitor pool. Moreover, most of the markers do not have obvious functional roles in adipocyte development, nor are they informative about the developmental origins of adipocytes. Therefore, in vivo lineage tracing has been adopted to understand developmental origins [Box 3].
Box 3. Lineage tracing tools and technical considerations.
For typical Cre/Lox-based lineage tracing, Cre is expressed under control of a cell/tissue-specific promoter to indelibly activate a reporter gene—usually a Lox-Stop-Lox fluorescent or LacZ reporter—that is used to track the fate of the original Cre-expressing cell. Although this is currently the gold standard for lineage tracing in mice [101], there are important caveats to consider when designing and interpreting such studies, particularly for adipocytes. Mature adipocytes contain little cytoplasm and are composed primarily of triacylglycerol. Having such little cytoplasm reduces the resolution of cytoplasmic reporters, such as LacZ or GFP. LacZ has an additional caveat of relying on indirect staining for imaging in which the XGal precipitate can “drift” from the cellular region in which it is generated, often clumping with lipid droplets where it is not expressed. Membrane targeted fluorescent reporters (such as the membrane targeted eGFP, tdTomato, and mTmG reporters) seem to be preferred tools for labeling precursor and mature adipocytes because they concentrate at the plasma membrane and can faithfully resolve individual adipocytes. These reporters are also useful for single cell purification experiments [29, 48, 58, 67, 102]. For example, in the dual fluorescent reporter system called the “LoxP-membrane targeted tdTomato-LoxP-membrane targeted GFP” or mTmG reporter, any cell expressing Cre and its descendants are indelibly labeled with membrane targeted GFP; all other cells are labeled with membrane targeted tdTomato.
There are also several considerations concerning the Cre driver. First, Cre activity is strictly a measure of promoter activity not of the gene product that the promoter normally encodes. The use of knock-in Cre drivers is ideal because Cre is under control of the endogenous promoter, while transgenic Cre drivers are more susceptible to influences by the genomic region in which they integrate. Second, while a constitutive Cre often has high recombination efficiency, it cannot be turned off, making it difficult to determine when and where Cre first expresses in a particular lineage. These caveats also apply to transgenic reporters (e.g. when a promoter is coupled to GFP and inserted into randomly into the genome). The use of tamoxifen or doxycycline inducible Cre systems can circumvent concerns with constitutive expression by allowing for transient labeling and subsequent tracking of the original labeled cells. In this approach, all existing mature adipocytes can be labeled tdTomato+ following a short tamoxifen-treatment; therefore, if new adipocytes formed after tamoxifen washout they should be tdTomatonegative. However, efficiency relies on penetration of the inducer. Moreover, tamoxifen may remain present in adipose tissue after the washout period and it has transient toxic effects on fat [77]. Doxycycline, by contrast, can affect mitochondrial function and is toxic to gut microbiota [103]. In sum, Cre-Lox based lineage tracing is a powerful method for tracking cell fate but should be interpreted cautiously.
Brown Adipocyte Development
Due to clear differences in metabolism and anatomical distribution it seemed empirically evident that brown adipocytes originate from a cellular lineage distinct from all white adipocytes. Early evidence supporting this model came from lineage tracing experiments in which embryonic mesenchymal precursor cells expressing the transcription factor Engrailed 1 (En1) were found to give rise to interscapular brown adipocytes, skeletal muscles, and dermis [53, 54]. Gene expression profiling studies further revealed that brown preadipocytes isolated from the stromal vascular fraction of the interscapular BAT depot have a signature more similar to skeletal muscles than to stromal vascular fraction cells from pgWAT, including expression of myf5 and myoD (which encode muscle differentiation factors) [55]. The independent lineage model of brown and white adipocyte development seemed incontrovertible following a lineage tracing study reporting that interscapular brown adipocytes and skeletal muscles but no inguinal or perigonadal white adipocytes were labeled by the Myf5-Cre knock-in allele [Box 3] [56]. Similar results were subsequently reported with Pax7-Cre [57] and it rapidly became widely assumed that brown adipocytes share a common myf5+ and pax7+ precursor cell with muscle while white adipocytes arise from a different lineage.
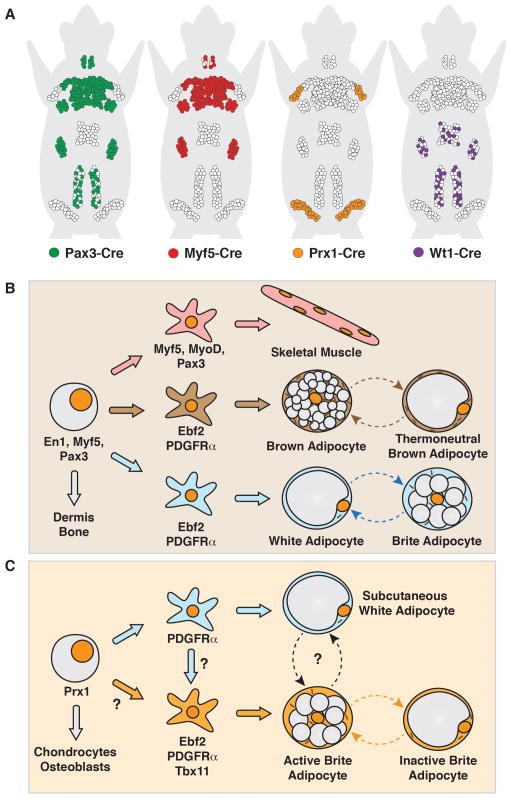
The independent myf5+ lineage model of brown and white adipocyte origins was elegant in its simplicity; however, recent studies indicate the situation is more complex. Genetic studies of BAT using the same Myf5-Cre knock-in allele observe targeting in the interscapular and anterior scWATs as well as the retroperitoneal WAT, depots not examined in earlier studies [27, 49, 58–63] [Figure 2A]. To comprehensively map the Myf5-Cre labeling pattern, Myf5-Cre was combined with a dual fluorescent reporter called the “LoxP-membrane targeted tdTomato-LoxP-membrane targeted GFP” or mTmG reporter, which is particularly useful for labeling adipocytes [Box 3] [50, 58, 64]. These studies confirm that white unilocular adipocytes in the anterior scWAT, interscapular scWAT, and retroperitoneal WAT also originate from Myf5-Cre expressing precursors. This approach also revealed that many brown adipocytes do not have a history of Myf5-Cre expression [58]. Indeed only about half of the adipocytes in cervical BAT are marked with Myf5-Cre, and no brown adipocytes in the periaortic or perirenal BAT depots are labeled, while all brown adipocytes in the interscapular and subscapular depots are labeled [58]. Similar observations in both BAT and WAT were made with a Pax3-Cre knock-in driver (Pax3 is muscle transcription factor) [58] [Figure 2A].
Figure 2. Current models depicting the developmental origins of adipocytes based on in vivo lineage tracing/cell labeling studies.
(A) Representation of Pax3-Cre, Myf5-Cre, Prx1-Cre, and Wt1-Cre labeling of mature adipocytes in young mice. It should be noted that Cre-labeling is only a measure of promoter activity. Although it is often assumed that Cre activity mirrors expression of the gene product controlled by that promoter [See Box 3]. (B) Lineage model depicting the origin of skeletal muscle, most brown adipocytes, and many white adipocytes from a common Myf5/Pax3+ multipotent precursor cell. It is alternatively possible that multiple lineages independently express Myf5/Pax3. Lipid droplets in both brown and white adipocytes can appear unilocular or multilocular depending upon the temperature. (C) Lineage model depicting subcutaneous white and brite adipocyte origins. It remains unclear if and when white and brite lineages diverge, and whether the primary mechanism of brite adipocyte formation is by de novo adipogenesis from precursors or by inter-conversion from existing mature adipocytes.
Collectively, these studies suggest distinct pools of brown and white adipocytes descend from embryonic En1+, Pax3+ and Myf5+ mesenchymal precursor cells [Figure 2B]. Being that these factors express early in embryonic development, it is likely that they express in multipotent precursors prior to adipocyte commitment. Notably however, the aforementioned studies cannot determine when these Cre drivers first express in adipocyte lineages, or exclude the possibility that Myf5/Pax3-Cre labeled brown and white adipocytes descend from different precursor cell pools that independently express them [Box 2]. Nevertheless, the evidence argues that there is likely more than one origin of both brown and white adipocytes, and that a lineage marker unique to only brown adipocyte precursors remains elusive.
Subcutaneous White and Brite (or Beige) Adipocyte Development
The unique browning capacity of scWAT [Figure 1C] raises several questions. Why do scWATs possess a higher capacity for browning than visceral pgWAT in response to cold? Are brite adipocytes a unique class of adipocytes that masquerade in scWAT as unilocular white adipocytes and acquire thermogenic properties only when stimulated; or rather do they arise de novo from precursor cells following stimulation? Do brite adipocytes originate from a unique lineage, different from that of both brown and white adipocytes? Can all white adipocytes become brite if appropriately stimulated? There are no definitive answers yet to these questions and the emerging picture suggests they are likely complex and multifactorial.
Is there a brite/beige adipocyte lineage?
Myf5/Pax3-Cre lineage tracing experiments indicate that brite adipocytes in the inguinal scWAT do not arise from a Myf5/Pax3 lineage [49, 56, 58]. However, most brite adipocytes in the anterior scWAT and retroperitoneal WAT are positively marked with Myf5-Cre and Pax3-Cre [58]. Thus, previous expression of Myf5-Cre and Pax3-Cre is not a distinguishing factor between brown and brite adipocyte origin. Transient labeling with the PDGFRα-CreERT2 driver led to the description of a bipotential progenitor cell residing in visceral WAT that could give rise to brite adipocytes in response to β3-adrenergic stimulation. However, later lineage tracing experiments could not confirm these findings, negating PDGFRα+ expression as a potential distinguishing feature of a brite adipocyte lineage [30, 48, 65].
The Paired Related Homeobox transcription factor 1 Cre (Prx1-Cre) transgene, which expresses in the early embryonic limb bud mesenchyme [66], is highly selective at labeling the pre- and mature adipocytes only in inguinal and anterior scWAT and not in visceral white or brown fat depots [Figure 2A][67, 68]. Consistent with its selective labeling of scWAT, conditional deletiion the SHP-2 phosphatase, a key adipogenic factor, with Prx1-Cre resulted in mice that lack subcutaneous fat [69, 70]. In response to β3-adrenergic receptor agonist (CL316,243) treatment, all multilocular adipocytes that arise in the posterior subcutaneous WAT also arise from a Prx1-Cre expressing cell. Thus, Prx1-Cre labeling, while selective for scWAT, does not distinguish between nascent brite adipocytes and existing white adipocytes. Prx1-Cre may express in an early precursor pool that gives rise to both subcutaneous brite and white adipocytes in addition to chondrocytes and osteoblasts. However, Prx1-Cre could also express in separate precursor pools that give rise to these different cell types.
Presently, the strongest evidence favoring the existence of a brite adipocyte lineage comes from clonal cell isolation studies. In these studies, adipogenic precursor cell lines were generated from the stromal vascular fraction of inguinal WAT and found to have different propensity to express either brite/beige or white adipocyte specific markers (such as ucp1) upon differentiation in culture [71]. However, definitive existence of a brite/beige lineage still awaits confirmation by in vivo lineage tracing.
Brite adipocytes reportedly possess a smooth muscle cell like gene expression profile not observed in brown adipocytes [43]. However, after two weeks at 4°C, constitutive and inducible Cre drivers under the control of the Myh11 promoter (a selective marker smooth muscle-like cells) labeled approximately 10% of the brite adipocytes in inguinal scWAT. Thus, a subset of brite adipocytes may originate from smooth muscle-like cells, but Myh11 is neither a universal nor dominant marker of a brite adipocyte lineage [43].
One candidate marker, though not a specific marker, for a committed brite adipocyte precursor, is the Ebf2 transcription factor [50]. Ebf2 is highly expressed in early brown adipogenic cells isolated from embryos based on positive Myf5-Cre labeling and PDGFRα expression. Ebf2 also regulates the expression of thermogenic genes in mature brown adipocytes [72]. Inducing WAT browning in mice via cold exposure increased the expression of Ebf2 in a subpopulation of scWAT stromal vascular fraction cells that also express PDGFRα [50]. Moreover, while all of the PDGFRα+ precursor cells in scWAT are adipogenic in vitro, only those expressing Ebf2 are capable of displaying a brown fat gene expression signature. Thus, Ebf2 may be an early functional marker of both brown and brite preadipocytes.
How do brite/beige adipocytes develop?
How brite adipocytes form is currently under debate. Most published studies favor a model in which brite adipocytes in the scWAT, regardless of whether they originate from a unique lineage or not, normally arise from preexisting unilocular/UCP1negative adipocytes. Fine imaging by light and electron microscopy, DNA content analysis, and BrdU labeling studies in cold exposed or β3-adrenergic receptor stimulated rodents support the hypothesis that brite adipocytes originate from preexisting white adipocytes—a process we refer to here as “inter-conversion” though it has also been referred to as “transdifferentiation” [30, 37, 65, 73–76].
One group tested the inter-conversion model in mice using a Cre-based cell labeling strategy [29]. The authors in this study generated a “UCP1-tracer” mouse model that can label cells currently expressing UCP1 as well as marked cells that had previously but no longer expressed UCP1 (i.e. former brite adipocytes) with different reporters. The study found that following an initial cold challenge, which induces UCP1+ brite adipocytes, returning mice to room temperature transforms the nascent brite adipocytes into UCP1negative unilocular white adipocytes. More importantly, 75% of the cells that induce UCP1 and become multilocular upon a second cold acclimation originate from the former brite adipocyte population. These results provide strong evidence that brite adipocytes that have turned white have a “memory” of their former self and can rapidly interconvert between a unilocular/UCP1negative white and multilocular/UCP1+ brown adipocyte-like states. This strategy however cannot determine how brite adipocytes arose in the first cold acclimation (e.g. by inter-conversion or de novo from precursor cells).
Using a different Cre-based labeling strategy, a second study observed that brite adipocytes forming in scWAT do not arise from PDGFRα+ precursor cells or incorporate the proliferation marker BrdU during their formation [30]. This led the authors to conclude that brite adipocytes must arise from existing unilocular adipocytes. To test this directly, the authors inducibly marked mature adipocytes (i.e. by combining the inducible Adipoq-CreERT2 driver with the ROSA26-tdTomato reporter mice [Box 3]) to determine whether nascent brite adipocytes retained the label after tamoxifen washout. The study found that all multilocular/UCP1+ adipocytes induced by cold or CL316,243 after tamoxifen washout retained the tdTomato+ label. The combination of these results supports the hypothesis that brite adipocytes in scWAT arise by inter-conversion from existing unilocular adipocytes. Similar observations were made using the mTmG reporter [30].
Despite strong evidence favoring inter-conversion, data equally as strong suggests the alternative possibility that brite adipocytes originate de novo from precursors. For example, another study employing a doxycycline-inducible Cre system to permanently label all mature adipocytes (coined the AdipoChaser mouse) reported that most brite/beige adipocytes arise de novo following a 3-day cold exposure or CL316,243 treatment [35]. Similar findings were noted using the mTmG reporter [77]. In the AdipoChaser mouse, several nascent brite/beige adipocytes are also LacZ+, suggesting brite/beige adipocytes might form both by de novo adipogenesis and inter-conversion in this model. Alternatively, the reporter could be leaky.
The reason for these different conclusions is puzzling. One proposed explanation is that detectable levels of tamoxifen remain in the adipose tissue several days after washout and that the CreERT2 protein remains in the nucleus of white adipocytes weeks after treatment, which would compromise the ability to use this approach to transiently label cells [77, 78]. However, this does not seem to impact the ability to shut off CreERT2 in BAT after tamoxifen washout, nor after 6 weeks in the scWAT [29, 30]. Another possibility is that the mechanism of browning could depend on whether the mice were previously cold stressed (e.g. in neonatal and/or early juvenile development). If this occurred, then some existing white adipocytes could be former brite adipocytes and therefore rapidly interconvert upon cold exposure. In contrast, mice that never had brite adipocytes might generate them de novo [29]. Another hypothesis is that a predetermined brite adipocyte lineage may give rise to white-like unilocular adipocytes that reside alongside non-thermogenic white adipocytes waiting for the appropriate stimulus. Further experiments are clearly required to test these hypotheses. Notably, a discussion largely missing from the debate is the role of the sympathetic nervous system and the vasculature. This should be further investigated.
Interestingly, de novo adipogenesis of UCP1negative cells occurs in pgWAT following cold exposure [35, 65] suggesting cold-induced preadipocyte activation in WAT is not specific to brite cells. Why new visceral white adipocytes form is presently unknown.
Visceral White Adipocyte Development
Although perigonadal WAT is largely resistant to browning induced by cold [Figure 1B]; the retroperitoneal WAT (rWAT) displays different metabolic characteristics. For instance, the capacity of rWAT to induce UCP1 and become multilocular after cold or CL316,263 treatment is quite high, on par with that of inguinal scWAT [49]. Interestingly, all of the mature white adipocytes in the rWAT of young (6 week old) mice are derived from Myf5- and Pax3-expressing precursors [58], suggesting they originate in the paraxial mesoderm along with most brown adipocytes and anterior WATs. With age, however, Myf5/Pax3-lineagenegative adipocytes increase in abundance in the rWAT, suggesting that two populations of adipocytes, one of embryonic origin and one derived from adult precursor cells, may contribute to this depot [58]. In addition, Pax3-Cre marked precursor cells, but not those labeled with Myf5-Cre, also constitute about half of the adipocytes in the pgWAT of male but not female mice, providing one of the first indications of a gender variation in origins [58]. Whether there is functional relevance to this observation is unclear and there is no correlation between Pax3-Cre labeling in male pgWAT and browning [58, 62]. Mesenteric WAT is completely Myf5 and Pax3 negative [58].
Some visceral white adipocytes in all major visceral depots (perigonadal, mesenteric, omental, retroperitoneal, and perirenal) derive from precursor cells in the lateral plate mesoderm that express the Wilm’s tumor (Wt1) gene promoter (ranging from 28% in the mesenteric WAT to 77% in the perigonadal WAT) as determined by Wt1-Cre and Wt1-CreERT2 lineage tracing [Figure 2A] [79]. Interestingly, no subcutaneous white or brown adipocytes arise from Wt1-Cre expressing precursors. Furthermore, a Wt1-GFP transgene is expressed in a population of visceral CD34+Sca1+ stromal vascular fraction that are enriched for adipogenic progenitors suggesting Wt1 might also function in visceral adipocyte development [79]. Wt1 precursors are multipotent, generating testicles, ovaries, kidneys, spleen, adrenal glands, the mesothelial layer of visceral organs, and endothelial cells [79, 80]. Thus, a lineage marker that specifically labels all visceral white adipocytes has not been identified. The picture emerging suggests that visceral adipocytes might have multiple origins.
Concluding Remarks
One goal going forward is to determine when lineages become adipocyte committed and the key determining factors (see also Outstanding Questions). Importantly, elucidating how different adipocyte precursor pools sense and respond to environmental, nutritional, and hormonal cues may be equally if not more crucial to understanding the adaptability of adipose tissue. For example, external factors in the microenvironment could be major drivers of adipocyte precursor identity and fate. Regardless, identifying fate determinants could lead to therapeutic strategies that selectively expand or activate favorable adipocyte lineages (such as brown or brite adipocytes). A major advance would be the definitive identification of a true (human and rodent) adipocyte stem cell and genetic tools such as Cre drivers that can be used in mice to selectively target them. Regarding the latter, a step in this direction is the finding that Prx1-Cre and Wt1-Cre can selectively target subcutaneous and visceral white preadipocytes respectively. However, in each case non-adipocyte lineages are also targeted. Whether markers truly unique to brown, white, and brite adipocyte precursors can be identified remains to be seen. Human adipocyte precursor cells are also abundantly available through minimally invasive procedures. Defining them may have implications for cell-based therapies.
Outstanding questions.
Is human BAT a good target for an anti-obesity therapy? Now that it is clear that adult humans have BAT, finding therapeutic strategies to increase BAT mass and/or activity to burn excess energy is an exciting goal. But it remains unclear if selectively activating human BAT is safely achievable, would be comfortably tolerated, and is attractive pharmaceutically.
What are all the lineages that give rise to brown and white adipocytes and when is adipocyte identity determined? Adipocyte development is complex. New tools and strategies will be important to determine when lineages become committed into adipocytes.
Which features of murine adipose tissue development and function are conserved in humans? The analysis of different kinds of adipocytes in mice is providing new insight and molecular markers of adipocyte origins and identity; however, many of these discoveries await validation in humans.
Does developmental origin impact adult adipose tissue distribution and function? What determines the set point for an individuals fat distribution pattern is not known but could be influenced by where specific depots originate from, as well as the adjacent tissues with which they develop.
What is the primary evolutionary function of WAT browning? Conventional wisdom favors thermogenesis; but whether WAT browning evolved to fight cold temperature or whether this is the primary function of BAT - and WAT browning has another purpose - is not clear.
Are brite adipocytes definable by a distinct lineage, or do other cues establish which adipocytes become thermogenic?
Do brite adipocytes arise by transdifferentiation, de novo from precursors, or both?
What other functions do adipocytes have, particularly those in less studied locations such as the bone marrow and skin?
The study of adipocyte development is a relatively new area and the ideal tools and techniques are still being devised. In addition, there are many, less studied depots for which interest is rapidly growing [Box 4]. The emerging picture is that adipocyte development is complex, with adipocytes likely having multiple developmental origins. It is noteworthy that many lipodystrophy disorders are often characterized by selective adipose tissue atrophy sometimes in combination with compensatory overgrowth in other depots [81], possibly reflecting different origins. Adding to this complexity, emerging evidence also suggests neonatal/juvenile adipogenesis may be mechanistically different from adipogenesis in adults [82, 83]. Characterizing adipocyte development is essential to understanding obesity and those most at risk for obesity-related complications, as well as the set point determinants for white versus brown or brite fat abundance, for which the depot distribution is highly variable in the human population. Although recent years have seen great progress in understanding adipose tissue development, we are just scratching the surface of everything that is to come.
Box 4. Other Adipocytes of Emerging Interest.
Head adipocytes
Significant fat deposits exist in the head that contribute to facial shape and insulation. A subset of these adipocytes originates from neural crest cells as indicated by lineage tracing with Sox10-Cre+ and Wnt1-Cre+ [104, 105]. Subsets of muscles, cartilage, and bones in the head also derive from neural crest derivatives, suggesting that some neural crest precursors undergo an epithelial to mesenchymal transition to make key structural components of the head [106]. Whether facial adipocytes have other functions is not clear.
Skin adipocytes
Adipocytes exist in the skin layered between the dermis and panniculus carnosus, called dermal white adipose tissue (dWAT). Emerging evidence suggests these adipocytes regulate the hair follicle cycle, function as a barrier against infection, and contribute to wound healing and thermo-insulation [107–110]. It has been calculated that dermal adipocytes may represent 7% of body fat in women [111]. Skin adipocytes appear to originate from a distinct subset of fibroblasts residing in the lower dermis that are also marked by Dlk1/Pref1-CreERT2 at E16.5 [112].
Intramuscular adipocytes
These adipocytes are highly abundant particularly in humans where they are interspersed in limb muscles between the muscle cells. Their function is not clear, though their presence correlates with insulin resistance and type 2 diabetes [113–115]. In mice, these adipocytes appear to originate from PDGFRα+, Myf5-Crenegative and Pax3-Crenegative precursors suggesting they do not share a common precursor with skeletal muscle [51, 116].
Bone Marrow Adipocytes
Marrow adipose tissue (MAT) is thought to function as a support matrix for bone marrow components and its relative amount may be related to fracture risk [117, 118]. Emerging studies suggest multiple functional types of MAT exist (called constitutive and regulated MAT) that differs in timing of adipocyte appearance, lipid composition, genetic determinants and gene expression [119]. MAT is Myf5-Crenegative and Vav1-Crenegative, which labels hematopoietic stem cells [120]. Moreover, only about half of the marrow adipocytes are marked with PDGFRα-Cre in contrast to all other adipocyte pools that have been examined using this Cre [48, 120]. Marrow adipocytes but no others are labeled with Osx1-Cre (Osx1 is a transcription factor essential for osteoblast differentiation) supporting their emergence from an embryonic precursor pool shared with bone [120, 121].
Trends Box.
The developmental origins of adipose tissue and the mechanisms controlling its expansion are just beginning to be revealed. Although the tools and techniques used to study adipocyte development still need refinement, the emerging picture is that adipocyte development is complex. Brown, brite (or beige) and white adipocytes may have multiple developmental origins.
In addition to giving rise to brown adipocytes and skeletal muscle cells, precursor cells expressing the Myf5 promoter also give rise to some white/brite adipocytes.
It is currently under debate as to whether subcutaneous brite/beige adipocytes arise by inter-conversion or transdifferentiation from certain existing mature adipocytes, or de novo from precursors, and strong evidence supports both models.
Whether brown or brite/beige adipocytes are more prevalent in adult humans is also unclear. Emerging data suggests heterogeneity dependent upon multiple factors.
Acknowledgments
DAG is supported by grants from the NIH (R01DK094004 & R01CA196986), the American Diabetes Association (ADA113BS066), and a Leukemia & Lymphoma Society Career Development Award. JSG is supported by a postdoctoral fellowship from the American Heart Association (15POST25550079). We thank the Guertin lab for valuable discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls DG. The physiological regulation of uncoupling proteins. Biochimica et biophysica acta. 2006;1757:459–466. doi: 10.1016/j.bbabio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Sluse FE, et al. Mitochondrial UCPs: new insights into regulation and impact. Biochimica et biophysica acta. 2006;1757:480–485. doi: 10.1016/j.bbabio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Lehr L, et al. The control of UCP1 is dissociated from that of PGC-1alpha or of mitochondriogenesis as revealed by a study using beta-less mouse brown adipocytes in culture. FEBS letters. 2006;580:4661–4666. doi: 10.1016/j.febslet.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Fedorenko A, et al. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labbe SM, et al. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:2046–2058. doi: 10.1096/fj.14-266247. [DOI] [PubMed] [Google Scholar]
- 7.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nature medicine. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 8.Villarroya J, et al. An endocrine role for brown adipose tissue? American journal of physiology. Endocrinology and metabolism. 2013;305:E567–572. doi: 10.1152/ajpendo.00250.2013. [DOI] [PubMed] [Google Scholar]
- 9.Hansen IR, et al. Contrasting effects of cold acclimation versus obesogenic diets on chemerin gene expression in brown and brite adipose tissues. Biochimica et biophysica acta. 2014;1841:1691–1699. doi: 10.1016/j.bbalip.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 10.de Jong JM, et al. A stringent validation of mouse adipose tissue identity markers. American journal of physiology. Endocrinology and metabolism. 2015;308:E1085–1105. doi: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- 11.Walden TB, et al. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. American journal of physiology. Endocrinology and metabolism. 2012;302:E19–31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 12.Cinti S. The adipose organ. Prostaglandins, leukotrienes, and essential fatty acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgibbons TP, et al. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. American journal of physiology. Heart and circulatory physiology. 2011;301:H1425–1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoneshiro T, et al. Age-Related Decrease in Brown Adipose Tissue and Obesity in Humans. Obesity. 2011;19:S79–S79. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- 15.Yoneshiro T, et al. Brown Adipose Tissue, Whole-Body Energy Expenditure, and Thermogenesis in Healthy Adult Men. Obesity. 2011;19:13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 16.Yoneshiro T, et al. Recruited brown adipose tissue as an antiobesity agent in humans. The Journal of clinical investigation. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Lans AA, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. The Journal of clinical investigation. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouellet V, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. Journal of Clinical Investigation. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanssen MJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nature medicine. 2015 doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 20.Nedergaard J, et al. Unexpected evidence for active brown adipose tissue in adult humans. American journal of physiology. Endocrinology and metabolism. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 21.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 23.Virtanen KA, et al. Brief Report: Functional Brown Adipose Tissue in Healthy Adults. New England Journal of Medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 24.Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kortelainen ML, et al. Immunohistochemical detection of human brown adipose tissue uncoupling protein in an autopsy series. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1993;41:759–764. doi: 10.1177/41.5.8468458. [DOI] [PubMed] [Google Scholar]
- 26.Cypess AM, et al. Brown fat in humans: consensus points and experimental guidelines. Cell metabolism. 2014;20:408–415. doi: 10.1016/j.cmet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung CM, et al. Rictor/mTORC2 loss in the Myf5 lineage reprograms brown fat metabolism and protects mice against obesity and metabolic disease. Cell reports. 2014;8:256–271. doi: 10.1016/j.celrep.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts-Toler C, et al. Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity (Silver Spring, Md) 2015;23:1765–1770. doi: 10.1002/oby.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenwald M, et al. Bi-directional interconversion of brite and white adipocytes. Nature cell biology. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, et al. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:286–299. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bukowiecki LJ, et al. Proliferation and differentiation of brown adipocytes from interstitial cells during cold acclimation. The American journal of physiology. 1986;250:C880–887. doi: 10.1152/ajpcell.1986.250.6.C880. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Annals of the New York Academy of Sciences. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pischon T, et al. General and Abdominal Adiposity and Risk of Death in Europe. New England Journal of Medicine. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 34.Meisinger C, et al. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg Cohort Study. American Journal of Clinical Nutrition. 2006;84:483–489. doi: 10.1093/ajcn/84.3.483. [DOI] [PubMed] [Google Scholar]
- 35.Wang QA, et al. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature medicine. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young P, et al. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS letters. 1984;167:10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 37.Cousin B, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. Journal of cell science. 1992;103( Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 38.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell metabolism. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Kozak LP. The genetics of brown adipocyte induction in white fat depots. Frontiers in endocrinology. 2011;2:64. doi: 10.3389/fendo.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidossis LS, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell metabolism. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petruzzelli M, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell metabolism. 2014;20:433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Tsoli M, et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer research. 2012;72:4372–4382. doi: 10.1158/0008-5472.CAN-11-3536. [DOI] [PubMed] [Google Scholar]
- 43.Long JZ, et al. A smooth muscle-like origin for beige adipocytes. Cell metabolism. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shabalina IG, et al. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell reports. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 45.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 46.Rutkowski JM, et al. The cell biology of fat expansion. The Journal of cell biology. 2015;208:501–512. doi: 10.1083/jcb.201409063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veniant MM, et al. Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell metabolism. 2015;21:731–738. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nature cell biology. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez-Gurmaches J, et al. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell metabolism. 2012;16:348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudak CS, Sul HS. Pref-1, a gatekeeper of adipogenesis. Frontiers in endocrinology. 2013;4:79. doi: 10.3389/fendo.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atit R, et al. beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Developmental biology. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 54.Sgaier SK, et al. Morphogenetic and cellular movements that shape the mouse cerebellum: Insights from genetic fate mapping. Neuron. 2005;45:27–40. doi: 10.1016/j.neuron.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 55.Timmons JA, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–U927. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lepper C, Fan CM. Inducible Lineage Tracing of Pax7-Descendant Cells Reveals Embryonic Origin of Adult Satellite Cells. Genesis (New York, NY: 2000) 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nature communications. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulz TJ, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harms MJ, et al. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell metabolism. 2014;19:593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez-Lopez N, et al. Autophagy in Myf5+ progenitors regulates energy and glucose homeostasis through control of brown fat and skeletal muscle development. EMBO reports. 2013;14:795–803. doi: 10.1038/embor.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W, et al. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. Journal of cell science. 2013;126:3527–3532. doi: 10.1242/jcs.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shan T, et al. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. Journal of lipid research. 2013;54:2214–2224. doi: 10.1194/jlr.M038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muzumdar MD, et al. A global double-fluorescent Cre reporter mouse. Genesis (New York, NY: 2000) 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 65.Lee YH, et al. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell metabolism. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Logan M, et al. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis (New York, NY : 2000) 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez-Gurmaches J, et al. Highly selective in vivo labeling of subcutaneous white adipocyte precursors with prx1-cre. Stem cell reports. 2015;4:541–550. doi: 10.1016/j.stemcr.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krueger KC, et al. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem cell reports. 2014;3:1147–1158. doi: 10.1016/j.stemcr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lapinski PE, et al. Deletion of SHP-2 in mesenchymal stem cells causes growth retardation, limb and chest deformity, and calvarial defects in mice. Disease models & mechanisms. 2013;6:1448–1458. doi: 10.1242/dmm.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He Z, et al. Nonreceptor tyrosine phosphatase Shp2 promotes adipogenesis through inhibition of p38 MAP kinase. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E79–88. doi: 10.1073/pnas.1213000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajakumari S, et al. EBF2 determines and maintains brown adipocyte identity. Cell metabolism. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barbatelli G, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. American journal of physiology. Endocrinology and metabolism. 2010;298:E1244–1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 74.Granneman JG, et al. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. American journal of physiology. Endocrinology and metabolism. 2005;289:E608–616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 75.Himms-Hagen J, et al. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. American journal of physiology. Cell physiology. 2000;279:C670–681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 76.Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. Journal of endocrinological investigation. 2002;25:823–835. doi: 10.1007/BF03344046. [DOI] [PubMed] [Google Scholar]
- 77.Ye R, et al. Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Molecular metabolism. 2015;4:771–778. doi: 10.1016/j.molmet.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reinert RB, et al. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PloS one. 2012;7:e33529. doi: 10.1371/journal.pone.0033529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chau YY, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nature cell biology. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner KD, et al. The Wilms’ tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nature communications. 2014;5:5852. doi: 10.1038/ncomms6852. [DOI] [PubMed] [Google Scholar]
- 81.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. The Journal of clinical endocrinology and metabolism. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang Y, et al. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell reports. 2014;9:1007–1022. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang QA, et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nature cell biology. 2015;17:1099–1111. doi: 10.1038/ncb3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zingaretti MC, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 85.Cypess AM, et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell metabolism. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lidell ME, et al. Evidence for two types of brown adipose tissue in humans. Nature medicine. 2013 doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 87.Sharp LZ, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS one. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shinoda K, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nature medicine. 2015;21:389–394. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cypess AM, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature medicine. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jespersen NZ, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell metabolism. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 91.Xue R, et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nature medicine. 2015;21:760–768. doi: 10.1038/nm.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. The Journal of experimental biology. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 93.Veniant MM, et al. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PloS one. 2012;7:e40164. doi: 10.1371/journal.pone.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Veniant MM, et al. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology. 2012;153:4192–4203. doi: 10.1210/en.2012-1211. [DOI] [PubMed] [Google Scholar]
- 95.Hondares E, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. The Journal of biological chemistry. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samms RJ, et al. Discrete Aspects of FGF21 In Vivo Pharmacology Do Not Require UCP1. Cell reports. 2015;11:991–999. doi: 10.1016/j.celrep.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 97.Kwon Michelle M, et al. FGF21-Mediated Improvements in Glucose Clearance Require Uncoupling Protein 1. Cell reports. doi: 10.1016/j.celrep.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 98.Stemmer K, et al. Thermoneutral housing is a critical factor for immune function and diet-induced obesity in C57BL/6 nude mice. International journal of obesity (2005) 2015;39:791–797. doi: 10.1038/ijo.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kokolus KM, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian XY, et al. Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell metabolism. 2015 doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kretzschmar K, Watt FM. Lineage tracing. Cell. 2012;148:33–45. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 102.Jeffery E, et al. Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte. 2014;3:206–211. doi: 10.4161/adip.29674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moullan N, et al. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell reports. 2015 doi: 10.1016/j.celrep.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Billon N, et al. The generation of adipocytes by the neural crest. Development (Cambridge, England) 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lemos DR, et al. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem cells (Dayton, Ohio) 2012;30:1152–1162. doi: 10.1002/stem.1082. [DOI] [PubMed] [Google Scholar]
- 106.Bronner-Fraser M. Neural crest cell formation and migration in the developing embryo. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1994;8:699–706. doi: 10.1096/fasebj.8.10.8050668. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L-j, et al. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science (New York, NY) 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development (Cambridge, England) 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kasza I, et al. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS genetics. 2014;10:e1004514. doi: 10.1371/journal.pgen.1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexander CM, et al. Dermal white adipose tissue: a new component of the thermogenic response. Journal of lipid research. 2015;56:2061–2069. doi: 10.1194/jlr.R062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Driskell RR, et al. Defining dermal adipose tissue. Experimental dermatology. 2014;23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gallagher D, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. The American journal of clinical nutrition. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schrauwen-Hinderling VB, et al. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring, Md) 2006;14:357–367. doi: 10.1038/oby.2006.47. [DOI] [PubMed] [Google Scholar]
- 115.Yim JE, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. International journal of obesity (2005) 2007;31:1400–1405. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uezumi A, et al. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 117.Doucette CR, et al. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. Journal of cellular physiology. 2015;230:2032–2037. doi: 10.1002/jcp.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fazeli PK, et al. Marrow fat and bone--new perspectives. The Journal of clinical endocrinology and metabolism. 2013;98:935–945. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scheller EL, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nature communications. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berry R, et al. Adipose Tissue-Residing Progenitors (Adipocyte Lineage Progenitors and Adipose-Derived Stem Cells (ADSC)) Curr Mol Bio Rep. 2015;1:101–109. doi: 10.1007/s40610-015-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen J, et al. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PloS one. 2014;9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]