Abstract
This longitudinal study investigated whether variation in the oxytocin receptor gene (OXTR) and early parent-child interactions predicted later empathic behavior in 84 toddlers at high or low familial risk for ASD. Two well-studied OXTR single nucleotide polymorphisms (SNPs), rs53576 and rs2254298, were examined. Parent-child interaction was measured at 15 and 18 months of age during free play sessions. Empathy was measured at 24 and 30 months using a response to parental distress paradigm. While there was no direct association between parent-child interaction quality or OXTR and empathy, rs53576 moderated the relation between interaction quality and empathy. Results suggest that the interplay between OXTR and early parent-child interactions predicts individual differences in empathy in children at varying risk for atypical social development. Findings are consonant with a differential susceptibility model in which an OXTR variant may increase the social salience of interaction processes for specific allele carriers. These results increase our understanding of predictors of empathy development in young children with a wide range of social outcomes.
Keywords: Oxytocin Receptor Gene, Parent-Child Interaction, Empathy, Autism Risk
Empathy, the ability to identify with and respond to others' emotional experiences, is a core component of social-emotional development. Individual differences in empathy are associated with prosocial behavior (Eisenberg & Miller, 1987), social competence (Zhou et al., 2002), and relationship quality (Cramer, 2003). Empathic behavior typically emerges between one and two years of age as children transition from exhibiting personal distress to demonstrating concern for others (Knafo, Zahn-Waxler, Van Hulle, Robinson, & Rhee, 2008; Zahn-Waxler, Radke-Yarrow, Wagner, & Chapman, 1992). However, predictors of variation in early empathy development are not well understood among children with related developmental risks. Adopting a developmental psychopathology approach, we investigated predictors of individual differences in empathy in children with a wide range of outcomes in social functioning.
Early Empathy Deficits in Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted and repetitive behaviors and interests (APA, 2013). The social deficits associated with ASD include difficulty empathizing with others (e.g., Sigman, Kasari, Kwon, & Yirmiya, 1992; Yirmiya, Sigman, Kasari, & Mundy, 1992). There is general agreement concerning the presence of cognitive empathy deficits in individuals with ASD, although there is debate as to whether there are impairments in emotional empathy associated with ASD (e.g., Rueda, Fernandez-Berrocal, & Baron-Cohen, 2015; Smith, 2009). ASD is typically not diagnosed until at least three years of age, making it difficult to understand the role of early developing abilities such as empathy in the emergence of the disorder. Approximately one-fifth of the younger siblings of children with ASD (high-risk siblings) will meet criteria for an ASD diagnosis at 36 months (Ozonoff et el., 2011) and another one-fifth will evidence subclinical symptoms of the disorder (Messinger et al., 2013). In this study, we prospectively follow these high-risk siblings to examine early predictors of empathy in the context of risk for atypical social outcomes.
Deficits in empathy are apparent as early as 12 months of age in high-risk siblings later diagnosed with ASD, as measured by children's behavioral responses to the simulated distress of an examiner or a parent (Hutman et al., 2010; McDonald & Messinger, 2012). In addition, high-risk children who responded more empathically to their parents' distress at 24 months of age exhibited lower ASD symptom severity at 30 months (McDonald & Messinger, 2012). Little is known, however, about contributors to empathy development among children at elevated risk for ASD. We included high-risk siblings to investigate whether predictors of empathy in low-risk children can be generalized to children at risk for disorders involving clinically relevant empathy deficits, such as ASD.
Genetic and Environmental Influences on Empathy Development
There is a robust literature describing early empathy development, altruism, and prosocial behavior, which contributed to the motivation and methodology of the current work. With regard to predictors of individual differences in empathy development, longitudinal studies of monozygotic and dizygotic twins suggest a substantial role for both heritable and non-shared environmental factors in the development of empathy. Responses to simulated distress measured in twins at 14 and 20 months of age indicated significant heritability at both ages for empathic behavior (e.g., displaying a sympathetic facial expression, engaging in helping behaviors; Zahn-Waxler, Robinson, & Emde, 1992). At 24 and 36 months, heritability was associated with one-third to one-half of the variation in children's empathic behaviors, with non-specific environmental factors believed to account for the remaining variance (Knafo et al., 2008). Two promising predictors of individual differences in early empathy development are characteristics of early parent-child interactions and genetic variation in the oxytocin system.
Parent-Child Interaction
Several longitudinal studies have found associations between early parent-child interactions and empathy in typically developing children. Both mutually responsive orientation and affective synchrony, theoretically similar constructs, predict empathy development in children (Feldman, 2007; Kochanska, 2002). Mutually responsive orientation reflects a close, mutually binding, cooperative, and positive parent-child relationship. Early mutually responsive orientation and its components predict empathic responding and other aspects of conscience development in early childhood (e.g., Kochanska, Forman, & Coy, 1999; Kochanska, Forman, Aksan, & Dunbar, 2005). Similarly, affective synchrony, the temporal matching of affective behavior during parent-child interactions, shows longitudinal relations with empathy development (Feldman, 2007). In addition to these dyadic characteristics, individual parenting behaviors, including emotional availability (Moreno, Klute, & Robinson, 2008), warmth (Robinson, Zahn-Waxler, & Emde, 1994; Zhou et al., 2002), and discourse about emotions (Garner, 2003) are predictively associated with higher observed empathy.
Despite evidence suggesting an important contribution of early parent-child interactions to empathy in typically developing children, little is known about the impact of early social interactions in children who are at risk for atypical empathy development (e.g., ASD). The present study examined the influence of early parent-child interaction qualities such as dyadic affective mutuality and parent supportiveness on later empathic behavior in children at varying risk for ASD. Research on family contributors to social cognition abilities indicates similar developmental pathways among children with developmental delays and typically developing children (Fenning, Baker, & Juvonen, 2011).
Oxytocin Receptor Gene (OXTR)
Oxytocin is synthesized in specialized cells of the hypothalamus, and functions as a neurotransmitter, neuromodulator, and peripheral hormone. Within the brain, oxytocin is released from axons connected directly and indirectly to several critical brain regions, including those important for social functioning (e.g., amygdala, anterior cingulate cortex, insula, striatum; MacDonald & MacDonald, 2010). Research in animals and humans suggests that oxytocin plays an important role in social behavior and social cognition, as well as in the formation and maintenance of social relationships (Donaldson & Young, 2008; Ebstein et al., 2009; Feldman, Gordon, & Zagoory-Sharon, 2011). Several administration studies have suggested that oxytocin enhances empathic perspective taking abilities in individuals with typical development and ASD (e.g., Bartz et al., 2010; Domes, Heinrichs, Michel, Berger, & Herpertz, 2007; Guastella et al., 2009).
OXTR, the oxytocin receptor gene, has been studied in relation to empathy and related social behaviors. The current study focused on two common OXTR single nucleotide polymorphisms (SNPs), rs53576 (A/G) and rs2254298 (A/G), with associations to empathy and autism (e.g., Rodrigues, Saslow, Garcia, John, & Keltner, 2009; Schneiderman et al., 2014; Wu et al., 2005). While multiple studies have found associations between OXTR and autism (Jacob et al., 2007; Lerer et al., 2008; Liu et al., 2010; Wu et al., 2005), others have not (Tansey et al., 2010). Additionally, the particular markers and alleles associated with autism have been somewhat inconsistent across studies. Markers including rs53576 and rs2254298 have been associated with ASD, although the relevant “risk” allele may differ by population (see Liu et al., 2010 and Wu et al., 2005 vs. Jacob et al., 2007). Some of the inconsistency in associations between OXTR and autism may be due to limitations in relating this gene to the heterogeneous behavioral profile of autism, rather than to more proximal behaviors such as empathy.
Rs53576 is associated with several aspects of social functioning, including empathy, in adults. In contrast with the major allele (rs53576G), the minor allele (rs53576A) has been associated with lower empathic perspective taking skills (Rodrigues et al., 2009), lower self-reported trait empathy (Rodrigues et al., 2009; Smith, Porges, Norman, Connelly, & Decety, 2014) and lower autonomic arousal in response to observed pain (Smith et al., 2014). Rs53576A has also been associated with diminished social and interpersonal functioning (Tost et al., 2010; Bakermans-Kranenburg & van IJzendoorn, 2008). Paradoxically, the A allele, has also been associated with higher levels of social-emotional functioning, such as higher levels of positive parenting (Michalska et al., 2014), increased social brain activity (Michalska et al., 2014), and higher empathy (Laursen et al., 2014; Lucht et al., 2012, differs from Rodrigues et al., 2009).
A similar pattern of empathy-related results has emerged for rs2254298. The GG genotype has been associated with lower observed empathic concern, affect matching, and social reciprocity (Schneiderman et al., 2014), lower salivary oxytocin (Apter-Levy, Feldman, Vakart, Ebstein, & Feldman, 2013), and lower empathic concern in individuals with schizophrenia (although not with controls; Montag et al., 2012). However, Slane et al. (2014) found that the AG genotype was associated with lower parent-reported social functioning in typically developing children.
Genotypic variation in both rs53576 and rs2254298 OXTR SNPs is associated with differences in social functioning, but the direction of the effect is sometimes variable. Examining interactions between OXTR variants and relevant environmental factors may help to elucidate apparently discrepant findings.
Gene × Environment Interaction
A prominent hypothesis concerning the process by which oxytocin influences social behavior, the social salience theory, suggests that oxytocin increases attention to the relative value of social (vs. non-social) environmental stimuli (Shamay-Tsoory et al., 2009). This theory was developed, in part, following findings that the effect of oxytocin administration depends on social context, as well as individual differences in domains such as social sensitivity and social motivation (Bartz, Zaki, Bolger, & Ochsner, 2011). Accordingly, it may be particularly important to consider environmental context when examining the role of OXTR in phenotypic outcomes.
Studies of rs53576 suggest that the G allele may contribute to increased susceptibility to environmental influences. For instance, rs53576G has been associated with both higher and lower levels of sensitivity in parents (Sturge-Apple, Cicchetti, Davies, & Suor, 2012), antisocial behavior in adolescents (Smearman, Winiarski, Brennan, Najman, & Johnson, 2014), and depressive symptoms in young adults (McQuaid, McInnis, Stead, Matheson, & Anisman, 2013), depending on environmental circumstances related to social stress and adversity. Research on rs2254298 is more limited, although Thompson, Parker, Hallmayer, Waugh, and Gotlib (2011) found that OXTR rs2254298A carried increased risk for depressive symptoms in girls who had experienced high levels of early adversity. Consistent with the social salience theory, these studies highlight the importance of investigating the role of environmental context in moderating the influence of OXTR on psychological outcomes.
The Current Study
Despite increasing evidence for the importance of oxytocin for social behavior and cognition, little is known about the influence of OXTR on empathic behavior in a developmental context. We asked whether environmental factors exhibited in early interaction and genetic factors predicted later empathic behavior in children at varying risk for ASD. Specifically, we investigated whether phenotypic variations in empathic behavior during the third year of life (24 and/or 30 months) were predicted by ASD risk status, genotypic variation in OXTR rs53576 and rs2254298, and qualities of parent-child interactions measured during the second year of life (15 and/or 18 months). We also tested for interaction effects in which we examined whether early parent-child interactions differentially predicted children's empathy depending on OXTR allelic variation and ASD risk status.
Method
Participants
Participants were part of a longitudinal study examining the early development of infants at high- and low-risk for ASD. High-risk infants had one or more older siblings with an ASD diagnosis. Older sibling diagnoses were confirmed by a licensed clinician based on DSM-IV-TR diagnostic criteria (American Psychiatric Association, 2000) and results from the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). Low-risk infants had older siblings who did not have an ASD diagnosis and showed no evidence of elevated ASD symptoms on the Social-Communication Questionnaire screener (Rutter, Bailey, & Lord, 2003).
Participants were recruited from a university-based autism service, brochure mailings to parents of infants whose addresses and names were obtained from county birth records, child care programs, and word of mouth. All portions of the study received IRB approval and informed consent was obtained from parents prior to beginning the study.
Participants contributed parent-child interaction data at 15 and/or 18 months of age, at least one successfully genotyped OXTR SNP, and empathy data at 24 and/or 30 months. A total of 84 participants (High-Risk n = 51, Low-Risk n = 33) met these inclusion criteria. There were no significant associations between ASD risk status and race/ethnicity, χ2 (4) = .26, p = .99, or maternal education, χ2 (4) = 1.02, p = .9l; however, there tended to be more boys in the high-risk than the low-risk group, χ2(1) = 3.71, p = .05. High-risk children had lower developmental scores, t(81) = 4.42, p < .01, and higher ADOS severity scores, t(81) = -3.87, p < .01, than low-risk children. See Table 1 for sample information by ASD risk status.
Table 1. Sample demographics by ASD risk status.
| Demographic variable | Total n (%) | Low-Risk n (%) | High-Risk n (%) |
|---|---|---|---|
| ASD risk status | 84 | 33 (39%) | 51 (61%) |
| Gender | |||
| Male | 49 (58%) | 15 (45%) | 34 (67%) |
| Female | 35 (42%) | 18 (55%) | 17 (35%) |
| Ethnicity | |||
| White/Caucasian | 29 (35%) | 11 (33%) | 18 (35%) |
| Black/African-American | 2 (2%) | 1 (3%) | 1 (2%) |
| Hispanic/Latino | 40 (48%) | 16 (48%) | 24 (47%) |
| Asian/Asian-American | 2 (2%) | 1 (3%) | 1 (2%) |
| Mixed Ethnicity/Other | 11 (13%) | 4 (12%) | 7 (14%) |
| Maternal Education | |||
| High school | 4 (5%) | 1 (3%) | 3 (7%) |
| Some college | 4 (4%) | 1 (3%) | 2 (4%) |
| 2-year college | 13 (16%) | 5 (16%) | 8 (15%) |
| 4-year college | 24 (26%) | 9 (26%) | 15 (26%) |
| Advanced/Professional degree | 39 (49%) | 17 (52%) | 22 (48%) |
| ASD Outcome* | |||
| ASD | 11 (14%) | 0 (0%) | 11 (23%) |
| No ASD | 70 (86%) | 33 (100%) | 37 (77%) |
|
| |||
| Measure | Mean (SD) | ||
|
| |||
| MSEL developmental scores** | |||
| Early Learning Composite (Standard score) | 94.1 (18.9) | 104.5 (14.0) | 87.5 (18.7) |
| Visual Reception (T-score) | 50.6 (15.0) | 56.2 (11.4) | 47.1 (16.0) |
| Fine Motor (T-score) | 44.2 (13.0) | 51.7 (12.0) | 39.5 (11.5) |
| Receptive Language (T-score) | 44.0 (11.6) | 49.4 (9.0) | 40.7 (11.8) |
| Expressive Language (T-score) | 47.8 (10.2) | 51.7 (8.1) | 45.3 (10.7) |
| ADOS severity score*** | 2.5 (2.1) | 1.6 (1.4) | 3.1 (2.2) |
ASD outcome diagnoses obtained at the 36-month assessment, or, when not available, the 4-6 year assessment (n = 4). Expert licensed clinician diagnosed based on ADOS, Autism Diagnostic Interview-Revised (Lord, Rutter, & Le Couteur, 1994), Mullen Scales of Early Learning (MSEL; Mullen, 1995), as well as clinical impression. ASD outcome was unavailable for 3 children.
Developmental scores were obtained from the MSEL, a normed, standardized measure of cognitive abilities for children from birth to 68 months. MSEL scores are from 36 months (n = 79) or, when not available, 24 months (n = 4). MSEL scores were unavailable for one participant.
ADOS severity scores are from 30 months (n = 75), or, when not available, 24 months (n = 1), 36 months (n = 4), or 4-6 years (n = 3). An ADOS severity score was missing for one participant. Autism Spectrum cutoff = 4; Autism cutoff = 6 (Gotham et al., 2009)
Procedure
Families visited the laboratory for approximately two hours to participate in observational and clinical assessments when the children were 15, 18, 24, and 30 months of age. Parent-child interaction was measured at 15 and 18 months and empathy was measured at 24 and 30 months. All observational data were video-recorded to allow for later behavioral coding.
Saliva was collected at one of the longitudinal assessments (ranging from the 15-month to a 4-6 year assessment). Younger children's saliva (∼4 years and under) was collected using swabs and older children's saliva (∼5 years and older) was collected by having the child spit directly into a container. Families of participants who were not able to provide saliva in the lab were mailed kits to collect saliva at home (n = 14). Genetic samples were then sent for extraction and analysis.
Measures
Parent-Child Interaction
During an unstructured five-minute free play session, parents were instructed to, “Play with [child's name] as you normally would at home.” Parents and children played on the floor with an array of age-appropriate toys available.
Parent and dyadic behaviors were rated using the NICHD Early Child Care Research Network scales (e.g., 2003; McElwain, Booth-LaForce, Lansford, Wu, & Dyer, 2008). Research associates, blind to child genotypes and empathy ratings, rated the free play interactions on scales of Affective Mutuality (dyadic), Parental Sensitivity, Respect for Autonomy, and Positive Regard. These scales were rated from 1 (the absence of a given behavior) to 7 (the clear and abundant presence of a behavior). The Affective Mutuality rating assessed the availability and mutuality of emotion between the child and parent, and the degree of shared positive affect and affective synchrony within the dyad (Baker et al., 2015). A Parent Supportiveness composite score was calculated using the mean of the Parental Sensitivity, Respect for Autonomy, and Positive Regard parent ratings (Baker et al., 2010). This score reflected the degree of parental warmth and acceptance, responsiveness to the child's needs, and the balance of parental involvement with respect for the child's desires and emerging independence. The Parent Supportiveness composite had good internal consistency in this sample (ICC = .85). Intra-class correlations revealed good reliability on all scales: 15-month Affective Mutuality (.80), 18-month Affective Mutuality (.80), 15-month Parent Supportiveness (.81), and 18-month Parent Supportiveness (.88).
There were no age differences in Affective Mutuality, t(68) = -1.04, p = .30 (15-month M = 4.29, SD = .90; 18-month M = 4.43, SD = .98), or Parent Supportiveness ratings, t(68) = -.08, p = .93 (15-month M = 4.97, SD = .84; 18-month M = 4.98, SD = .98). Parent-child interaction variables were correlated between ages (Affective Mutuality: r(67) = .31, p = .01; Parent Supportiveness: r(67) = .58, p < .01). The mean of the 15- and 18-month ratings were utilized in analyses. Prior to calculating the mean ratings for each participant, both rating scales were mean-centered within age. For participants missing parent-child interaction data at 15 months (n = 9) or 18 months (n = 6), individual data points were used in lieu of mean scores. There was no difference in Affective Mutuality, t(82) = .35, p = .73, or Parent Supportiveness, t(82) = .95, p = .35, when participants with one missing data point (Affective Mutuality: M = -.07, SD = .85; Parent Supportiveness: M = -.20, SD = .94) were compared to mean scores of participants with data at both ages (Affective Mutuality: M = .01, SD = .76; Parent Supportiveness: M = .02, SD = .81). Although highly correlated (see Table 2 for correlations among study variables), the Affective Mutuality and Parent Supportiveness scores are theoretically distinct (i.e., dyadic vs. parenting) constructs that were analyzed separately.
Table 2. Bivariate correlations among study variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Gender | - | .21† | .12 | .01 | -.01 | .08 | -.20† |
| 2. ASD Risk Status | - | .15 | -.21† | -.01 | -.04 | -.21† | |
| 3. rs53576 | - | -.08 | .01 | .00 | .15 | ||
| 4. rs2254298 | - | -.14 | -.17 | -.10 | |||
| 5. Affective Mutuality (AM) | - | .76** | .19† | ||||
| 6. Parent Supportiveness (PS) | - | .06 | |||||
| 7. Empathy | - |
p < .10
p < .05
p < .01
Oxytocin Receptor Gene
Genetic data were obtained through saliva using Oragene DNA collection kits (DNA Genotek). To ensure quality data collection, we consulted with DNA Genotek and followed recommended procedures for saliva collection in young children. The use of swabs was necessary for the younger children in our sample who were not yet able to spit. This method has been shown to yield DNA that is sufficient in quality and quantity for genetic analysis (e.g., Koni et al., 2011).
Genotyping was conducted for the following OXTR SNPs: rs53576 (A/G) and rs2254298 (A/G). Due to the limited sample size, two relatively well-studied SNPs were chosen to minimize the risk of type 1 error. These SNPs have shown associations with both empathy (e.g., Rodrigues et al., 2009; Schneiderman et al., 2014) and autism (e.g., Jacob et al., 2007; Wu et al., 2005). Given the relatively low numbers of individuals expected to be homozygous for the minor alleles, we made an a priori decision to compare those with at least one minor allele to those dominant for the major allele for both SNPs.
Saliva and DNA extraction
Genetic material was extracted using standard procedures recommended by DNA Genotek for samples that include DNA collected from young children using sponges. The samples were incubated at 50°C for two hours in an air incubator. The free liquid was removed from the sponges by centrifugation in a 6 ml syringe suspended on a 15 μL conical centrifuge tube. The device was then centrifuged at 200 × g (e.g., 1,000 rpm in a Beckman Coulter Allegra® X-15R centrifuge) for 10 minutes at 20°C. The free saliva was then extracted using DNA Genotek prepIT-L2P protocol.
DNA amplification
Due to low saliva yields (< 2μg) in approximately one-third (n = 31) of the total genotyped sample (n = 93), the extracted samples underwent multiple displacement amplification prior to genotyping (Qiagen, Repli-G Midi Kit). For samples with sufficient DNA yields (n = 60), both genomic and amplified DNA were genotyped to assess for concordance. Genotype concordance rates between genomic and amplified DNA were 100% for both markers, indicating reliable genotyping of the amplified DNA. The remaining 33 samples were genotyped using amplified DNA only.
Genotyping
Genotyping was conducted using Taqman allelic discrimination assays from Applied Biosystems (ABI). Cycling was performed on GeneAmp PCR Systems 9700 thermocyclers, with conditions recommended by ABI. End-point fluorescence was measured on the ABI 7900 HT system. Genotype discrimination of experimental results was then conducted using ABI's 7900 HT Sequence detection Systems version 2.3 analysis software. To ensure genotyping accuracy, one negative and seven positive quality control samples per 96 sample well plate were included.
For rs53576, 84 samples were successfully genotyped and nine were undetermined. For rs2254298, 88 samples were successfully genotyped and five were undetermined. The relatively high level of participants with inconclusive genotypes is likely related to lower yields resulting from the predominant use of swabs, rather than direct saliva collection, which was necessary with this study's young sample. Participants who were undetermined for both markers (n = 9) were not included in the study sample.
Empathy
Child empathy was measured using a response to parental distress paradigm that occurred during a play session (McDonald & Messinger, 2012). Prior to the session, an examiner gave the parent the following instructions: “After you and [child's name] play for a while, I will step into the room to alert you to begin pretending that you have something in your eye. Act like it really bothers you by saying ‘Oh, I have something in my eye.’ Carry on like this for a while, but don't say your child's name or suggest your child do anything to help you feel better.” The task was terminated after approximately one minute when the examiner re-entered the room and instructed the parent to tell the child that his or her eye felt better.
An empathy coding system originally used with typically developing toddlers was utilized (Young, Fox, & Zahn-Waxler, 1999) with minor adaptations to remove bias toward higher scores for verbal responses (McDonald & Messinger, 2012). Research associates, blind to ASD risk status, genotypes, and parent-child interaction ratings, rated the episodes. Each episode was given an Empathy rating of 1 to 7, which captured the overall quality of the child's empathic responding. A 1 indicated no signs of empathy or concern and a 7 strong expressions of concern and caring behavior. Examples of empathic behaviors included facial or vocal concern (e.g., brow furrowing, sympathetic tone, “I'm sorry”), prosocial behavior (e.g., providing comfort to parent), paying attention to the parent, and inquiring about the problem. Approximately 25% of the episodes were double coded to assess inter-rater reliability. Intra-class correlations (absolute agreement, single measures) were .90 at 24 months and .94 at 30 months.
The mean of the 24- and 30-month Empathy ratings were utilized in analyses. Empathy was correlated between ages, r(62) = .36, p < .01. There was a significant difference between 24- and 30-month Empathy ratings, t(63) = -2.23, p < .05 (24-month M = 2.98, SD = 1.45; 30-month M = 3.47, SD = 1.61); children displayed more empathic behavior at 30 months than 24 months. The Empathy ratings were thus standardized as z-scores within the 24- and 30-month time points, which removed age-related differences, t(63) = -.39, p = .70. A mean of the standardized scores was then calculated to create a single Empathy score. For participants missing Empathy data at 24 months (n = 11) or 30 months (n = 9), individual data points were used in lieu of mean scores. There was no difference in Empathy when the individual scores of participants with one missing data point (M = -.11, SD = 1.06) were compared to mean scores of participants with data at both ages (M = .02, SD = .82), t(82) = .55, p = .59.
Results
Preliminary Analyses
Parent-Child Interaction
There were no differences in parent-child interaction ratings based on child Gender (Affective Mutuality, t(82) = .07, p = .95; Parent Supportiveness, t(82) = -.73, p = .47) or ASD Risk Status (Affective Mutuality, t(82) = .11, p = .91; Parent Supportiveness, t(82) = .36, p = .72).
OXTR Genotypes
Genotype distributions were consistent with Hardy-Weinberg equilibrium (rs53576: χ2 (2) = .04, p = .98; rs2254298: χ2 (2) = .88, p = .64) (http://www.oege.org/software/hwe-mr-calc.shtml). Table 3 reports genotype frequencies and mean Empathy scores. Due to the expected low frequencies of the minor allele groups for both SNPs, participants with AA genotypes were grouped with the AG genotypes for analyses.
Table 3. Genotype information for rs53576 and rs2254298.
| Genotype | |||
|---|---|---|---|
| rs53576 | |||
| AA | AG | GG | |
| Total n | 7 | 34 | 37 |
| High-Risk n | 4 | 23 | 19 |
| Low-Risk n | 3 | 11 | 18 |
| Empathy M (SD) | .10 (1.26) | .16 (.71) | -.12 (.95) |
| AG/AA | GG | ||
| n | 41 | 37 | |
| High-Risk n | 27 | 19 | |
| Low-Risk n | 14 | 18 | |
| Empathy M (SD) | .15 (.81) | -.12 (.95) | |
|
| |||
| rs2254298 | |||
| AA | AG | GG | |
| Total n | 3 | 19 | 60 |
| High-Risk n | 2 | 8 | 41 |
| Low-Risk n | 1 | 11 | 19 |
| Empathy M (SD) | -.96 (.44) | -.07 (.76) | .01 (.89) |
| AG/AA | GG | ||
| Total n | 22 | 60 | |
| High-Risk n | 10 | 41 | |
| Low-Risk n | 12 | 19 | |
| Empathy M (SD) | -.19 (.78) | .01 (.89) | |
Note. M = mean; SD = standard deviation
There were 78 participants included in analyses involving rs53576 and 82 participants for rs2254298. Genotypes for both SNPs were available for 76 participants. There was no association between participants' genotypes on rs53576 (GG vs. AG/AA) and rs2254298 (GG vs. AG/AA), χ2 (1) = .43, p = .51. There were no differences in parent-child interaction ratings for rs53576 (Affective Mutuality, t(76) = -.12, p = .90; Parent Supportiveness, t(76) = -.00, p = .99) or rs2254298 (Affective Mutuality, t(80) = 1.23, p = .22; Parent Supportiveness, t(80) = 1.57, p = .12). There was no association between ASD Risk Status and Genotype for rs53576, χ2(1) = 1.47, p = .23. For rs2254298, there was a tendency for high-risk siblings to be over-represented in the GG group, χ2 (1) = 3.39, p = .07.
Empathy
Females tended to show higher levels of Empathy than males (Female M = .19, SD = .82; Male M = -.16, SD = .89), t(82) = 1.82, p = .07. Low-risk siblings showed higher levels of Empathy than high-risk siblings (Low-Risk M = .22, SD = .71; High-Risk M = -.16, SD = .95), t(82) = 1.97, p = .05. Gender was included as a covariate in subsequent analyses.
Empathy Predictors
Four multiple regressions were conducted to examine predictors of Empathy, including ASD Risk Status, Parent-Child Interaction variables, and OXTR SNPs. All models had the following structure: Step 1 = Covariate (Gender), Step 2 = Main effects (ASD Risk Status, Affective Mutuality (AM) or Parent Supportiveness (PS), rs53576 or rs2254298), Step 3 = 2-way interactions (ASD Risk Status × AM or PS, ASD Risk Status × rs53576 or rs2254298, AM or PS × rs25376 or rs2254298). Three-way interactions were not assessed due to limited power associated with relatively small sizes. For both rs53576 and rs2254298, the major (G) allele groups were dummy coded 0 and the minor (A) allele groups were dummy coded 1. Interaction terms were computed by multiplying the two variables of interest (e.g., Genotype (0 or 1) × Affective Mutuality score). We followed up significant interactions between SNPs and parent-child interaction variables by examining the correlation of the parent-child interaction variables with Empathy within each genotype group. See Table 4 for results. Results had the same pattern of significance when the effect of ASD severity scores was examined in lieu of ASD Risk Status.
Table 4. Prediction of Empathy by ASD Risk Status, Genotype, and Parent-Child Interaction variables.
| Covariate | Main Effects | Interaction Effects | ||||
|---|---|---|---|---|---|---|
| Analysis 1 (n = 78) | β | p | β | p | β | p |
|
| ||||||
| Empathy | ||||||
| Gender | -.22 | .05 | -.20 | .07 | -.22 | .05 |
| ASD Risk Status | -.20 | .08 | -.23 | .14 | ||
| Affective Mutuality (AM) | .16 | .15 | .20 | .40 | ||
| rs53576 | .20 | .07 | .25 | .17 | ||
| Status × AM | .27 | .20 | ||||
| Status × rs53576 | -.02 | .94 | ||||
| AM × rs53576 | -.37 | .02 | ||||
| R2 | .05 | .14 | .22 | |||
|
| ||||||
| Analysis 2 (n = 82) | β | p | β | p | β | p |
|
| ||||||
| Empathy | ||||||
| Gender | -.21 | .07 | -.17 | .13 | -.22 | .05 |
| ASD Risk Status | -.16 | .16 | -.03 | .83 | ||
| Affective Mutuality (AM) | .17 | .12 | .20 | .43 | ||
| rs2254298 | -.11 | .32 | -.04 | .84 | ||
| Status × AM | .10 | .68 | ||||
| Status × rs2254298 | -.17 | .33 | ||||
| AM × rs2254298 | -.23 | .08 | ||||
| R2 | .04 | .11 | .18 | |||
|
| ||||||
| Analysis 3 (n = 78) | β | p | β | p | β | p |
|
| ||||||
| Empathy | ||||||
| Gender | -.22 | .05 | -.21 | .06 | -.22 | .06 |
| ASD Risk Status | -.20 | .09 | -.20 | .22 | ||
| Parent Supportiveness (PS) | .02 | .85 | .03 | .90 | ||
| rs53576 | .21 | .07 | .26 | .15 | ||
| Status × PS | .27 | .29 | ||||
| Status × rs53576 | -.05 | .82 | ||||
| PS × rs53576 | -.26 | .14 | ||||
| R2 | .05 | .12 | .15 | |||
|
| ||||||
| Analysis 4 (n = 82) | β | p | β | p | β | p |
|
| ||||||
| Empathy | ||||||
| Gender | -.21 | .07 | -.17 | .13 | -.22 | .06 |
| ASD Risk Status | -.17 | .16 | -.06 | .67 | ||
| Parent Supportiveness (PS) | .02 | .83 | -.02 | .94 | ||
| rs2254298 | -.13 | .25 | -.09 | .64 | ||
| Status × PS | .18 | .45 | ||||
| Status × rs2254298 | -.14 | .46 | ||||
| PS × rs2254298 | -.18 | .19 | ||||
| R2 | .04 | .08 | .13 | |||
Analysis 1 tested the main and interaction effects of ASD Risk Status, Affective Mutuality, and rs53576, explaining 22% of the variance in Empathy (see Table 4). There were marginally significant main effects of ASD Risk Status and rs53576 on Empathy. The main effect of Affective Mutuality on Empathy did not reach significance; however, there was a significant interaction effect of Affective Mutuality and rs53576 on Empathy, β = -.37, p = .02. Follow-up of this interaction revealed a significant positive correlation between Affective Mutuality and Empathy for children with the GG genotype, r(35) = .37, p = .02, but not for children with at least one A allele, r(39) = -.03, p = .87 (see Figure 1). Interactions including ASD Risk Status were non-significant.
Figure 1. Empathy by rs53576 Genotype and Affective Mutuality.
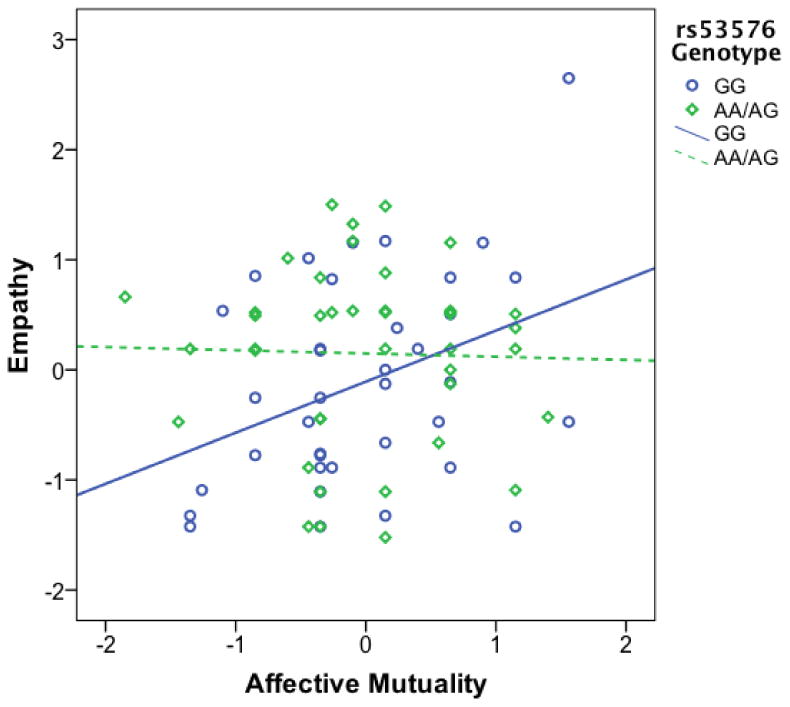
Note. There was a significant correlation between Affective Mutuality (mean of mean-centered 15- & 18-month ratings) and Empathy (mean of standardized 24- & 30-month ratings) for children with the GG rs53576 genotype, r(35) = .37, p = .02, but not for children with at least one A allele, r(39) = -.03, p = .87.
Analysis 2 tested the main and interaction effects of ASD Risk Status, Affective Mutuality, and rs2254298, explaining 18% of the variance in Empathy (see Table 4). Main effects of ASD Risk Status, Affective Mutuality, and rs2254298 on Empathy did not reach significance in this analysis; however, there was an interaction between Affective Mutuality and rs2254298, β = -.23, p = .08. Although the interaction was significant at a trend level only, we examined the correlations between Affective Mutuality and Empathy within each group as a follow-up analysis, given the relatively low sample size of the A allele group. Follow-up of this interaction revealed a significant positive correlation for children with the GG genotype, r(58) = .29, p = .02, but not for children with at least one A allele, r(20) = -.31, p = .16. Interactions including ASD Risk Status were again non-significant.
Analyses 3 and 4 tested the main and interaction effects of ASD Risk Status, Parent Supportiveness, and rs53576 or rs2254298, explaining 15% (rs53576) and 13% (rs2254298) of the variance in Empathy (see Table 4). These analyses revealed no significant main or interaction effects associated with the Parent Supportiveness variable.
Discussion
We investigated the influence of two common OXTR polymorphisms and early parent-child interaction quality on individual differences in empathy in children at varying risk for empathy deficits. This appears to be the first study to investigate predictors of empathy in a sample including children at elevated risk for ASD. In comparison to children with at least one A allele, children with the GG rs53576 genotype were especially vulnerable to the influence of lower quality early dyadic interactions.
Early Social Interactions and Empathy Development
We examined main and interaction effects of parent behaviors and dyadic interaction quality on later empathic behavior. Contrary to expectations, there were no main or interaction effects indicating that variation in parenting behaviors explained individual differences in children's empathy. This result is somewhat inconsistent with previous findings that parenting qualities, such as warmth and responsivity, were related to individual differences in children's empathy (Kochanska et al., 1999; Zhou et al., 2002). One potential explanation for this discrepancy is the context of the interaction. The free play measure may have been ideal for capturing dyadic affective synchrony, but not as well suited to measuring parenting variables, such as sensitivity. Rather, more challenging tasks, such as a problem solving task, may be required to more accurately assess parent sensitivity and supportiveness (Fenning & Baker, 2012). There was evidence, however, that affective mutuality, a dyadic variable, interacted with child genotype to predict empathy in some children.
OXTR and Empathy
The association between dyadic affective mutuality and empathy varied by OXTR genotype. Children homozygous for the major allele (G) on rs53576 had lower levels of empathy in the context of lower quality early parent-child interactions. This was not the case for children with at least one minor allele (A), who as a group showed similar levels of empathy regardless of early interaction quality. This finding may appear inconsistent with studies suggesting that rs53576A is the “risk” allele (Bakermans-Kranenburg & van IJzendoorn, 2008; Rodrigues et al., 2009; Smith et al., 2014; Tost et al., 2010); however, it is consistent with a growing literature suggesting that rs53676G denotes increased susceptibility to environmental influences. For instance, Sturge-Apple et al. (2012) found evidence for increased susceptibility to interparental conflict on parental sensitivity among mothers with the GG genotype on OXTR rs53576. Similarly, G allele carriers had higher levels of antisocial behavior in the context of high social stress (Smearman et al., 2014), were more at risk for depressive symptoms when they had experienced high early adversity (McQuaid et al., 2013), and were more responsive to the effects of oxytocin inhalation (Marsh et al., 2012).
Studies examining direct effects of OXTR rs2254298A have typically indicated associations between the G allele and more optimal social-emotional functioning (e.g., Schneiderman et al., 2014; Apter-Levy et al., 2013). Although there is limited research on interaction effects involving rs2254298, Thompson et al. (2011) found that OXTR rs2254298A was associated with increased risk for depressive symptoms in girls who had experienced high levels of early adversity. In the current study, the interaction between rs2254298 and affective mutuality did not reach significance, although follow-up analyses revealed that children homozygous for the major allele (G) on rs2254298 had higher levels of empathy in the context of higher quality early parent-child interactions, while early interaction quality and later empathy were not significantly associated in children with at least one minor allele (A). Paradoxically, rs2254298A allele carriers appeared to have lower levels of empathy in the context of higher quality early interactions. Given the lack of significance for the overall interaction and relatively small group size (n = 22), it is difficult to interpret these data; however, this underscores the diversity of findings common in the oxytocin literature and suggests the importance of follow-up with a larger cohort.
We did not find a direct effect of OXTR allelic variation on empathy. Likewise, findings from a recent meta-analysis of the direct effects of OXTR rs53576 and rs2254298 suggest that these SNPs may not independently explain a substantial portion of social behavior (Bakermans-Kranenburg & van IJzendoorn, 2014). Consistent with probabilistic epigenesis, which holds that genetic variation exerts effects in concert with the environment (Gottlieb, 2007), the current findings highlight the importance of investigating the role of common genetic variants such as OXTR in moderating the influence of context on behavioral outcomes.
The social salience theory posits that oxytocin increases attention to the relative value of social (vs. non-social) environmental stimuli (Shamay-Tsoory et al., 2009). By this account, genotypic variations related to increased sensitivity to endogenous oxytocin could increase the salience of both positive and negative environmental circumstances (Tabak, 2013), which may help to explain the difficulty in clearly defining a “risk” allele for many OXTR polymorphisms. This is consistent with the differential susceptibility hypothesis, which argues that particular genotypes predispose individuals to be more sensitive to their environments, allowing for more positive and more negative outcomes (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007). Accordingly, it may be that the inconsistency in previous OXTR findings is at least partially due to a focus on examining direct effects of variation in OXTR SNPs without considering potential interactions with environmental variables. In this study, participants with GG genotypes appear to be sensitive to both positive and negative variation in the quality of parent-child interactions. For children with GG genotypes on rs53576 (and, perhaps, rs2254298), genetic differences in OXTR may be associated with a stronger influence of early social interactions on empathy development.
Research on the mechanisms by which allelic variation in rs53576 and rs2254298 may influence social-emotional functioning is relatively preliminary. With regard to peripheral measures of oxytocin, some studies suggest that individuals with GG genotypes on rs53576 (Moons, Way, & Taylor, 2014) and rs2254298 (Apter-Levy et al., 2013; Feldman et al., 2012) have lower levels of plasma or salivary oxytocin, while others have found no difference in plasma or salivary oxytocin for either SNP (Bhandari et al., 2014; Parker et al., 2014). There is initial evidence of differences in social brain structure and function associated with allelic variation, although with some inconsistency in findings. For rs53576, the A allele has been associated with reduced amygdala and hypothalamus volumes and lower amygdala activation in response to emotionally salient social cues, effects which were largely driven by males (Tost et al., 2010). Associations with amygdala volume have also been found for rs2254298; girls with the GG genotype had lower amygdala volumes (Furman, Chen, & Gotlib, 2011) and the number of A alleles was proportionally associated with larger amygdala volume in Japanese adults (Inoue et al., 2010).
Both OXTR SNPs studied are in the intron 3 (non-coding) region of the gene, the functions of which are not yet well understood (Inoue et al., 1994). It is possible that SNPs in intron 3 of OXTR do not have direct effects on empathy but rather are in linkage disequilibrium with other parts of the gene, or other genes, with more influential functions (Lerer et al., 2008). Conversely, direct effects on phenotype are also possible. A functional study of OXTR revealed that the third intron may be involved in transcriptional suppression or downregulation of the gene (Mizumoto et al., 1997), suggesting a possible function of OXTR's intron 3 that may be related to phenotypic outcomes.
OXTR and ASD
The current study did not directly assess the role of OXTR in ASD. Rather, variations in OXTR were examined in relation to a common symptom of ASD (i.e., reduced empathy), in a sample enriched with respect to risk for social impairments. Our findings suggest that there may be similar developmental processes across high- and low-risk groups, which is consistent with previous literature comparing the social cognitive development of children with typical development and developmental delays (Fenning et al., 2011). Although findings do not speak directly to the role of oxytocin in the development and/or treatment of ASD (e.g., Lerer et al., 2008; Liu et al., 2010; Jacob et al., 2007), they do imply that OXTR may be related to ASD in part through its effects on specific social behaviors such as empathy. Some OXTR genotypes may put children at risk for adverse outcomes, such as deficits in empathy, which in combination with other genetic and environmental factors are associated with increased levels of ASD symptomatology.
Limitations and Future Directions
Findings from this study should be considered in light of its limitations. The genetic findings should be interpreted with caution given the study's relatively small sample size. Not only might a larger sample be used to replicate the current findings, but it would allow for the investigation of a broader group of OXTR variants. A larger sample would also allow for investigation of children homozygous for the minor allele who might differ from heterozygous children, as well as further examination of potential moderation effects by ASD risk status. A strength of the current study was the use of observational measures of parent-child interaction and empathy, in comparison to previous genetic studies that have often relied on questionnaire data. Nevertheless, future studies may benefit from including parent-child interaction measures that challenge the dyad (e.g., a problem solving or compliance task) or use a micro-coding approach, which might reveal associations between parent behavior and child outcome. Follow-up studies would also benefit from measuring empathy in response to multiple social partners and situations. Likewise, a more nuanced examination of empathy that includes discrimination between cognitive and emotional empathic dimensions would be of interest. Finally, it is difficult to fully interpret the OXTR findings, given the general lack of knowledge available regarding underlying physiological mechanisms of variation in the SNPs studied; an important area of future research.
This study provides evidence that a common OXTR polymorphism (rs53576) plays a moderating role in the relation between early parent-child interactions and empathy in a sample of children at varying risk for ASD. This finding has implications for intervention. In particular, oxytocin administration may eventually prove to be a useful tool for improving outcomes in children at risk for empathy deficits (Green & Hollander, 2010), particularly when used in concert with interventions focused on facilitating more affectively synchronous early parent-child interactions (e.g., Dawson et al., 2010).
Acknowledgments
The research reported in this article was supported by grants from the National Institute of Child Health and Development (R01HD057284 & R01HD047417), the National Institute of General Medical Sciences (R01GM105004), and the Fred C. and Helen Donn Flipse Research Support Fund, Department of Psychology, University of Miami. We thank the families who participated in this research.
Footnotes
Author Note: Nicole M. McDonald is now at the Child Study Center, Yale School of Medicine.
Contributor Information
Nicole M. McDonald, Department of Psychology, University of Miami
Jason K. Baker, Department of Child and Adolescent Studies, California State University, Fullerton
Daniel S. Messinger, Department of Psychology, University of Miami
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. Impact of maternal depression across the first 6 years of life on the child's mental health, social engagement, and empathy: The moderating role of oxytocin. American Journal of Psychiatry. 2013;170:1161–1168. doi: 10.1176/appi.ajp.2013.12121597. [DOI] [PubMed] [Google Scholar]
- Baker JK, Fenning RM, Howland MA, Baucom BR, Moffitt JM, Erath SA. A pilot study of parent-child biobehavioral synchrony in autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015 doi: 10.1007/s10803-015-2528-0. [DOI] [PubMed] [Google Scholar]
- Baker JK, Messinger DS, Lyons KK, Grantz CJ. A pilot study of maternal sensitivity in the context of emergent autism. Journal of Autism and Developmental Disorders. 2010;40:988–999. doi: 10.1007/s10803-010-0948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatry Genetics. 2014;24:45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, Ochsner KN. Oxytocin selectively improves empathic accuracy. Psychological Science. 2010;21:1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Science. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Bhandari R, Bakermans-Kranenburg MJ, van der Veen R, Parsons CE, Young KS, Grewen KS, van IJzendoorn MH. Physiology and Behavior. 2014;131:123–128. doi: 10.1016/j.physbeh.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Cramer D. Facilitativeness, conflict, demand for approval, self-esteem, and satisfaction with romantic relatiosnhips. Journal of Psychology: Interdisciplinary and Applied. 2003;137:85–98. doi: 10.1080/00223980309600601. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves ‘mind-reading’ in humans. Biological Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I, Yirmiya N. Arginine vasopressin and oxytocin modulate human social behavior. Annals of the New York Academy of Sciences. 2009;1167:87–102. doi: 10.1111/j.1749-6632.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Miller PA. The relation of empathy to prosocial and related behaviors. Psychological Bulletin. 1987;101:91–119. doi: 10.1037/0033-2909.101.1.91. [DOI] [PubMed] [Google Scholar]
- Feldman R. Mother-infant synchrony and the development of moral orientation in childhood and adolescence: Direct and indirect mechanisms of developmental continuity. American Journal of Orthopsychiatry. 2007;77:582–597. doi: 10.1037/0002-9432.77.4.582. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: Considering stress and affiliation components of human bonding. Developmental Science. 2011;14:752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biological Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Fenning RM, Baker JK. Mother-child interaction and resilience in children with early developmental risk. Journal of Family Psychology. 2012;26:411–420. doi: 10.1037/a0028287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenning RM, Baker BL, Juvonen J. Emotion discourse, social cognition, and social skills in children with and without developmental delays. Child Development. 2011;82:717–731. doi: 10.1111/j.1467-8624.2010.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36:891–897. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner PW. Child and family correlates of toddlers' emotional and behavioral responses to a mishap. Infant Mental Health Journal. 2003;24:580–596. doi: 10.1002/imhj.10076. [DOI] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Probabilistic epigenesis. Developmental Science. 2007;10:1–11. doi: 10.1111/j.1467-7687.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Green JJ, Hollander E. Autism and oxytocin: New developments in translational approaches to therapeutics. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2010;7:250–257. doi: 10.1016/j.nurt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry. 2009;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Hutman T, Rozga A, DeLaurentis AD, Barnwell JM, Sugar CA, Sigman M. Response to distress in infants at risk for autism: A prospective longitudinal study. Journal of Child Psychology and Psychiatry. 2010;51:1010–1020. doi: 10.1111/j.1469-7610.2010.02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Yamasue H, Tochigi M, Abe O, Liu X, Kawamura Y, Kasai K. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biological Psychiatry. 2010;68:1066–1072. doi: 10.1016/j.biopsych.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kimura T, Azuma C, Inazawa J, Takemura M, Kikuchi T, Saji F. Structural organization of the human oxytocin receptor gene. Journal of Biological Chemistry. 1994;269:32451–32456. [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience Letters. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo A, Zahn-Waxler C, Van Hulle C, Robinson JL, Rhee SH. The developmental origins of a disposition toward empathy: Genetic and environmental contributions. Emotion. 2008;8:737–752. doi: 10.1037/a0014179. [DOI] [PubMed] [Google Scholar]
- Kochanska G. Mutually responsive orientation between mothers and their young children: A context for the early development of conscience. Current Directions in Psychological Science. 2002;11:191–195. doi: 10.1111/1467-8721.00198. [DOI] [Google Scholar]
- Kochanska G, Forman DR, Aksan N, Dunbar SB. Pathways to conscience: Early mother-child mutually responsive orientation and children's moral emotion, conduct, and cognition. Journal of Child Psychology and Psychiatry. 2005;46:19–34. doi: 10.1111/j.1469-7610.2004.00348.x. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Forman DR, Coy KC. Implications of the mother-child relationship in infancy for socialization in the second year of life. Infant Behavior and Development. 1999;22:249–265. doi: 10.1016/S0163-6383(99)00009-0. [DOI] [Google Scholar]
- Koni AC, Scott RA, Bailey MES, Peplies J, Bammann K, Pitsiladis YP. DNA yield and quality of saliva samples and suitability for large-scale epidemiological studies in children. International Journal of Obesity. 2011;35:113–118. doi: 10.1038/ijo.2011.43. [DOI] [PubMed] [Google Scholar]
- Laursen HR, Siebner HR, Haren T, Madsen K, Gronlund R, Hulme O, Henningsson S. Variation in the oxytocin receptor gene is associated with behavioral and neural correlates of empathic accuracy. Frontiers of Behavioral Neuroscience. 2014;8:1–10. doi: 10.3389/fnbeh.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: Relationship to Vineland Adaptive Behavior Scales and cognition. Molecular Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, Sasaki T. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. Journal of Human Genetics. 2010;55:137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Ulrich I, Grabe HJ, Schroeder W, Rosskopf D. Associations between the oxytocin receptor gene (OXTR) and “mind-reading” in humans—An exploratory study. Nordic Journal of Psychiatry. 2013;67:15–21. doi: 10.3109/08039488.2012.700731. [DOI] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM. The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Gorodetsky EK, Goldman D, Blair RJ. The influence of oxytocin administration on responses to infant faces and potential moderation by OXTR genotype. Psychopharmacology. 2012;224:469–476. doi: 10.1007/s00213-012-2775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald NM, Messinger DS. Empathic responding in toddlers at risk for an autism spectrum disorder. Journal of Autism and Developmental Disorders. 2012;42:1566–1573. doi: 10.1007/s10803-011-1390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwain NL, Booth-LaForce C, Lansford JE, Wu XY, Dyer WJ. A process model of attachment-friend linkages: Hostile attribution biases, language ability, and mother-child affective mutuality as intervening mechanisms. Child Development. 2008;79:1891–1906. doi: 10.1111/j.1467-8624.2008.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Stead JD, Matherson K, Anisman H. A paradoxical association of an oxytocin receptor gene polymorphism: Early-life adversity and vulnerability to depression. Frontiers in Neuroscience. 2013;7:1–7. doi: 10.3389/fnins.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, Sigman M. Beyond autism: A baby sibling research consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Decety J, Liu C, Chen Q, Martz ME, Jacob S, Lahey BB. Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Frontiers in Behavioral Neuroscience. 2014;8:1–10. doi: 10.3389/fnbeh.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto Y, Kimura T, Ivell R. A genomic element within the third intron of the human oxytocin receptor gene may be involved in transcriptional suppression. Molecular Cell Endocrinology. 1997;135:129–138. doi: 10.1016/s0303-7207(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Montag C, Brockmann E, Lehmann A, Muller DJ, Rujescu D, Gallinat J. Association between oxytocin receptor gene polymorphisms and self-rated ‘empathic concern’ in schizophrenia. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0051882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons WG, Way BM, Taylor SE. Oxytocin and vasopressin receptor polymorphisms interact with circulating neuropeptides to predict human emotional reactiosn to stress. Emotion. 2014;14:562–572. doi: 10.1037/a0035503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AJ, Klute MM, Robinson JL. Relational and individual resources as predictors of empathy in early childhood. Social Development. 2008;17:613–637. doi: 10.1111/j.1467-9507.2007.00441.x. [DOI] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- NICHD Early Child Care Research Network. Early child care and mother-child interaction from 36 months through first grade. Infant Behavior and Development. 2003;26:345–370. doi: 10.1016/S0163-6383(03)00035-3. [DOI] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128:488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, Hardan AY. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Zahn-Waxler C, Emde RN. Patterns of development in early empathic behavior: Environmental and child constitutional influences. Social Development. 1994;3:124–145. doi: 10.1111/j.1467-9507.1994.tb00032.x. [DOI] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda P, Fernandez-Berrocal P, Baron-Cohen S. Dissociation between cognitive and affective empathy in youth with Asperger Syndrome. European Journal of Developmental Psychology. 2015;12:85–98. doi: 10.1080/17405629.2014.950221. [DOI] [Google Scholar]
- Rutter M, Bailey A, Lord C. SCQ: The Social Communication Questionnaire Manual. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Schneiderman I, Kanat-Maymon Y, Ebstein RP, Feldman R. Cumulative risk on the oxytocin receptor gene (OXTR) underpins empathic communication difficulties at the first stages of romantic love. Social Cognitive and Affective Neuroscience. 2014;9:1524–1529. doi: 10.1093/scan/nst142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Hariri AR, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biological Psychiatry. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Sigman MD, Kasari C, Kwon J, Yirmiya N. Responses to the negative emotions of others by autistic, mentally retarded, and normal children. Child Development. 1992;63:796–807. [PubMed] [Google Scholar]
- Smith KE, Porges EC, Norman GJ, Connelly JJ, Decety J. Oxytocin receptor (OXTR) gene variation predicts empathic concern and autonomic arousal while perceiving harm to others. Social Neuroscience. 2014;9:1–9. doi: 10.1080/17470919.2013.863223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slane MM, Lusk LG, Boomer KB, Hare AE, King MK, Evans DW. Social cognition, face processing, and oxytocin receptor single nucleotide polymorphisms in typically developing children. Developmental Cognitive Neuroscience. 2014;9:160–171. doi: 10.1016/j.dcn.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smearman EL, Winiarski DA, Brennan PA, Najman J, Johnson KC. Social stress and the oxytocin receptor gene interact to predict antisocial behavior in an at-risk cohort. Development and Psychopathology. 2014:1–10. doi: 10.1017/S0954579414000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. The empathy imbalance hypothesis of autism: A theoretical approach to cognitive and emotional empathy in autistic development. The Psychological Record. 2009;59:489–510. [Google Scholar]
- Sturge-Apple ML, Cicchetti D, Davies PT, Suor JH. Differential susceptibility in spillover between interparental conflict and maternal parenting practices: Evidence for OXTR and 5-HTT genes. Journal of Family Psychology. 2012;26:431–442. doi: 10.1037/a0028302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA. Oxytocin and social salience: A call for gene-environment interaction research. Frontiers in Neuroscience. 2013;7:1–3. doi: 10.3389/fnins.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey KE, Brookes KJ, Hill MJ, Cochrane LE, Gill M, Skusec D, Anney RJ. Oxytocin receptor (OXTR) does not play a major role in the aetiology of autism: Genetic and molecular studies. Neuroscience Letters. 2010;474:163–167. doi: 10.1016/j.neulet.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36:144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Meyer-Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Sigman MD, Kasari C, Mundy P. Empathy and cognition in high-functioning children with autism. Child Development. 1992;63:150–160. doi: 10.1111/j.1467-8624.1992.tb03603.x. [DOI] [PubMed] [Google Scholar]
- Young SK, Fox NA, Zahn-Waxler C. The relations between temperament and empathy in 2-year-olds. Developmental Psychology. 1999;35:1189–1197. doi: 10.1037/0012-1649.35.5.1189. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Radke-Yarrow M, Wagner E, Chapman M. Development of concern for others. Developmental Psychology. 1992;28:126–136. [Google Scholar]
- Zahn-Waxler C, Robinson JL, Emde RN. The development of empathy in twins. Developmental Psychology. 1992;28:1038–1047. doi: 10.1037/0012-1649.28.6.1038. [DOI] [Google Scholar]
- Zhou Q, Eisenberg N, Losoya SH, Fabes RA, Reiser M, Guthrie IK, Shepard SA. The relations of parental warmth and positive expressiveness to children's empathy-related responding and social functioning: A longitudinal study. Child Development. 2002;73:893–915. doi: 10.1111/1467-8624.0044. [DOI] [PubMed] [Google Scholar]


