Abstract
The infrequent use ABO incompatible (ABOi) kidney transplantation in the US may reflect concern about the costs of necessary preconditioning and post-transplant care. Medicare data for 26,500 live donor kidney transplant recipients (2000 to March 2011), including 271 ABOi and 62 A2-incompatilbe (A2i) recipients, were analyzed to assess pre-transplant, transplant episode, and 3 year post-transplant cost impacts. The marginal costs of ABOi and A2i vs. ABO compatible transplant (ABOc) were quantified by multivariate linear regression including adjustment for recipient, donor and transplant factors. Compared with ABOc transplantation, patient (93.2% vs 88.15, P=0.0009) and death-censored graft survival (85.4% vs 76.1%, P<0.05) at three years were lower after ABOi but not A2i. The average overall cost of the transplant episode was significantly higher for ABOi ($65,080) compared with A2i ($36,752) and ABOc ($32,039) transplantation (P<.001) excluding organ acquisition. ABOi was independently associated with incrementally higher post-transplant spending (year 1: $25,044, year 2: $10,496, year 3: $7,307; P<0.01). ABOi transplantation provides a clinically effective method to expand access to transplantation. While more expensive, the modest increases in total spending are easily justified by avoiding long term dialysis and its associated morbidity and costs.
Introduction
Kidney transplantation across blood group incompatibilities (ABOi) was initially believed to result in universal hyperacute rejection of the allograft and subsequent graft loss. In 1987, however, successful live donor ABOi transplantation was introduced in Japan using pre-transplant antibody depletion, to expand access to transplantation in the absence of legal recognition of brain death.(1-3) Since that time, ABOi transplantation evolved into routine practice and constituted nearly 14% of living donor transplant procedures performed in Japan in 2011. ABOi transplantation remains a rare procedure in the west, where it has been sporadically used in a limited number of centers.(4-6) A 2011 United States (US) registry analysis identified 738 patients who had undergone ABOi transplant from 1995-2010, representing approximately 1.5% of all US living donor transplant procedures.
Concerns limiting broader dissemination of ABOi transplantation include diminished long term graft outcomes, regulatory risk, and the significant resources required to successfully transplant across the ABO barrier. Contemporary data from large registry analyses in Japan, Europe, and the US demonstrate that, on average, long term outcomes following ABOi are comparable with ABO compatible (ABOc) transplantation at 10 years, although the incidence of some early complications is higher.(4, 6, 7) ABOi transplant recipients may even have a lower incidence of antibody mediated rejection as a result of early antibody depletion with anti-CD20 antibody therapy.(8) The logistical and financially onerous nature of early ABOi protocols has been reduced as routine splenectomy has been eliminated in favor of anti-CD20 antibody administration (rituximab) to diminish rebound of anti-blood group antibody after depletion.(6, 9, 10) Recently protocols have reported the successful elimination of anti-CD20 treatment (11) and limiting immunoadsorption or plasmapheresis to cases with high titers of anti-donor blood group antibodies.(12) Living donor transplantation using donors with the A2 blood type into blood group O and B recipients with low level anti A2-antibodies have been safely performed without any preconditioning treatments as the A2 antigen has a low level of expression on the cell membrane and similar to a blood group O kidney.(13)
Despite evidence of clinical success, many programs remain concerned about the short- and long-term costs of performing this procedure in an era of decreasing reimbursement. Unfortunately, data on the economic implications of ABOi transplantation have been limited to single center analyses of specific protocols with small sample sizes and unclear generalizability.(14) To address these concerns, the objective of this study was to conduct a retrospective registry analysis of Medicare-beneficiaries undergoing ABOi transplant in the US to examine of ABOi transplant, with and without concomitant splenectomy, on Medicare payments when compared with ABOc transplantation and A2i transplantation.
Materials and Methods
Data Sources and Study Samples
Study data were drawn from records of the United States Renal Data System (USRDS) which integrate Organ Procurement and Transplantation Network (OPTN) records with Medicare claims. The primary study sample comprised recipients of live donor kidney transplants in the U.S. in 2000 to March 2011 with Medicare as the primary payer at time of transplantation. The similarities and differences of patients in the USRDS with and without Medicare as their primary payer are displayed according to donor-recipient blood-type compatibility for the current sample in Supplementary Table 1. This study was approved by the Saint Louis University Institutional Review Board.
Definitions of Blood Type Compatibility and other Baseline Factors
Blood type compatibility was ascertained using donor and recipient ABO blood types as reported to the national registry. A (non-A2)-to-(O or B), B-to-(O or A), and AB to (O, A, or B) were considered ABOi. A2-to-(O or B) and A2B-to-B transplants were categorized as an additional comparison group (A2i), as previously described (7), due to increasing data that A2i transplantation may be safe without preconditioning (13, 15). Recipients of ABOc live donor transplants were considered as the reference group. Use of splenectomy was ascertained by submission of a procedure (International Classification of Diseases Code 9th Edition Clinical Modification (ICD9-CM) procedure or Common Procedure Terminology (CPT)) code any time before transplant through the end of transplant hospitalization. Information on other baseline recipient clinical and demographic traits, donor characteristics and transplant factors were drawn from the OPTN Transplant Candidate Registration and Transplant Recipient Registration forms incorporated in the USRDS, as summarized in Table 1.
Table 1. Baseline demographic and clinical characteristics of the study sample of Medicare-insured live donor kidney transplant recipients according to ABO compatibility.
| ABOi (N =271) | A2i (N =62) | Compatible(N = 26504) | ||
|---|---|---|---|---|
| Recipient Characteristics | % or mean +/- SD | % or mean +/- SD | % or mean +/- SD | |
| Age | * | |||
| <=18 | 2.21 | 3.23 | 4.89 | |
| 19-30 | 14.02 | 19.35 | 15.74 | |
| 31-44 | 18.08 | 29.03 | 23.64 | |
| 45-59 | 32.84 | 17.74 | 29.51 | |
| >=60 | 32.84 | 30.65 | 26.22 | |
| Female sex | 37.64 | 41.94 | 40.45 | |
| Race | ||||
| White | 64.94 | 70.97 | 69.33 | |
| Black | 20.66 | 17.74 | 17.71 | |
| Other | 14.39 | 11.29 | 12.97 | |
| BMI (kg/m2) | ||||
| 10 to 18.5 | 4.43 | 4.84 | 5.25 | |
| 18.5 to 25 | 33.21 | 40.32 | 34.64 | |
| 25 to 30 | 31.37 | 25.81 | 29.87 | |
| >30 | 21.77 | 19.35 | 24.58 | |
| Cause of ESRD | ||||
| Diabetes | 23.25 | 19.35 | 22.15 | |
| Glomerulonephritis | 17.34 | 33.87 | 21.18 | |
| Hypertension | 15.13 | 12.90 | 18.19 | |
| Polycystic kidney disease | 7.38 | 4.84 | 6.62 | |
| Other | 36.90 | 29.03 | 31.87 | |
| Previous transplant | 22.51‡ | 12.90 | 12.02 | |
| Pretransplant dialysis | ||||
| Preemptive | 15.87 | 11.29 | 16.66 | |
| >0-24 | 35.06 | 37.10 | 41.24 | |
| 25-60 | 28.41 | 35.48 | 26.73 | |
| >60 | 18.45 | 14.52 | 13.84 | |
| Peak panel reactive antibody level | ‡ | |||
| < 10 | 58.30 | 64.52 | 74.80 | |
| 10–79 | 20.30 | 20.97 | 16.47 | |
| >=80 | 11.44 | 6.45 | 4.35 | |
| Missing | 9.96 | 8.06 | 4.39 | |
| HLA mismatches | ||||
| Zero A, B, and DR | 10.33 | 6.45 | 8.30 | |
| Zero DR | 18.82 | 8.06 | 18.44 | |
| Cytomegalovirus | ||||
| Recipient + / Donor + | 37.27 | 38.71 | 36.13 | |
| Recipient + / Donor - | 25.83 | 20.97 | 19.21 | |
| Recipient - / Donor + | 9.59 | 16.13 | 14.14 | |
| Recipient - / Donor - | 17.34 | 17.74 | 18.10 | |
| Year of Transplant | ‡ | * | ||
| 2000-2005 | 35.42 | 41.94 | 55.61 | |
| 2006-2011 | 64.58 | 58.06 | 44.39 | |
| Donor Characteristics | ||||
| Age, mean (SD), yr | 43.58 (12.12)‡ | 40.71 (11.97) | 39.97 (11.30) | |
| Female | 60.52 | 66.13 | 59.84 | |
| Race | ||||
| White | 69.00 | 80.65 | 70.65 | |
| Black | 17.34 | 11.29 | 15.71 | |
| Other | 13.65 | 8.06 | 13.65 | |
| Induction | 67.16 | 88.71‡ | 63.39 | |
| Steroids at discharge | 74.91 | 75.81 | 72.71 | |
| Maintenance ISx at discharge | ‡ | * | ||
| Tacrolimus and MMF | 79.34 | 82.26 | 62.63 | |
| Rapamycin-based | 5.17 | 1.61 | 9.95 | |
| Other | 15.50 | 16.13 | 27.41 | |
P-values for comparisons of trait distribution in ABOi or A2-to-O groups versus compatible transplants:
P-values:
P 0.002–0.04;
P 0.0001–0.001;
P < 0.0001
Economic Outcomes
The primary economic analysis was conducted from the health system perspective and calculated using payments for all healthcare services made by Medicare (paid claims) exclusive of organ acquisition. Payments were evaluated in the following exclusive periods: 30 days prior to transplantation, the transplant hospitalization, and then annual intervals during the first, second and third years post-transplant. The cost analysis was limited to 3 years, as Medicare transplant benefits expire at three years except in the cases of people age ≥65 years or with certain disabilities. Organ acquisition costs were excluded as they are not available through Medicare claims as they are paid separately via the Medicare cost report. Patient costs were included in analysis of an interval if: 1) the recorded Medicare eligibility extended continuously from the beginning to the end of the period, or if 2) Medicare eligibility ended in an interval because of death or graft loss. Costs for 2 and 3 year follow-up were determined for patients transplanted prior to 2010 and 2009, respectively. The reported transplant episode costs do not include payments made via the Medicare cost report for kidney acquisition. Monetary figures were adjusted to the prices in the year 2012 Medical Care Component of the Consumer Price Index. We did not include indirect costs such as copayments, lost income, transportation, or need for home support services not covered by Medicare.
To compare the cost of ABOi with the alternative of waiting for an ABOc donor, maintenance dialysis was determined from published USRDS data and the median waiting time for an ABO compatible kidney transplant. The incremental cost of ABOi transplant was compared with ABOc transplant over 10 years to adjust for higher costs in later years assuming a constant increment in costs from years 3-10.
Clinical Outcomes
Mortality was defined as death from any cause. Graft failure was defined as the earliest reported date of return to maintenance dialysis or “preemptive” re-transplantation. Events were censored from survival analyses at the date of each individual's last expected follow-up or end of study (December 2011).
Statistical Analyses
Data management and analysis were performed with SAS for Windows software, version 9.2 (SAS Institute Inc., Cary, NC). Continuous data were summarized as means and standard deviations, and categorical data were summarized as proportions. Distributions of baseline traits among ABOi and A2O recipients versus recipients of blood type compatible transplants were compared with Chi-square and t-tests. The marginal cost associated with ABOi and A2O transplantation in each of the cost periods were computed by OLS regression equations as: E(Y) = β1X1 + β 2X2 +… β kXk, where E(Y) = Medicare payments within a period of interest, Xk = the value of a given predictor variable, and βk = the marginal costs associated with a 1-unit change in a given variable after adjustment for other observed factors in the model. Thus, the βk parameters quantify the marginal costs incurred by ABOi compared with compatible recipients, adjusted for the recipient, donor and transplant factors in the models. Post-transplant costs were also adjusted for death and graft failure events in the period, as per our previous methods. (16, 17) Average expected costs for a typical patient according to blood type compatibility were computed from these multivariable regression models, with values of adjustment covariates set to the average characteristics of the study sample.
Observed patient and death-censored graft survival according to blood type compatibility was compared by the Kaplan-Meier method, and the Log-Rank test was used to assess the statistical significance of differences in graft and patient survival. Multivariate cox proportional hazard modeling was also performed to adjust survival associations for donor and recipient characteristics.
Results
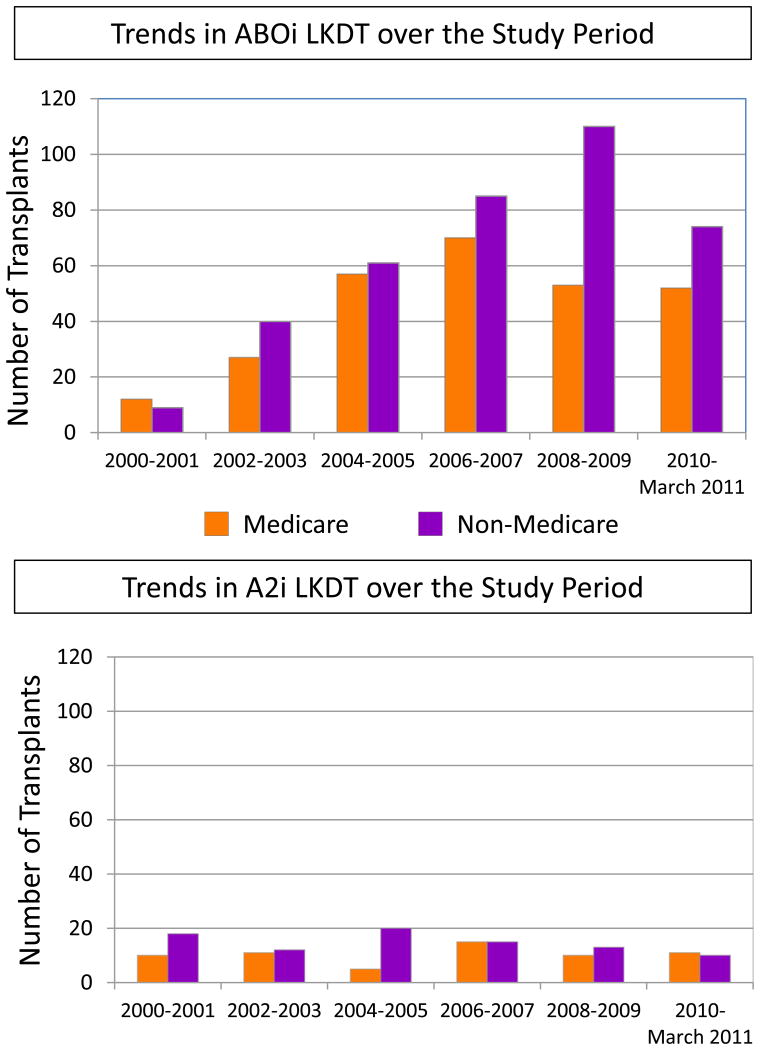
From 2000 to March 2011, 271 Medicare beneficiaries in the US underwent ABOi kidney transplantation, 62 underwent A2i transplantation, and 26,504 underwent ABOc transplantation. Use of ABOi appears to have increased over the study period (Figure 1). The ABOi patients were older and more likely to be male, African American, and re-transplant recipients. ABOi recipients were more likely than ABOc recipients to be highly sensitized (PRA>80%) (11.44% vs. 4.35%) (Table 1). Compared with donors for ABOc transplants, ABOi donors were older but there were no other significant difference in characteristics.
Figure 1.
Trends in the use of ABO incompatible transplant and A2-incompatible live donor kidney transplantation in the U.S. over the study period, among beneficiaries of Medicare and of other payers. ABOi, ABO incompatible; LDKT, live donor kidney transplantation
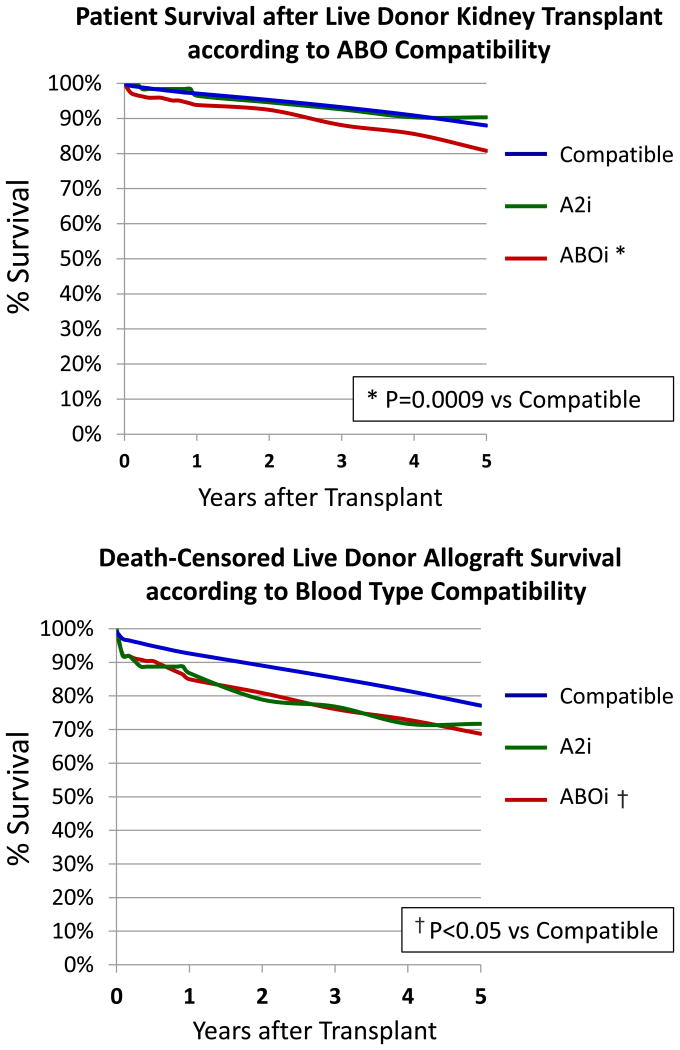
Post-transplant patient and graft survival was excellent for all three groups, although the ABOi had lower survival than the ABOc group (Figure 2). Patient survival was lower at 3 years for the ABOi group (88.1%) compared to the ABOc (93.2%) and A2i (92.6%) groups (P=0.009 vs. ABOc). These survival differences reflected early post-transplant mortality in the ABOi group. In multivariate analysis, ABOi transplant was associated with an 85% increase in the hazard of death (adjusted hazard ratio [aHR] 1.85 P=0.001 (Supplementary Table 2). Graft survival appeared consistently higher in the ABOc versus ABOi groups at 3 years (85.4% vs. 76.1%, P=0.05). In multivariate analysis, ABOi transplant increased the risk of graft failure (aHR 1.93 p<.001) which appears to be largely the result of early graft failures in the incompatible group, after which the survival curves are similar (Supplementary Table 2). Unadjusted patient and graft survival in the A2i group were not significantly different than that of ABOc patients, although A2i was associated with a higher rate of graft failure at 3 years in the multivariate model (aHR 1.97 P=.01).
Figure 2.
Post-transplant patient and allograft survival after live donor kidney transplantation according to donor-recipient blood type compatibility.
Pretransplant Costs
ABOi transplant recipients were significantly more expensive to care for than either ABOc or A2i transplants. Average spending for the 30 days prior to transplantation was $10,600 for ABOi transplants vs. $3,590 for ABOc (p<0.001) and $5,947 for A2i transplantation (p>0.05) (Supplementary Figure 1). Despite reported reductions in desensitization protocol intensity, 30-day pre-transplant Medicare payments for ABOi transplants have increased over time ($9,086 in 2000-2005 to $11,394 in 2006-2011, p=0.02). In the multivariate analysis, ABOi transplant was associated with $6,583 higher incremental spending after adjusting for baseline recipient, donor and transplant characteristics (Table 2). Other factors associated with increased pre-transplant costs included prior kidney transplant, prolonged dialysis exposure, high PRA, and female gender (Supplementary Table 3). Pre-emptive transplant was associated with lower spending, presumably because patients did not require pre-transplant dialysis in this window.
Table 2. Adjusted associations of ABOi compatibility with Medicare payments in the pre-, peri- and post-transplant periods a.
| 30 Days Pre-Transplant | Transplant | One-Year Post-Transplant | Two-Year Post-Transplant | Three-Year Post-Transplant | |
|---|---|---|---|---|---|
| Clinical Factors | Parameter Estimate, $ per 30 days | Parameter Estimate, $ episode | Parameter Estimate, $ per period | Parameter Estimate, $ per period | Parameter Estimate, $ per period |
| ABOi | 6,584 | 32,536‡ | 25,044‡ | 10,496 ‡ | 7,379* |
| A2i | 2,189 | 6,343* | 4,266 | -2,601 | -2,980 |
| ABOc | Reference | Reference | Reference | Reference | Reference |
P-values: * P 0.002–0.04;
P 0.0001–0.001;
P < 0.0001
Estimates adjusted for all other baseline recipient, donor and transplant factors in Table 1. Please see Supplementary Table 1 for complete cost regression results.
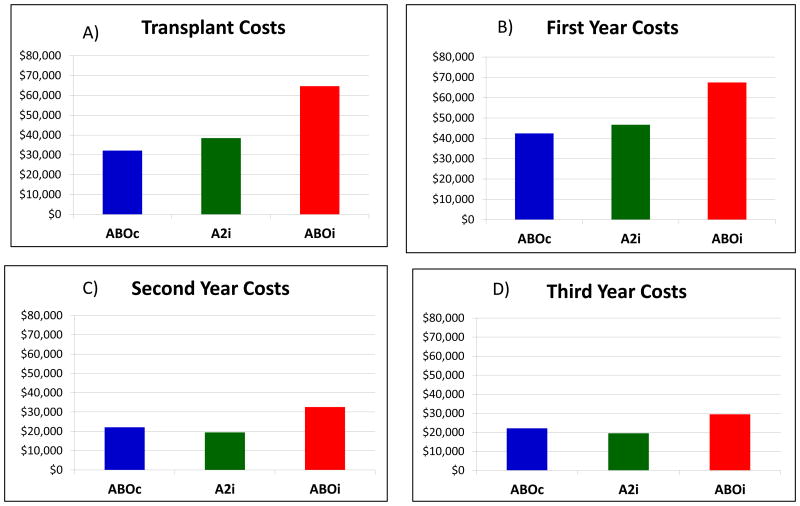
Transplant Episode Costs
The average total cost of an ABOi transplant was $65,080 compared with $36,753 for A2i and $32,039 for ABOc (Figure 3) exclusive of organ acquisition. The cost of the ABOi transplant episode was consistent over time: $64,735 in 2000-2005 and $65,256 in 2006-2011. In the multivariate model of Medicare spending for the kidney transplant event, ABOi was associated with $32,356 (P<0.001) higher payments for the transplant episode than ABOc transplantation (Table 2). A2i transplant was independently associated with a marginal increase in spending of $6,342 (P=0.04). Other factors associated with higher transplant costs included age < 18, prior transplant, longer dialysis duration, and higher PRA at the time of transplant (P<0.05 for all). Transplantation in the later part of the study period was associated with a reduction in average adjusted Medicare spending of $3,362.
Figure 3.
Average expected costs according to donor-recipient blood type compatibility during the transplant events and post-transplant periods. *adjusted for average characteristics of living donor transplant recipients in this study sample.
Post-Transplant Costs
ABOi transplant remained significantly more expensive in the each of the post-transplant periods (transplant to 1 year, 1-2 years, 2-3 years) (Table 2). After adjusting for the cost of death or graft failure, ABOi increased the cost of care by $25,044 in year 1, $10,496 in year 2, and $7,307 in year 3 (P<0.05 for all). A2i transplant was not associated with increased spending in periods after the transplant event. Recipient death was associated with incremental spending of $35,403 to $53,159 depending upon graft function at the time of death. Graft failure was associated with a $23,659 to $56,052 increase in the spending during the year in which it occurred.
The impact of splenectomy on the cost of ABOi transplant was separately analyzed. Among the 271 patients who underwent ABOi during this period, a splenectomy was performed in 40. After covariate adjustment, ABOi with splenectomy was associated with $12,633 higher pre-transplant costs and $47,465 incremental spending during the transplant event compared to ABOc (P<0.0001, Table 3). In comparison, ABOi without splenectomy was only $5,445 and $29,563 more expensive than ABOc transplantation during the pre-transplant period and during the transplant procedure, respectively. Incremental costs related to ABOi transplant with versus without splenectomy were minimal after the first year post transplant.
Table 3. Associations of ABOi compatibility including splenectomy with Medicare payments in the pre-, peri- and post-transplant periods.a.
| 30 Days Pre-Transplant | Transplant | One-Year Post-Transplant | Two-Year Post-Transplant | Three-Year Post-Transplant | |
|---|---|---|---|---|---|
| Blood type compatibility | Parameter Estimate, $ per 30 days | Parameter Estimate, $ episode | Parameter Estimate, $ per period | Parameter Estimate, $ per period | Parameter Estimate, $ per period |
| ABOi with splenectomy | 12,633‡ | 47,465‡ | 33,923‡ | 15,009* | 6,533 |
| ABOi without splenectomy | 5,445‡ | 29,563‡ | 23,296‡ | 9,527† | 7,567* |
| A2i | 2,189 | 6,339* | 4,264 | -2,601 | -2,979 |
| ABOc | Reference | Reference | Reference | Reference | Reference |
P-values:
P 0.002–0.04;
P 0.0001–0.001;
P < 0.0001
Estimates adjusted for all other baseline recipient, donor and transplant factors in Table 1.
Discussion
In contemporary practice, ABOi transplantation in the US is associated with acceptable post-transplant outcomes despite higher rates of early complications and, in some reports, higher rates of graft loss. This national study of Medicare payments shows that ABOi remains infrequently performed and is associated with increased use of health care resources compared with ABOc. Elimination of splenectomy was associated with reductions in pre-transplant and transplant event spending, but overall it appears that the incremental cost of ABOi transplants has remained consistent over time despite increased experience with this procedure. ABOi transplants also appear to be more expensive over the first three years post-transplant, although marginal cost impacts declined from $25,044 to $7,307-per year when compared with ABOc transplants. A2i transplants were somewhat more expensive than ABOc transplants during the pre-transplant phase and transplant events, but incurred no significant increases in post-transplant spending.
ABOi transplantation has evolved from an innovative practice to a well-established and highly effective solution to the ongoing donor shortage, particularly for blood group O and B recipients.(1, 6) In 2015, Aikawa and colleagues reported the results of 2,434 ABOi transplants performed in Japan since 1989. Patient and graft survival rates were exceptional at 1 year (97%, 94%), 5 years (93%, 86%), and 10 years (90%, 71%). The authors noted that the results improved with experience: 5 year patient and graft survival rates increased from 90% and 70% among patients transplanted from 1989-2000, to 95% and 90% among patients transplanted in 2001-2012 (P<0.01). The authors speculated that the improvement was principally due to a reduction in the rate of graft loss from antibody-mediated rejection. Similar clinical outcomes have also been reported in the western literature. Opelz et al. summarized the results of 1,420 ABOi transplants performed in 101 largely European centers in 2005 to 2012. This study compared ABOi and ABOc matched controls and noted no significant differences in graft survival (89.9% vs. 90.1%, p=0.44) or patient survival (95.6% vs. 96.3% p=0.15). Furthermore, the rates of rejection and development of donor HLA specific antibodies were similar between the groups, although the rate of early death from infection was higher in the ABOi group (p=0.037). The reported graft and patient survival in the US Medicare cohort noted in this study was not equal to that achieved in Japan and Europe. However, there are substantial differences in recipient characteristics including a greater use of ABOi in patients on dialysis (vs. pre-emptive therapy in Japan and Europe), a higher number of African Americans and patients with high BMIs, and a greater diversity of socioeconomic status in the American series. These differences in recipient characteristics render direct comparison of outcomes problematic.
ABOi transplant protocols have evolved to eliminate the necessity for the most invasive aspects of the protocol. Splenectomy has been routinely omitted since the introduction of antibody removal protocols utilizing plasmapheresis or immunoadsorption columns and pre-transplant anti-CD20 monoclonal antibody treatment in 2004.(12, 18) This protocol, followed by induction therapy with IL-2 receptor antibody and three drug maintenance (corticosteroids, mycophenolate mofetil, and tacrolimus) has been successfully used in the US, Japan, and Europe, confining splenectomy as a salvage technique. Recent reports, however, have suggested that anti-CD20 treatment may also be safely eliminated, particularly in low titer recipients (19). In the European experience, anti-CD20 treatment (n=804) was compared with ABOi transplants without B cell reduction (n=96), and there was no statistically significant difference in outcome (p=0.08), although there was a trend in favor of rituximab treatment overall.(6) In Australia, Flint and colleagues transplanted 37 carefully selected low-titer patients without rituximab, using pre-treatment with MMF and plasma exchange.(20) Patient and graft survival was 100% in this sample and the incidence of antibody mediated rejection was < 8%. Recently, Masterson et al. report a series of 20 ABOi transplant performed using only conventional immunosuppression without either plasmapheresis or anti-CD20 treatment, with 100% graft and patient survival.(12) All patients had initial anti-donor blood group antibody titers of < 1:16 and received pre-operative therapy with MMF for 7-14 days followed by triple therapy (corticosteroids, tacrolimus, MMF) with basiliximab induction. The elimination of rituximab treatment offers the opportunity to decrease the cost and morbidity of ABOi even further.
Previous reports examining the incremental cost of ABOi transplantation were confined to single center reports utilizing institutional cost accounting data. Schwartz et al. assessed clinical and economic implications of Mayo clinic's ABOi program in 2006.(14) They assessed the cost of transplant in 77 ABOc patients 40 consecutive ABOi patients who received four pre-transplant pheresis treatments. Splenectomy was performed in 25% of the ABOi cohort. ABOi transplantation was associated with an average increase in hospital costs of $37,800 compared with ABOc ($90,300 vs. $52,500), which is nearly identical to our current findings of the national experience. ABOi recipients had longer lengths of stay (15.3 vs. 10.4 days P=0.12), more thymoglobulin administration (1001 mg vs. 686 mg P<0.001), and underwent more frequent kidney biopsies (4 vs. 1.2 P<0.001). Overall length of stay, pharmacy costs, and apheresis treatments accounted for more than 50% of the increase in spending among ABOi patients. The increase in spending reflects, in part, the impact of pre-transplant conditioning regimens including plasmapheresis and intensified immunosuppression on the risks of surgical and medical complications. In the Mayo experience, ABOi patients appeared more likely to experience complications than ABOc transplant recipients (mean 3.6 complications vs. 2.7 p=0.018). Recently, we reported a higher rate of complications in ABOi transplant compared ABOc using Medicare claims.(7) Specifically, wound infections (12.7% vs. 7.3%), pneumonia (7.6% vs. 3.8%), urinary tract infections (24.5% vs. 15.3%) were all more common among recipients of ABOi transplants (P<0.05 for all). Perioperative hemorrhage rates were higher in patients undergoing splenectomy only.
Despite the higher initial and maintenance cost of ABOi transplantation, this technique appears to be clinically useful and, when compared to maintenance hemodialysis, is cost effective. Patients without a ABOc donor who forgo ABOi transplant will wait an average of 4.5 years with Medicare payments for dialysis ($87,500/year) exceeding $390,000 followed by the cost of transplant and maintenance medications.(21, 22) By comparison, we estimate that the incremental cost of ABOi transplant over a standard ABOc transplant over the 10 years (assuming years 4-10 are the same as year 3) was $126,313, and may be less as ABOc deceased donor may be more expensive than ABOc living donor transplant. Using the Mayo clinical cost estimates, Schnitzler and Machnicki concluded that ABOi transplant was approximately 15% less expensive than dialysis over a 20 year time horizon including the cost of re-transplantation in the case of a greater incidence of graft failure.(23) This saving is likely greater today, given the longer waiting times for deceased donor transplantation.
Given the increased costs and inferior outcomes associated with ABOi transplant when compared to ABOc, alternatives including living donor kidney donor paired (KPD) exchange should be considered as the first option. However, KPD exchange programs are enriched with blood group O recipients and non-blood group O donors, leaving many patients unable to identify an ABOc match.(24, 25) Combining ABOi with KPD programs, allows an expanded options for KPD and direct donation. Ferrari et al. reported that the inclusion of ABOi doubled the number of transplants performed in their KPD registry. Li and colleagues at the Mayo clinic have recently expanded their KPD program to include ABOi transplants, as 100% of the high PRA candidates who were unable to be matched within their KPD program were blood group O.(26) ABOi programs can be combined with desensitization to address HLA incompatibility by identifying donors to whom the recipient has low level anti-HLA antibodies and low anti-donor blood group antibody levels.
Despite the successful use of ABOi transplant outside of the US and a more favorable cost-effectiveness analysis, ABOi transplantation remains much less common in the US than in Japan or Europe.(4) In a previous registry analysis, only 43% of active US programs had performed even a single ABOi transplant between 1995-2010, and 11 centers contributed 57% of the ABOi transplants performed. Although many centers had only a limited experience with ABOi transplant, the authors were not able to identify a significant association between ABOi transplant volume and post-transplant outcomes. From these data, the authors argue that ABOi transplantation can, and should, be more widely offered in the transplant community. Wider adoption is likely limited by several factors including the potential for increased cost without commensurate reimbursement given DRG based payments; a higher incidence of graft failure when compared to ABOc transplants; and the potential that greater graft loss could impact publically reported program outcomes as ABOi transplant is not adjusted for in current risk adjustment models. Fear that ABOi transplants, like other innovative protocols, may impact the center's survival statistics is likely to pose an ongoing barrier to adoption of this technique unless the survival models are properly adjusted or these patients are excluded from review.(27)
This analysis has several key limitations inherent to registry analysis. First, the cost data here were drawn from Medicare claims which are limited in precision as Diagnosis-Related Group (DRG) payments reduce differences in payment for inpatient costs. However, the estimates provided here are very similar to those reported previously in single center studies. Medicare claims data also do not capture indirect costs including copayments, lost wages, or need for uncovered home support services. Second, we were not able to quantify titer levels for anti-A or anti-B antibodies. Third, pre-transplant antibody removal protocols have been rapidly evolving. While this study represents contemporary data, it is not possible to stratify the analysis on protocol choices beyond the use or avoidance of splenectomy. Fourth, the cost of ABOi transplantation was not directly compared with other options to achieve transplantation including KPD programs. However, as noted, KPD programs tend to be enriched with blood group O recipients who cannot find donors.(28) Furthermore, KPD often requires higher costs for travel and logistics, payment to alternative centers for donation and recovery, and multiple evaluations to identify a compatible pair. Finally, with respect to the structure of our economic models, alternatives to ordinary least-squares (OLS) models, such as regressions estimating the determinants of the natural log of Medicare payments, may be more efficient but also may produce biased estimates and are difficult to interpret. Because we have access to cost data for very large samples, we employ the unbiased estimator. Our past work has demonstrated nearly identical results with OLS cost regression and regressions on the natural log of Medicare payments (29), and OLS has become our standard in analyses of the economic impact of complications in transplantation (16, 17).
In conclusion, the use of ABOi transplantation to expand the organ supply and facilitate living donor transplant appears clinically and economically appropriate. The incremental costs of ABOi transplantation are lower than the annual cost of hemodialysis. While KPD programs offer one method of overcoming blood group incompatibility, most programs have disproportionately low number of blood group O donors, resulting in decreased access for blood group O and B recipients. By combining KPD and ABOi transplantation, it may be possible to overcome this deficit. Further analysis is need to assess economic and long term clinical implications of novel protocols for low anti-donor blood group antibody titer patients without pre-transplant plasmapheresis or anti-CD20 antibody treatments.
Supplementary Material
Figure S1: Average expected costs according to donor-recipient blood type compatibility during the 30-day pre-transplant period adjusted for average characteristics of living donor transplant recipients in this study sample.
Table S1. Comparisons of live donor transplant recipients with and without Medicare insurance, according to blood type compatibility.
Table S2. Adjusted associated of donor-recipient blood type compatibility and other baseline clinical factors with all-cause graft failure and post-transplant mortality by multivariate Cox regression.
Table S3. Associations of ABO compatibility and other baseline clinical factors with Medicare payments in the pre-, peri- and post-transplant periods. Death without function refers to patients who developed graft failure prior to death. In the period refers to events that occur in the period (0-1 year, 1-2 years, 2-3 years). Graft failure in the prior period reflects the incremental cost of care for patients in year 2 who graft failed in year 1 above the average cost of all patients.
Acknowledgments
An abstracts describing portions of this work was accepted for presentation at the 2013 American Transplant Congress in Seattle, WA. This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981 and R01DK098431.
Abbreviations
- ABOc
ABO compatible
- ABOi
ABO incompatible
- A2i
A2 incompatible
- CMV
Cytomegalovirus
- ESRD
End-stage renal disease
- MMF
Mycophenolate mofetil
- OLS
Ordinary least squares
- OPTN
Organ Procurement and Transplantation Network
- PRA
panel reactive antibody
- USRDS
United States Renal Data System
Footnotes
Publisher's Disclaimer: Disclaimer: The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Institution at which work was performed: Saint Louis University, St. Louis, MO, USA
Supporting Information: Additional Supporting Information may be found in the online version of this article.
References
- 1.Aikawa A, Saito K, Takahashi K. Trends in ABO-incompatible kidney transplantation. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2015;13(Suppl 1):18–22. [PubMed] [Google Scholar]
- 2.Aikawa A, Kawamura T, Shishido S, Saito K, Takahashi K members AB-ITC. ABO-incompatible living-donor pediatric kidney transplantation in Japan. Clinics. 2014;69(Suppl 1):22–27. doi: 10.6061/clinics/2014(Sup01)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, et al. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(7):1089–1096. doi: 10.1111/j.1600-6143.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93(6):603–609. doi: 10.1097/TP.0b013e318245b2af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker LE, Siebert D, Susal C, Opelz G, Leo A, Waldherr R, et al. Outcomes Following ABO-Incompatible Kidney Transplantation Performed After Desensitization by Nonantigen-Specific Immunoadsorption. Transplantation. 2015 doi: 10.1097/TP.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 6.Opelz G, Morath C, Susal C, Tran TH, Zeier M, Dohler B. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation. 2015;99(2):400–404. doi: 10.1097/TP.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 7.Lentine KL, Axelrod D, Klein C, Simpkins C, Xiao H, Schnitzler MA, et al. Early clinical complications after ABO-incompatible live-donor kidney transplantation: a national study of Medicare-insured recipients. Transplantation. 2014;98(1):54–65. doi: 10.1097/TP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohei N, Hirai T, Omoto K, Ishida H, Tanabe K. Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2):469–476. doi: 10.1111/j.1600-6143.2011.03830.x. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(8):1315–1322. doi: 10.1111/j.1600-6143.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 10.Morath C, Becker LE, Leo A, Beimler J, Klein K, Seckinger J, et al. ABO-incompatible kidney transplantation enabled by non-antigen-specific immunoadsorption. Transplantation. 2012;93(8):827–834. doi: 10.1097/TP.0b013e31824836ae. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, et al. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87(8):1246–1255. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 12.Masterson R, Hughes P, Walker RG, Hogan C, Haeusler M, Robertson AR, et al. ABO incompatible renal transplantation without antibody removal using conventional immunosuppression alone. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(12):2807–2813. doi: 10.1111/ajt.12920. [DOI] [PubMed] [Google Scholar]
- 13.Redfield RR, Parsons RF, Rodriguez E, Mustafa M, Cassuto J, Vivek K, et al. Underutilization of A2 ABO incompatible kidney transplantation. Clinical transplantation. 2012;26(3):489–494. doi: 10.1111/j.1399-0012.2011.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz J, Stegall MD, Kremers WK, Gloor J. Complications, resource utilization, and cost of ABO-incompatible living donor kidney transplantation. Transplantation. 2006;82(2):155–163. doi: 10.1097/01.tp.0000226152.13584.ae. [DOI] [PubMed] [Google Scholar]
- 15.Gloor JM, Stegall MD. ABO incompatible kidney transplantation. Current opinion in nephrology and hypertension. 2007;16(6):529–534. doi: 10.1097/MNH.0b013e3282f02218. [DOI] [PubMed] [Google Scholar]
- 16.Schnitzler MA, Johnston K, Axelrod D, Gheorghian A, Lentine KL. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation. 2011;91(12):1347–1356. doi: 10.1097/TP.0b013e31821ab993. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghian A, Schnitzler MA, Axelrod DA, Kalsekar A, L'Italien G, Lentine KL. The implications of acute rejection and reduced allograft function on health care expenditures in contemporary US kidney transplantation. Transplantation. 2012;94(3):241–249. doi: 10.1097/TP.0b013e318255f839. [DOI] [PubMed] [Google Scholar]
- 18.Tyden G, Kumlien G, Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76(4):730–731. doi: 10.1097/01.TP.0000078622.43689.D4. [DOI] [PubMed] [Google Scholar]
- 19.Segev DL, Simpkins CE, Warren DS, King KE, Shirey RS, Maley WR, et al. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(10):2570–2575. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 20.Flint SM, Walker RG, Hogan C, Haeusler MN, Robertson A, Francis DM, et al. Successful ABO-incompatible kidney transplantation with antibody removal and standard immunosuppression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(5):1016–1024. doi: 10.1111/j.1600-6143.2011.03464.x. [DOI] [PubMed] [Google Scholar]
- 21.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(Suppl 1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 22.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;65(6 Suppl 1):A7. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnitzler M, Machnicki G. ABO-incompatible living donor transplantation: is it economically “compatible”? Transplantation. 2006;82(2):168–169. doi: 10.1097/01.tp.0000226242.10027.e7. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari P, Hughes PD, Cohney SJ, Woodroffe C, Fidler S, D'Orsogna L. ABO-incompatible matching significantly enhances transplant rates in kidney paired donation. Transplantation. 2013;96(9):821–826. doi: 10.1097/TP.0b013e3182a01311. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery RA, Zachary AA, Ratner LE, Segev DL, Hiller JM, Houp J, et al. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA : the journal of the American Medical Association. 2005;294(13):1655–1663. doi: 10.1001/jama.294.13.1655. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Stegall MD, Dean PG, Casey ET, Reddy KS, Khamash HA, et al. Assessing the efficacy of kidney paired donation--performance of an integrated three-site program. Transplantation. 2014;98(3):300–305. doi: 10.1097/TP.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 27.Abecassis MM, Burke R, Klintmalm GB, Matas AJ, Merion RM, Millman D, et al. American Society of Transplant Surgeons transplant center outcomes requirements--a threat to innovation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(6):1279–1286. doi: 10.1111/j.1600-6143.2009.02606.x. [DOI] [PubMed] [Google Scholar]
- 28.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA : the journal of the American Medical Association. 2005;293(15):1883–1890. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 29.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(5):590–598. doi: 10.1034/j.1600-6143.2003.00082.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Average expected costs according to donor-recipient blood type compatibility during the 30-day pre-transplant period adjusted for average characteristics of living donor transplant recipients in this study sample.
Table S1. Comparisons of live donor transplant recipients with and without Medicare insurance, according to blood type compatibility.
Table S2. Adjusted associated of donor-recipient blood type compatibility and other baseline clinical factors with all-cause graft failure and post-transplant mortality by multivariate Cox regression.
Table S3. Associations of ABO compatibility and other baseline clinical factors with Medicare payments in the pre-, peri- and post-transplant periods. Death without function refers to patients who developed graft failure prior to death. In the period refers to events that occur in the period (0-1 year, 1-2 years, 2-3 years). Graft failure in the prior period reflects the incremental cost of care for patients in year 2 who graft failed in year 1 above the average cost of all patients.