Abstract
Objective
To determine if a threshold of a 1-hour glucose challenge test (GCT) eliminates the need for a 3-hour glucose tolerance test (GTT).
Study Design
A retrospective cohort of patients undergoing GTT after GCT was ≥140 mg/dL. GDM was diagnosed using National Diabetes Data Group (NDDG) and Carpenter Coustan(CC) criteria. Sensitivity, specificity, and predictive values were calculated for 1-hour GCT values of 160 to 220 mg/dL.
Result
Of 6218 patients, 988(15.9%) had an elevated GCT and 753(12.1%) underwent a GTT. 165(2.7%) were diagnosed with GDM using NDDG criteria, and 250(4.0%) by CC criteria. The positive predictive value of a 1-hour GCT≥200 mg/dL for GDM was 68.6% by NDDG and 80.0% for GDM by CC criteria.
Conclusion
Although the predictive value of an elevated 1-hour ≥200 mg/dL for GDM was high, 1 in 3 to 1 in 5 women would be overdiagnosed with GDM if the 3-hour GTT were omitted.
Introduction
Gestational diabetes mellitus (GDM) is a common complication of pregnancy, affecting nearly 6% of all pregnancies.1 Various screening strategies for GDM exist. The American College of Obstetricians and Gynecologists (ACOG) recommends a 2 step screening process, using a 50 gram glucose challenge test (GCT) for screening, followed by a diagnostic three hour glucose tolerance test (GTT) using 100 grams of glucose for those individuals with one hour glucose levels ≥130–140 mg/dL.1 Two main diagnostic criteria can be used for the diagnosis of GDM, the National Diabetes Data Group (NDDG) criteria, or the more stringent lower thresholds of the Carpenter-Coustan criteria (CC). Although use of the CC criteria results in approximately 50% more diagnoses of GDM, neither criteria has been shown to more favorably improve pregnancy outcomes and both are acceptable in current clinical practice.1
Some studies suggested that women with a very high 1-hour GCT might not need a 3-hour GTT to diagnose GDM.2,3,4,5 As would be expected, higher 1-hour GCT thresholds result in lower sensitivity but increased specificity and decreased false positive rates in diagnosing GDM. However, the positive predictive value of an extremely elevated 1-hour GCT has varied widely across studies, ranging from 50%–95% for a threshold of 180 mg/dL in some reports and from 79%–100% for a threshold of 200 mg/dL or greater in others.2,3,4,5,6,7,8,9 These studies are limited by their small sample sizes, by the use of single-ethnicity populations, and by the lack of contemporary data evaluating this question.
Because current data are unclear, there are varied clinical practices regarding patients with extremely elevated 1-hour results, with some institutions managing those patients as diabetics without further testing and others proceeding with the 3-hour GTT for definitive diagnosis.3 Although forgoing the 3-hour GTT in those with a very high 1-hour could allow for earlier treatment of GDM, eliminate the inconvenience and cost of the additional test, and avoid extremely elevated blood glucose levels induced by a 3-hour GTT, it could also lead to over diagnosis with unnecessary treatment of those who would not actually have GDM based on 3-hour testing. Our aim was to estimate if a threshold of a 1-hour GCT, alone or in combination with maternal risk factors, could achieve high enough specificity and positive predictive value to eliminate the need for a 3-hour GTT.
Materials and Methods
This was a retrospective cohort study of all consecutive patients undergoing a 1-hour, 50 gram GCT at Barnes Jewish Hospital between 2004 and 2008. Women were included in the study if they had a singleton gestation, did not have Type I or Type II diabetes, and completed 1-hour GCT testing followed by 3-hour GTT testing as appropriate after 20 weeks gestation. Women were excluded if there were no 3-hour GTT values available in the medical record. The study was conducted after approval from the Washington University School of Medicine Human Research Protection Office. Given the retrospective nature of the study, the need for informed consent was waived.
Our university-based tertiary care center employs a policy of universal GDM screening. Screening was conducted between 24–28 weeks unless risk factors suggested need for earlier testing, although only those with testing performed after 20 weeks were included for this analysis. Risk factors leading to early testing included a history of previous GDM, obesity with body mass index (BMI) ≥30.0 kg/m2, history of macrosomic infant in a prior pregnancy, first degree relative with diabetes mellitus, or glycosuria. For women with a normal early 1-hour GCT, screening was repeated between 24–28 weeks and only the second was included for analysis. For those with an elevated 1-hour GCT ≥140 mg/dL, prompt diagnostic testing with a 3-hour GTT was completed, generally within 1 week of initial screening test. GDM was diagnosed by having 2 or more abnormal values using National Diabetes Data Group (NDDG) criteria (fasting ≥105 mg/dL, one hour ≥190 mg/dL, two hour ≥165 mg/dL, three hour ≥145 mg/dL).1 Analysis was also performed for GDM as diagnosed using more stringent Carpenter Coustan (CC) criteria (fasting ≥95 mg/dL, one hour ≥180 mg/dL, two hour ≥155 mg/dL, three hour ≥140 mg/dL).1
Information on maternal baseline characteristics, obstetric history, including prior gestational diabetes (based on patient report or available medical records from prior pregnancy), medical history of comorbid conditions that are associated with GDM, body mass index at time of presentation to prenatal care, and laboratory data were obtained from the prenatal record.
Descriptive statistics were used to characterize the cohort. Baseline characteristics and outcomes were compared between women with and without an extremely elevated 1-hour. As originally suggested by Carpenter and Coustan, an extremely elevated 1-hour was defined as >=180 mg/dL.2 Student’s t test or Mann Whitney-U test were used for continuous variables and chi-squared tests were used for dichotomous variables as appropriate with a two-sided alpha of 0.05 considered significant. For continuous variables, normality was tested using histogram technique and confirmed using the Kolmogorov-Smirnoff test.
One hour GCT results were then categorized by 20 mg/dL increments between 160 mg/dL and 220 mg/dL. Receiver Operating Characteristic Curve (ROC) analysis was used to evaluate the test characteristic. The c-statistic, or area under the curve (AUC), is used to evaluate the efficacy of screening tests, with AUC approaching 1.0 and the far left corner of a ROC graph for more effective tests, and AUC paralleling the diagonal and nearing 0.5 for tests that are not better than chance.9 The AUC was calculated for each of the thresholds between 160 mg/dL and 220 mg/dL for the 1-hour GCT to diagnose GDM using both CC and NDDG thresholds. The optimal cutpoint was identified using the Youden index which maximizes the sum of sensitivity and specificity.10 Sensitivity, specificity, positive and negative predictive values were also calculated for each of the thresholds. Analysis was then repeated for each of the thresholds amongst women with individual and combinations of specific risk factors, including maternal BMI ≥30 kg/m2, history of GDM, and maternal age. These calculations were performed for both NDDG and CC criteria for diagnosis of GDM. All statistical analyses were performed using STATA 12, special edition (STATA, College Station, TX, USA).
Results
Of 6218 women screened, 988 (15.9%) had an elevated 1-hour GCT and 5230 (84.1%) did not (Figure 1). Of the 988 women with an elevated 1-hour, 235 (23.8%) were excluded, with 152 (15.3%) lost to follow up and 59 (6.0%) patients treated as GDM without a 3-hr GCT based on provider preference (Figure 1). The cohort was 51.3% African American, 33.6% Caucasians, 8.0% Hispanic, and 5.0% Asian (Figure 1). Of the eligible 753 women with an elevated 1-hour, 165 women, or 2.7% of the total cohort were diagnosed with GDM by NDDG criteria, and 250 (4.0%) were diagnosed with GDM by CC criteria. This increase of GDM in our cohort by 51.5% using the more inclusive CC criteria is similar to other published results.11
Figure 1. Study Population.
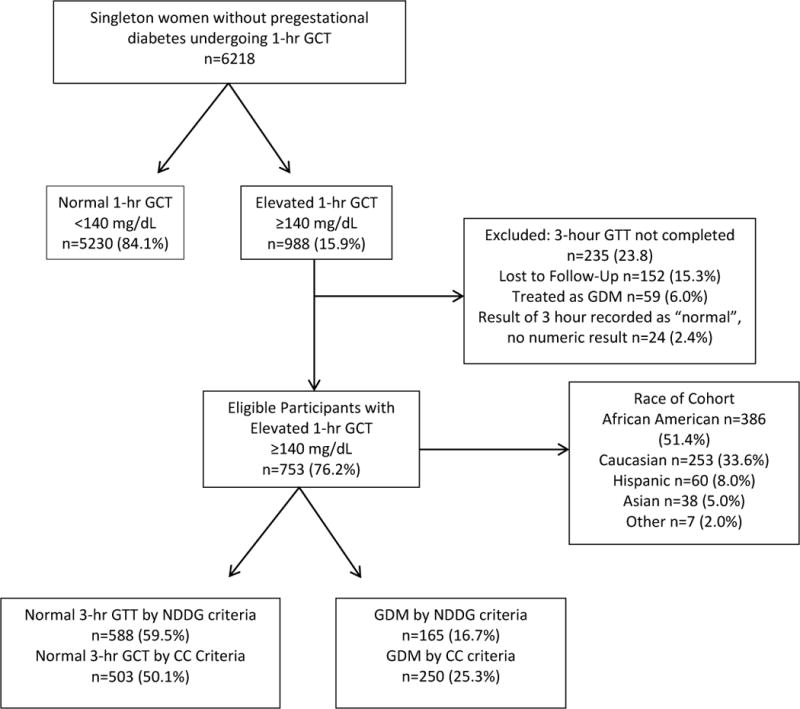
Study population, with included and excluded participants undergoing the glucose challenge test (GCT) with subsequent glucose tolerance test (GTT)
Women with an extremely elevated 1-hour ≥180 mg/dL were similar with respect to race, BMI, history of chronic hypertension, and alcohol, tobacco, and drug use to those with a 1-hour between 140 mg/dL and 180 mg/dL, the groups (Table 1). Women with an extremely elevated 1-hour ≥180 mg/dL were more likely to be older than 30 (RR 1.30, 95% CI 1.05, 1.62) and more likely to have a history of GDM (RR 3.48, 95% CI 2.40, 5.02) than those with a 1-hour between 140 mg/dL and 180 mg/dL (Table 1).
Table 1.
Characteristics of Women With and Without Extremely Elevated One Hour (≥180 mg/dL)
| Characteristic n=753 |
One Hour GCT between 140 mg/dL and 180 mg/dL (n=657) n(%) or Mean±SD |
One Hour GCT ≥180 mg/dL (n=96) n(%) or Mean±SD |
Risk Ratio (95% Confidence Interval) |
|---|---|---|---|
| Age (years) | 27.9 ± 6.3 | 28.7 ± 6.6 | – |
| Age over 30 | 257 (39.1) | 49 (51.0) | 1.52 (1.05,2.21) |
| African American Race | 336 (51.1) | 50 (52.1) | 1.03 (0.71,1.50) |
| Tobacco Use | 105 (16.0) | 18 (18.8) | 1.18 (0.74, 1.90) |
| Maternal Body Mass Index (kg/m2) | 34.10 ± 8.2 | 35.91 ± 8.6 | – |
| Normal BMI | 213 (33.3) | 27 (29.0) | 0.84 (0.55,1.28) |
| Obese | 427 (66.7) | 66 (71.0) | 1.19 (0.78,1.81) |
| Morbid Obesity | 129 (20.2) | 23 (24.7) | 1.25 (0.81,1.94) |
| Chronic Hypertension | 37 (5.63) | 8 (8.33) | 1.43 (0.74, 2.76) |
| History of GDM | 144 (21.9) | 53 (55.2) | 3.48 (2.40,5.02) |
ROC analysis was used to evaluate the predictive ability of a 1-hour GCT to detect GDM by both NDDG and CC criteria in the cohort of women with elevated 1-hour results (Figures 2 and 3). The area under the curve (AUC) was for 0.730 for GDM as diagnosed by NDDG criteria and 0.693 for CC criteria, with the Youden maximal cutpoint 157.5 mg/dL for NDDG criteria and 158.5 for CC criteria (Figures 2 and 3). Analysis was performed for each 20 mg/dL threshold between 160 mg/dL and 220 mg/dL with increasing specificity, decreasing sensitivity, at each threshold using both NDDG and CC criteria (Table 2). As evidenced by the non-overlapping 95% confidence intervals, these changes in sensitivity and specificity were statistically significant moving from 160 mg/dL to 180 mg/dL and to 200 mg/dL, but were not significantly different at 220 mg/dL, possibly due to smaller sample sizes (Table 2). Additionally, the AUC decreased at each threshold, although the AUC were not significantly different from one another based on the 95% confidence intervals. The specificity increased at higher thresholds, ranging from 92.2%–99.6% for values from 180–220 mg/dL using both diagnostic criteria (Table 2). The positive predictive values also increased at each threshold, ranging from 52.1% at 180 mg/dL to 72.7% at 220 mg/dL using NDDG criteria (Table 3). Positive predictive values were higher using CC criteria, ranging from 64.6% at 180 mg/dL to 81.8% at 200 mg/dL (Table 3).
Figure 2.
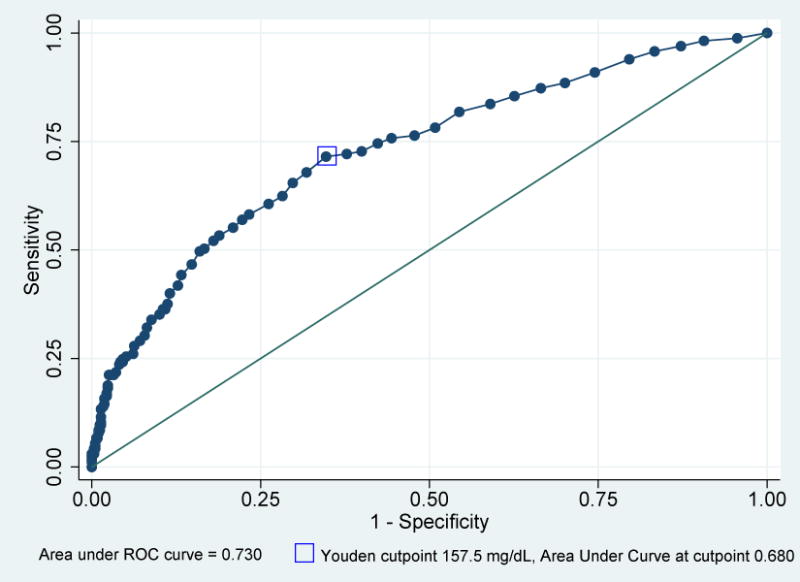
Receiver Operating Characteristic Curve for One Hour Glucose Challenge Test (GCT) to predict Gestational Diabetes Mellitus (GDM) using National Diabetes Data Group (NDDG) criteria for 753 participants.
Sensitivity, false positive rate, and area under the receiver operating curve for the prediction of GDM based on the one hour GCT using NDDG criteria for diagnosis of GDM, with Youden cutpoint identified.
Figure 3.
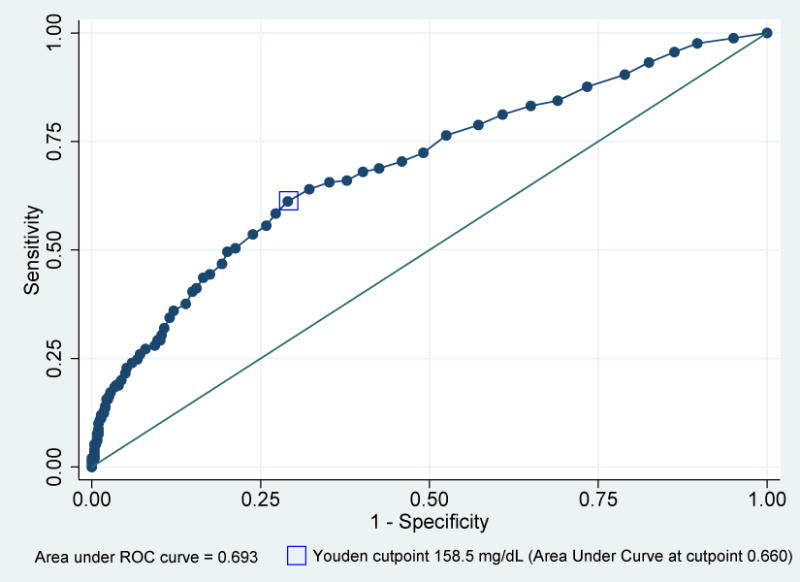
Receiver Operating Characteristic Curve for One Hour Glucose Challenge Test (GCT) to predict Gestational Diabetes Mellitus (GDM) using Carpenter Coustan criteria for 753 participants.
Sensitivity, false positive rate, and area under the receiver operating curve for the prediction of GDM based on the one hour GCT using CC criteria for diagnosis of GDM, with Youden cutpoint identified.
Table 2.
Selected test characteristics for 283 participants with 1 hour GCT ≥160 mg/dL using 1 hour GCT values, maternal BMI, age over 30, and history of GDM.
| 1 hour GCT Value (mg/dL), n |
National Diabetes Data Group Criteria for GDM (95% Confidence Interval) |
Carpenter – Coustan Criteria for GDM (95% Confidence Interval) |
||||
|---|---|---|---|---|---|---|
| Sens | Spec | AUC | Sens | Spec | AUC | |
| ≥160, n=283 |
65.5% (57.7, 72.7) |
70.2% (66.4, 73.9) |
0.678 (0.638, 0.719) |
58.4% (52.0,64.6) |
72.8% (68.6, 76.6) |
0.656 (0.620,0.692) |
| ≥160 and history of GDM, n=115 |
66.7% (57.6,74.9) |
55.4% (43.4, 67.0) |
0.610 (0.540, 0.681) |
59.2% (51.5, 66.6) |
47.8% (26.8, 69.4) |
0.535 (0.425, 0.646) |
| ≥160 and history of GDM, age≥30, BMI≥30 kg/m2, n=40 |
65.3% (50.4,78.3) |
65.2% (42.7, 83.6) |
0.653 (0.532, 0.773) |
56.9% (44.0,69.2) |
57.1% (18.4,90.1) |
0.570 (0.363, 0.777) |
| ≥180, n=96 |
30.3% (23.4, 37.9) |
92.2% (89.7, 94.2) |
0.612 (0.576, 0.649) |
24.8% (19.6, 30.6) |
93.2% (90.7, 95.3) |
0.590 (0.561,0.619 |
| ≥180, and history of GDM, n=53 |
32.5% (24.4, 41.6) |
82.4% (71.8, 90.3) |
0.575 (0.514, 0.635) |
27.6% (21.1, 34.9) |
78.3% (56.3, 92.5) |
0.529 (0.437, 0.622) |
| ≥180, and history of GDM, age≥30, BMI≥30 kg/m2, n=23 |
38.8% (25.2, 53.8) |
82.6% (71.8, 90.3) |
0.607 (0.502, 0.712) |
32.3% (21.2,45.1) |
71.4% (29.0, 96.3) |
0.519 (0.329, 0.708) |
| ≥200, n=35 |
14.5% (9.6, 20.9) |
98.1% (96.7,99.1) |
0.563 (0.536, 0.591) |
11.2% (7.6, 15.8) |
98.6% (97.2, 99.4) |
0.549 (0.529, 0.569) |
| ≥200, and history of GDM, n=23 |
16.3% (10.2, 24.0) |
95.9% (88.6,99.2) |
0.561 (0.521, 0.601) |
12.6% (8.1,18.5) |
95.7% (78.1, 99.9) |
0.541 (0.492, 0.591) |
| ≥200, and history of GDM, age≥30, BMI≥30 kg/m2, n=13 |
22.4% (11.8, 36.6) |
91.3% (72.0,98.9) |
0.569 (0.485, 0.652) |
18.5% (9.9, 30.0) |
85.7% (42.1,99.6) |
0.521 (0.373, 0.669) |
| ≥220, n=11 |
4.9% (2.1, 9.3) |
99.5% (98.5,99.9) |
0.522 (0.505, 0.538) |
3.6% (1.7, 6.7) |
99.6% (98.6, 100.0) |
0.516 (0.504, 0.528) |
| ≥220, and history of GDM, n=7 |
5.7% (2.3, 11.4) |
100.0% (95.1, 100.0) |
0.528 (0.508, 0.549) |
4.0% (1.6, 8.1) |
100.0% (85.2, 100.0) |
0.520 (0.505,0.535) |
| ≥220, and history of GDM, age≥30, BMI≥30 kg/m2, n=4 |
8.2% (2.3,19.6) |
100.0% (85.2, 100.0) |
0.541 (0.502, 0.580) |
6.2% (1.7,15.0) |
100.0% (59.0, 100.0) |
0.531 (0.051,0.560) |
Sensitivity (Sens), Specificity (Spec), and Area Under the Curve (AUC) for each threshold.
Table 3.
Positive Predictive Values for 283 participants with 1 hour GCT ≥160 mg/dL using 1 hour GCT values, maternal BMI, age over 30, and history of GDM.
| 1 hour GCT Value | Positive Predictive Value National Diabetes Data Group Criteria for GDM (95% Confidence Interval) |
Positive Predictive Value Carpenter – Coustan Criteria for GDM (95% Confidence Interval) |
|---|---|---|
| ≥160, n=283 |
38.2% (32.5, 44.1) |
51.6% (45.6, 57.5) |
| ≥160, and history of GDM, n=115 |
71.3% (62.1, 79.4) |
89.6% (82.5, 94.5) |
| ≥160, and history of GDM, age≥30, BMI≥30 kg/m2, n=40 |
80.0% (64.4, 90.9) |
92.5% (79.6, 98.4) |
| ≥180, n=96 |
52.1% (41.6, 62.4) |
64.6% (54.2, 74.1) |
| ≥180, and history of GDM, n=53 |
75.5% (61.7, 86.2) |
90.6% (79.3, 96.9) |
| ≥180, and history of GDM, age≥30, BMI≥30 kg/m2, n=23 |
82.6% (61.2, 95.0) |
91.3% (72.0, 98.9) |
| ≥200, n=35 |
68.6% (50.7,83.1) |
80.0% (63.1, 91.6) |
| ≥200, and history of GDM, n=23 |
87.0% (66.4, 97.2) |
95.7% (78.1, 99.9) |
| ≥200, and history of GDM, age≥30, BMI≥30 kg/m2, n=13 |
84.6% (54.6, 98.1) |
92.3% (64.0, 99.8) |
| ≥220, n=11 |
72.7% (39.0, 94.0) |
81.8% (48.2, 97.7) |
| ≥220, and history of GDM, n=7 |
100.0% (59.0, 100.0) |
100.0% (59.0, 100.0) |
| ≥220, and history of GDM, age≥30, BMI≥30 kg/m2, n=4 |
100.0% (39.8, 100.0) |
100.0% (39.8, 100.0) |
The predictive characteristics of each threshold were evaluated in women with individual and combinations of specific risk factors. Diagnostic performance of each threshold was evaluated in women with the addition of clinical risk factors for GDM, including age over 30, history of GDM, and obesity (Table 3). For each threshold amongst women with high risk characteristics, sensitivities and specificities were similar for both criteria. The positive predictive values increased for each threshold in the higher risk subgroups, with the most significant improvements in predictive value coming with the addition of history of GDM, and nominal increases in predictive value with the addition of other characteristics (Table 3). When considering women who were obese (BMI ≥ 30.0 kg/m2) in addition to being older than age 30 and having a history of GDM, the positive predictive value at 180 mg/dL increased to 82.6% for GDM as diagnosed by NDDG criteria and 91.3% for GDM as diagnosed by CC criteria (Table 3). The positive predictive value increased to 100% for those with a history of GDM and an elevated 1-hour ≥220 mg/dL, but this included only 7 women in our cohort, yielding a wide 95% confidence interval (Table 3).
Discussion
In an institution that employs universal GDM screening, we found that even with extremely elevated 1-hour GCT results from 180 mg/dL to 220 mg/DL, the positive predictive values ranged from 52.1%–81.8%. The predictive value of an extremely elevated 1-hour GCT was not substantially improved by applying CC criteria although the CC criteria did predictably increase the rates of diagnosis of GDM. The addition of maternal characteristics that place women at higher risk of GDM improved the positive predictive value to 100%, but only for those with a 1-hour result ≥220 mg/dL. These results suggest that a 3-hour GTT should be performed even in the setting of an extremely elevated 1-hour GCT to avoid over diagnosis and treatment of GDM in those who might not need it.
Although Carpenter and Coustan described a greater than 95% positive predictive value for GDM in those patients with elevated 1-hour ≥182 mg/dL, subsequent studies have found mixed results.2 Bobrowski et al, Friedman et al, and Landy et al found a 100% positive predictive value for GDM with elevated 1-hour results greater than 220 mg/dL, 200 mg/dL, and 220 mg/dL, respectively.4,6,7 Our positive predictive values are more similar to the contrasting findings of Shivvers et al, who found that only 81% of those with a 1-hour greater than 200 mg/dL were ultimately diagnosed with GDM.8 Similarly, Yogev et al found that only 34% of Mexican-Americans with 1-hour results ≥200 mg/dL had GDM.3 Some of these studies have been limited by small sample size which has precluded the statistical approach used in the current study. Most of the studies only evaluate the 1-hour with use of the higher thresholds of the NDDG criteria. Additionally, the studies of Yogev and Friedman were in single ethnic populations, limiting their generalizability to multi-ethnic populations such as ours.3,4 Finally, other studies are largely based on databases from the 1990s, with the most recent including data from 2004. Given the intervening rise in obesity as well as rates of gestational diabetes, our results add evidence on the predictive value of an extremely elevated 1-hour in a more modern cohort.
This study has several strengths. It is a large, diverse cohort of patients with elevated 1-hour GCT results. Additionally, the overall prevalence of GDM in our cohort, which was 2.7% using NDDG criteria and 4.0% using CC criteria, shows our prevalence to be in the range of findings from other studies, where estimates for prevalence of GDM in the United States range from 2.0–14% depending on which criteria are used.11 This makes our data externally generalizable to populations with similar GDM prevalence and similar ethnic makeup. Additionally, we attempted to use maternal clinical and demographic data to provide more robust and clinically relevant prediction of the diagnosis of GDM and compared these predictive criteria to both diagnostic approaches currently used in the United States.
Nevertheless, this study is not without limitations. First, it is based on an available retrospective cohort at our institution from 2004–2008. Although this represents older data, guidelines for GDM diagnosis at our institution have not changed since that time. Second, 235 people, or nearly 24% of those with elevated 1-hour GCT were excluded. These included some patients lost to follow-up as well as 59 (6.0%) patients who were treated for GDM without further diagnostic testing. As expected, these included patients with extremely elevated 1-hour GCT results; 43 (4.6%) patients had a 1-hour GCT ≥180 mg/dL. However, in a sensitivity analysis comparing those with an extremely elevated 1-hour who were treated as GDM without further testing to those who underwent traditional 3-hour testing and were not diagnosed with GDM, there were no significant differences in baseline characteristics between the two groups. Nevertheless, the inclusion of these patients, had they undergone diagnostic testing and tested positive for GDM, likely would have increased the predictive values of an extremely elevated 1-hour result. Including these patients as if they all tested positive for GDM raises the predictive value of a 1-hour GCT ≥180 mg/dL to 66.9% using NDDG criteria and 75.5% using CC criteria. Additionally, even in this large cohort, having an extremely elevated 1-hour result was still a relatively rare event. The group with high risk characteristics for whom the 1-hour result was 100% predictive included only 7 women. These results may not be applicable to a population with a much higher prevalence of women with extremely elevated 1-hour GCTs or with a much higher prevalence of GDM. It further should be noted that significant hyperglycemia ≥250 mg/dL occurred during diagnostic testing of 10 patients with a 1-hour GCT ≥200 mg/dL who were subsequently diagnosed with GDM. This hyperglycemia would have been avoided if no further testing had been performed on patients with fasting blood glucose on the day of the 3-hr GTT test ≥120 mg/dL.
Finally, the current study does not address pregnancy outcomes in women with an extremely elevated 1-hour GCT. Previous studies have shown that GDM is associated with higher rates of pregnancy induced hypertension, cesarean deliveries, operative deliveries, shoulder dystocia, and macrosomia.1 Additionally, the Hyperglycemia and Adverse Pregnancy Outcome Study confirmed a continuous relationship between maternal glucose levels and adverse pregnancy outcomes including cesarean delivery and macrosomia.12 Therefore, some have suggested that those with an extremely elevated 1-hour result may have some degree of glucose intolerance and may benefit from treatment for GDM.7 Further study is needed to evaluate pregnancy outcomes in those with an extremely elevated 1-hour who do not have GDM on diagnostic testing.
Despite these limitations, our study adds to the literature by demonstrating that even with an extremely elevated 1-hour GCT result ≥ 200 mg/dL, 20%–33% of patients would be over diagnosed with GDM if the 3-hour GTT was omitted. Although the addition of maternal risk factors marginally improves the specificity and positive predictive value of an extremely elevated 1-hour, it would only eliminate the need for a 3-hour GTT in a few select patients, making this less practical. These findings support the need for a diagnostic 3-hour GTT even in those patients with extremely elevated 1-hour results.
Acknowledgments
Dr. Cahill was a Robert Wood Johnson Foundation Faculty Physician Scholar which partially supported this work. Dr. Temming is supported by a NIH T32 training grant (5T32HD055172-07). This publication was also made possible by Grant Number UL1 TR000448 from the NIH National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
Footnotes
The authors report no conflict of interest.
Presented as Poster #354 at the 36th Annual Meeting of the Society of Maternal and Fetal Medicine, February 5–7, 2015 San Diego, CA
References
- 1.Committee on Practice Bulletins–Obstetrics. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013 Aug;122(2 Pt 1):406–16. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982 Dec 1;144(7):768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 3.Yogev Y, Langer O, Xenakis EM, Rosenn B. Glucose screening in Mexican-American women. Obstet Gynecol. 2004 Jun;103(6):1241–5. doi: 10.1097/01.AOG.0000124781.98059.fe. [DOI] [PubMed] [Google Scholar]
- 4.Friedman S, Khoury-Collado F, Dalloul M, Sherer DM, Abulafia O. Glucose challenge test threshold values in screening for gestational diabetes among black women. Am J Obstet Gynecol. 2006 May;194(5):e46–8. doi: 10.1016/j.ajog.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Cheng YW, Esakoff TF, Block-Kurbisch I, Ustinov A, Shafer S, Caughey AB. Screening or diagnostic: markedly elevated glucose loading test and perinatal outcomes. J Matern Fetal Neonatal Med. 2006 Nov;19(11):729–34. doi: 10.1080/14767050600926546. [DOI] [PubMed] [Google Scholar]
- 6.Bobrowski RA, Bottoms SF, Micallef JA, Dombrowski MP. Is the 50-gram glucose screening test ever diagnostic? J Matern Fetal Med. 1996 Nov-Dec;5(6):317–20. doi: 10.1002/(SICI)1520-6661(199611/12)5:6<317::AID-MFM5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Landy HJ, Gómez-Marín O, O’Sullivan MJ. Diagnosing gestational diabetes mellitus: use of a glucose screen without administering the glucose tolerance test. Obstet Gynecol. 1996 Mar;87(3):395–400. doi: 10.1016/0029-7844(95)00460-2. [DOI] [PubMed] [Google Scholar]
- 8.Shivvers SA, Lucas MJ. Gestational diabetes. Is a 50-g screening result > or = 200 mg/dL diagnostic? J Reprod Med. 1999 Aug;44(8):685–8. [PubMed] [Google Scholar]
- 9.Lanni S, Barrett D. The predictive value of the 1-h 50-g glucose screen for diagnosing gestational diabetes mellitus in a high-risk population. J Matern Fetal Neonatal Med. 2004 Jun;15(6):375–9. doi: 10.1080/14767050410001724308. [DOI] [PubMed] [Google Scholar]
- 10.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimalcut-point estimated from observations affected by a lower limit of detection. Biomedical Journal. 2008 Jun;50(3):419–30. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care. 2002 Sep;25(9):1625–30. doi: 10.2337/diacare.25.9.1625. [DOI] [PubMed] [Google Scholar]
- 12.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008 May 8;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]


