Abstract
Allergic reactions to mosquito bites are an increasing clinical concern. Due to lack of availability of mosquito salivary allergens, they are under-diagnosed. Here, we reported a newly cloned mosquito Aedes (Ae.) aegypti salivary allergen and its clinical relevance.
A cDNA encoding a 30-kDa Ae. aegypti salivary protein, designated Aed a 3, was isolated from an expression library. The full-length cDNA was cloned into baculovirus expression vector and recombinant Aed a 3 (rAed a 3) was expressed, purified and characterized. Skin prick tests with purified rAed a 3, and Ae. aegypti mosquito bite tests were performed in 43 volunteers, some of whom had serum rAed a 3-specific IgE levels measured.
The primary nucleotide sequence, deduced amino acid sequence, and IgE binding sites of Aed a 3 were identified. rAed a 3-selected antibodies recognized a 30 kDa Ae. aegypti saliva protein. rAed a 3 bound IgE in mosquito-allergic volunteers and the binding could be inhibited by addition of natural mosquito extract dose-dependently. Immediate skin test reactions to rAed a 3 correlated significantly with reactions produced by mosquito bites. Of the bite-test positive volunteers, 32% had a positive rAed a 3 skin test and 42% had specific IgE. No bite-test negative volunteers reacted to rAed a 3 in either the skin tests or the IgE assays, confirming the specificity of the assay.
Aed a 3 is a major mosquito salivary allergen. Its recombinant form has biological activity and is suitable for use in skin tests and serum IgE assays in mosquito-allergic individuals.
Keywords: Aed a 3, Aedes aegypti, Insect allergy, Mosquito, Salivary allergen/antigen, allergens and epitopes, dermatology, IgE, immunologic tests, venom
Introduction
Mosquitos are a global problem, not only because they facilitate transmission of potentially fatal diseases such as dengue and yellow fever, but also because their bites can cause large local skin reactions and, less commonly, systemic reactions including urticaria, angioedema, and anaphylaxis.1–8 Inhalation of mosquito allergens can cause asthma and/or allergic rhinoconjunctivitis symptoms in sensitized individuals.9, 10 In patients with Epstein-Barr virus-associated T/natural-killer cell-associated lymphoproliferative disorders, reactions to mosquito bites involve an intense skin reaction associated with systemic symptoms.11
Allergic reactions to mosquito bites are caused by salivary proteins introduced into the skin when adult female mosquitos probe and ingest a blood meal. Mosquito saliva proteins elicit both IgE-mediated immediate hypersensitivity and lymphocyte-mediated delayed hypersensitivity.3, 10, 12 IgG, especially IgG4, also may be involved in the development of mosquito bite-induced skin reactions.1, 3
Currently, only mosquito whole body extracts, containing multiple antigens not present in mosquito saliva, but few actual salivary allergens,13 are commercially available for diagnosis and immunotherapy of mosquito allergy. We compared the UniCAP assy that utilizes mosquito whole-body extract with our mosquito saliva-capture enzyme-linked immunosorbent assay (ELISA) for measurement of mosquito-specific IgE. The results indicated that the mosquito saliva-capture ELISA is significantly more sensitive and specific (less likely to give false-negative and false-positive results) than the UniCAP test.14 However, collection of mosquito saliva is time-consuming, labor-intensive, and impractical. Utilization of molecular techniques to clone and express pure allergens and even allergen-derived peptides has been successful for treating allergic patients.15–17 Development of recombinant mosquito salivary proteins circumvents the problems involved in collecting mosquito saliva, potentially facilitating accurate and safe clinical evaluation and immunotherapy in mosquito-allergic individuals.
Aedes (Ae.) aegypti is an important mosquito species with a worldwide cosmotropical distribution. The saliva of an adult female Ae. aegypti contains a complex of proteins in which at least 8 are allergens.18 We have previously expressed, purified, and clinically evaluated two recombinant Ae. aegypti mosquito salivary proteins19, 20 from two cDNAs previously isolated.21, 22 These two recombinant proteins bind to human IgE and elicit skin reactions in people who are allergic to mosquito bites. In accordance with allergen nomenclature, the 68 kDa protein (biochemical name “apyrase”) is called Aed a 1 19 and the 37 kDa protein (biochemical name “D7”) is called Aed a 2.20
Here, we report the isolation of a new cDNA encoding a 30 kDa IgE-binding protein from an Ae. aegypti salivary gland cDNA expression library using mouse anti-Ae. aegypti saliva serum and the evaluation of its clinical relevance in volunteers with Ae. aegypti bite tests. This 30 kDa salivary protein, designated Aed a 3, will facilitate studies of mosquito allergy and the mechanisms of pathogen transmission through mosquito saliva.
Methods
Participants
This project was approved by the University of Manitoba Research Ethics Board. The participants gave written, informed consent before study enrollment. Forty-three healthy volunteers (22 female and 21 male), ages 20 to 54 with a history of skin reactions to mosquito bites that ranged from highly sensitive to no reaction were selected from people who responded to advertisements. No participants had taken antihistamines within 5 days prior to the study. All 43 volunteers had a bite test from a laboratory-reared mosquito and a skin prick test with rAed a 3; and 23 also had a 10 ml blood sample taken for assay of rAed a 3-specific IgE.
Mosquitos, mosquito antigens, and anti-saliva sera
The Ae. aegypti colony was obtained from the Department of Entomology, University of Manitoba and maintained in our laboratory. Mosquito saliva extracts and head and thorax extracts were prepared as described previously.18, 23 Mouse anti-Ae. aegypti saliva serum was produced in our laboratory by immunization of mice with Ae. aegypti saliva and adjuvants.24 Mosquito-allergic human serum was obtained by pooling sera from participants who had large local reactions to mosquito bites and high titers of mosquito salivary gland-specific IgE. Control human serum was obtained by pooling sera from participants with negative mosquito bite tests.
Molecular cloning, DNA sequencing, and molecular modeling
The salivary gland λgt11 cDNA library of adult female mosquito Ae. aegypti (provided by Dr. Anthony James, University of California, Irvine, CA) was screened and two sets of PCR primer pairs were designed to combine two overlapping cDNA clones into a full-length Aed a 3 cDNA clone (Supplementary Data). The cDNA was subcloned into the vector pBluescript II SK (Stratagene, La Jolla, CA, USA) and sequenced using a kit supplied by US Biochemicals (Cleveland, OH, USA).
The Amersham Staden Plus software package was used to analyze the nucleotide sequence of the cDNA for open reading frames (ORF) and deduce the amino acid sequence. To identify the antigenic epitope, homology modeling of the C-terminus sequence of Aeda3 was carried out using the Schrodinger modeling package (Schrodinger, LLC, New York, NY). The X-ray crystallographic structure of Anopheles anti-platelet aggregation protein (apap) fragment in complex with a mouse Fab antibody (PDB 4OKV)25 was used as the structural template for homology modeling of the C-terminus sequence of Aed a 3 187-253. All missing sidechains and hydrogen atoms were added using the standard protein preparation protocols at physiological pH, followed by energy minimization in implicit solvent to optimize all hydrogen-bonding networks
Expression and purification of rAed a 3
The full-length protein encoded by the Aed a 3 cDNA was expressed using a baculovirus expression system (Supplementary Data). Concentrated rAed a 3 supernatant was loaded onto a DEAE-Sephacel column and then eluted with a linear NaCl gradient (0 to 0.6 M in Tris buffer). Protein concentrations were monitored by OD595 nm and rAed a 3 content assayed using ELIS A and immunoblots (Supplementary data). Fractions containing rAed a 3 were pooled and concentrated to a final protein concentration of 0.2 mg/ml for use in skin tests and serum rAed a 3-specific IgE assay.
Preparation of fusion protein-selected antibodies
Nitrocellulose membranes absorbed previously with the fusion protein produced by the cloned gene were used to purify specific antibodies from mouse anti-mosquito saliva serum and also from mosquito-allergic human serum using methods reported previously (Supplementary Data). These clone-specific antibodies were used in immunoblot analyses to detect their binding to the native protein present in the saliva of Ae. aegypti.
Immunoblotting
To identify which protein in the mosquito saliva reacts with the fusion protein-selected mouse antibodies, proteins in Ae. aegypti saliva extract were separated by 12% SDS-PADE, transferred to nitrocellulose membranes and immunoblotting analyses performed as described previously.18 Membranes were incubated with fusion protein-selected mouse antibodies or mouse anti-saliva serum as a positive control or PBST containing 1% BSA, followed by incubation with peroxidase-conjugated goat anti-mouse IgG (Calbiochem Corporation, San Diego, CA) and then with ECL detecting reagents (Amersham Life Science, Buckinghamshire, England). For the human IgE binding test, the membranes containing saliva proteins were incubated with fusion protein-selected human antibodies or a mosquito-allergic serum or the control buffer, followed by incubations with monoclonal anti-human IgE (ascites, clone No. 7.12, provided by Dr. A. Saxon, University of California, Los Angeles, CA), then with peroxidase conjugated goat anti-mouse IgG and finally with ECL detecting reagents.
To identify whether rAed a 3 reacts with saliva-induced antibodies in humans, proteins in purified rAed a 3 and in Ae. aegypti saliva (positive control) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated with human mosquito-allergic serum, followed by incubation with monoclonal anti-human IgE and then with alkaline phosphatase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Lab Inc., West Grave, PA). After washing, the color was developed by incubation of the membranes with an enzyme substrate solution.
ELISA and ELISA inhibition tests
An ELISA was developed to detect rAed a 3-specific IgE in human sera using the technique described previously.20, 26 In brief, microtiter plates were coated with 0.05 µg/well of purified rAed a 3 protein. After blocking the free binding sites, the plates were incubated with human sera (1:20 diluted). The bound serum Aed a 3-specific IgE was detected by incubation of the plates with goat anti-human IgE, followed by incubations with alkaline phosphatase-conjugated rabbit anti-goat IgG and finally with the enzyme substrate solution. Optical absorbance at 410 nm was read using a THERMO-max microplate reader.
An ELISA inhibition test was performed to determine whether the binding of human IgE to rAed a 3 could be inhibited by natural Aed a 3 in the saliva and to determine the specificity of the rAed a 3-specific IgE assay. The mosquito head and thorax extract was diluted 10-fold. Each dilution was mixed with an equal volume of a 1:25 diluted mosquito-allergic serum. Serum diluted with PBST served as a positive control. After incubation at 4°C overnight, rAed a 3-specific IgE contained in the mixtures was measured by ELISA as described above.
Mosquito bite tests and skin prick tests
In 43 healthy volunteers, the mosquito bite test was performed on the volar aspect of the left forearm using a laboratory-raised Ae. aegypti female mosquito.27 Skin prick tests were carried out on the right forearm with purified rAed a 3 (0.2 mg/ml), histamine (1 mg/ml) as a positive control, and saline as a negative control. The histamine-induced wheal and flare was traced with pen after 10 minutes and the other test sites were traced after 20 minutes and at 24 hours post-test. All tracings were transferred to paper and the areas measured.27 An immediate wheal or a delayed induration of ≥ 0.3 cm2 was considered to be a positive reaction.
Statistical analyses
Values were expressed as mean ± SD. The unpaired student t-test was used to compare the differences. Linear regressions were used to determine correlations. All statistical analyses were performed using GraphPad Prism version 5 (GraphPad Prism, USA). P values < 0.05 were considered statistically significant.
Results
cDNA cloning and sequence analysis
A total of 39 positive plaques were identified following screening of ~120,000 plaques from the whole salivary gland cDNA library of adult Ae. aegypti with mouse anti-saliva serum. Three clones with different sizes of cDNA inserts were subcloned. One clone (λAA22) with a complete 3′ terminus with a poly-A tail was selected for further analysis. This fragment was determined to contain the major coding region of the 30 kDa protein because the cloned cDNA (0.73 kb) has an open reading frame of 217 amino acids, the molecular weight of which is estimated to be 26 kDa. This clone lacked the 5′ terminal sequence because no initiation codon was observed.
Gene amplification techniques were used to clone the 5′ terminal fragment of the full-length cDNA. The full-length cDNA is 0.85 kb, encodes a protein of 254 amino acid residues with a relative molecular weight of ~30 kDa. The primary nucleotide sequence of the Aed a 3 cDNA and its deduced amino acid sequence are described in Supplementary data and the sequence data were deposited in GenBank (AF001927).
Molecular antigen binding site analysis
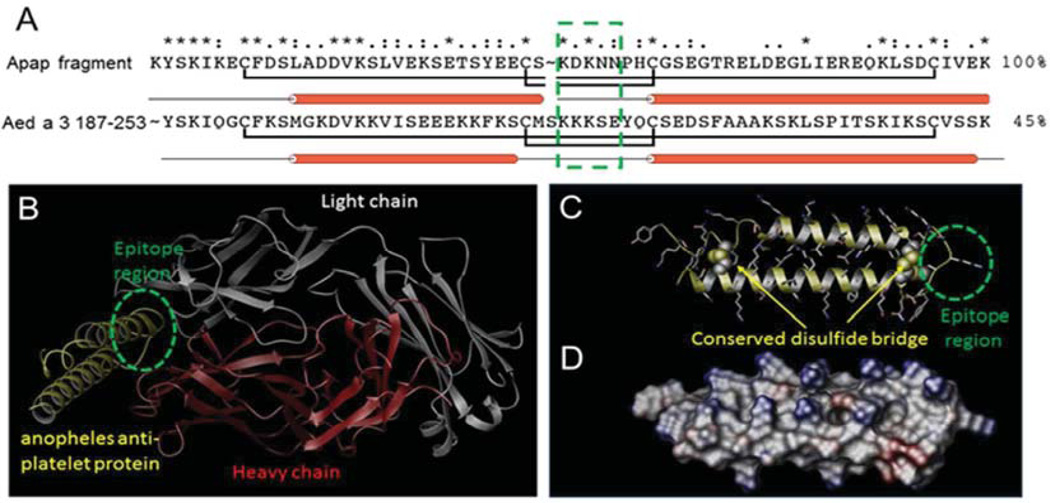
The x-ray-resolved crystallographic structure of a mosquito (Anopheline) anti-platelet aggregation protein (apap) fragment was identified using sequence analysis.25 This apap fragment has 45% sequence similarity to the C-terminal region of Aed a 3 (amino acids 187-253) (Figure 1A). The structure of the apap fragment is resolved in complex with the mouse Fab antibody at 1.8 Å resolution and consists primarily of two alpha helices connected by a turn region with two disulfide bridges located at the two opposing ends of each helix. Examination of the antigen binding site with the nearest apap residues identifies the KDKNN sequence motif located at the inter-helical turn as the antigenic epitope site for antigen binding (Figure 1B). Based on these observations, homology modeling of the C-terminal region of Aed a 3 was carried out. The final model showed all four cysteine residues essential for the formation of the two disulfide bridges in apap are conserved within the Aed a 3 C-terminus region (Figure 1C). There is >30% reduction in the number of ionizable residues located on the surface of Aed a 3 C-terminus region as compared to apap, two-thirds of which comprise primarily lysine residues (Figure 1D). Subsequently, the KKKSE sequence motif located within the turn region was identified as the most probable antigenic epitope region for antigen binding.
Figure 1.
Homology model of the Aed a 3 C-terminal region. (A) Sequence alignment between the apap fragment and Aed a 3 C-terminal region. The two conserved disulfide bridges are highlighted with the antigenic epitope region boxed in green. (B) X-ray structure of apap fragment (yellow) in complex with mouse FAB antibody consisting of a light chain (white) and a heavy chain (red). (C) Homology model of the Aed3 C-terminal region 187-253 with structurally conserved disulfide bridges. The antigenic epitope region is highlighted in green. (D) Electrostatic potential surface model of the Aed a 3 C-terminal region with the positively- and negatively-charged surfaces in blue and red, respectively.
IgE binding property of the 30 kDa protein
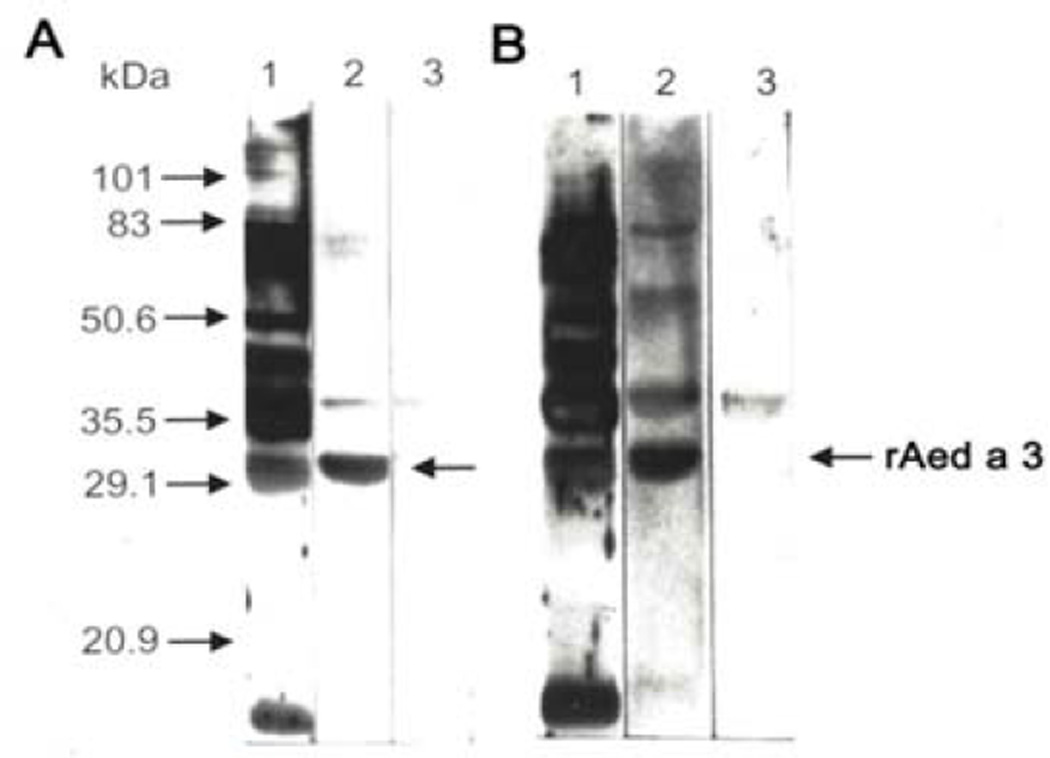
Both the mouse IgG (A) and human IgE (B) antibodies selected by the λAA22 clone fusion protein recognize a 30 kDa protein in the saliva (Figure 2, lane 2). This indicates that the cDNA that we isolated encodes the 30 kDa salivary protein and that it can induce a specific IgG response in mice and a specific IgE response in mosquito-allergic volunteers.
Figure 2.
Fusion protein-selected antibodies recognize a 30 kDa Ae. aegypti salivary protein.
A. Mouse antibodies. Ae. aegypti saliva proteins were separated by SDS-PAGE and immunoblotted with mouse anti-saliva serum (lane 1), fusion protein-selected mouse antibodies (lane 2), or PBST (lane 3), followed by incubation with peroxidase-conjugated goat anti-mouse IgG.
B. Human antibodies. Ae. aegypti salivary proteins were separated by SDS-PAGE and immunoblotted with a pooled human mosquito-allergic serum (lane 1), fusion protein-selected human antibodies (lane 2), or PBST (lane 3), followed by incubation with mAb anti-human IgE.
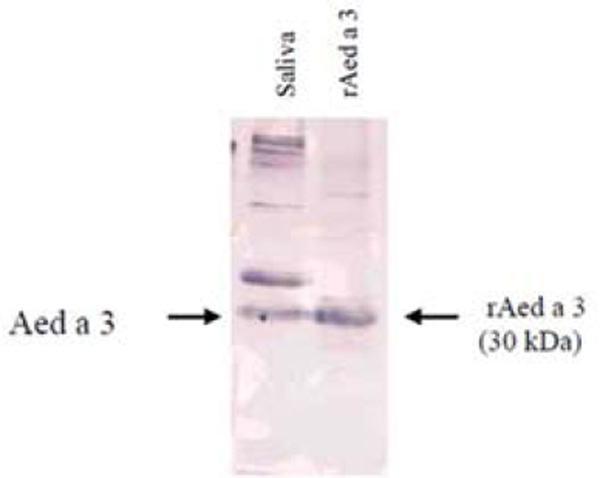
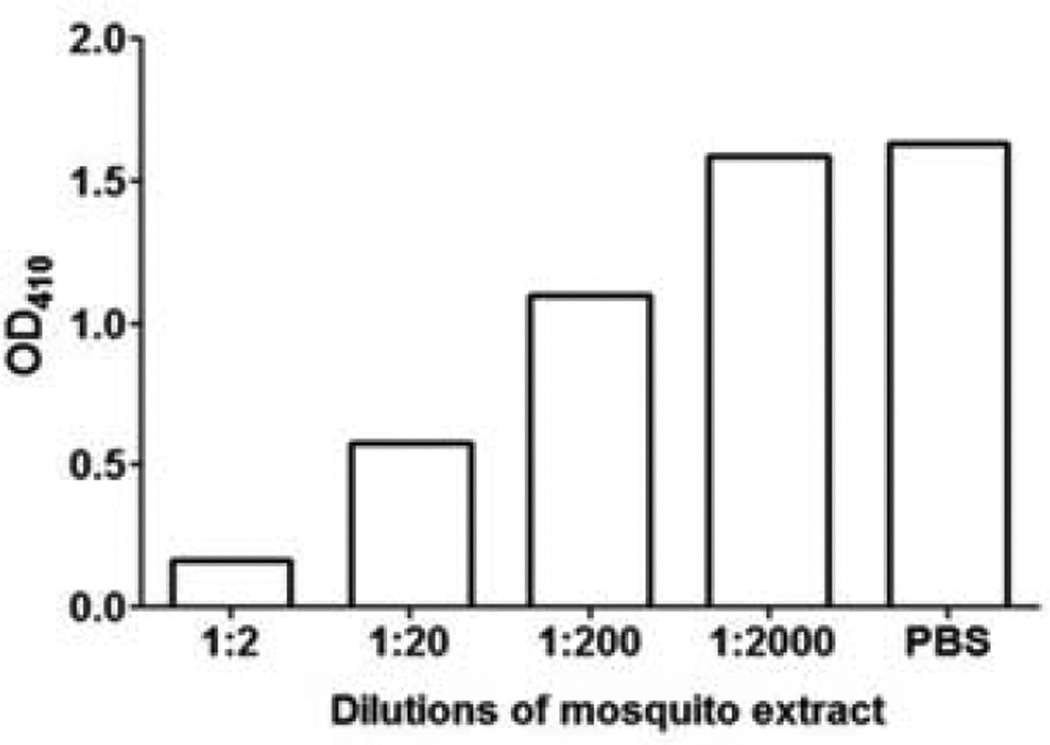
As shown in Figure 3, the Aed a 3 fusion protein binds directly to IgE in mosquito-allergic human sera. Further, the binding of serum rAed a 3-specific IgE was successfully inhibited in the ELISA inhibition test in a dose-dependent manner by the addition of mosquito head and thorax extract (Figure 4). This supports the conclusions that the recombinant Aed a 3 allergen and native Aed a 3 allergen in the salivary gland extract have identical antigenicity, and that the rAed a 3 captured-ELISA is specific for the detection of Aed a 3-specific IgE in human serum.
Figure 3.
rAed a 3 protein binds to the IgE in mosquito-allergic human serum. Proteins in Ae. aegypti saliva (lane 1) and purified rAed a 3 (lane 2) separated by 10% SDS-PAGE were transferred to nitrocellulose membrane. The membrane was immunoblotted with a pooled mosquito-allergic human serum and incubated with monoclonal anti-human IgE.
Figure 4.
Binding of rAed a 3 to human IgE can be inhibited in a dose-dependent manner by a natural Ae. aegypti head and thorax extract.
Skin testing and clinical relevance
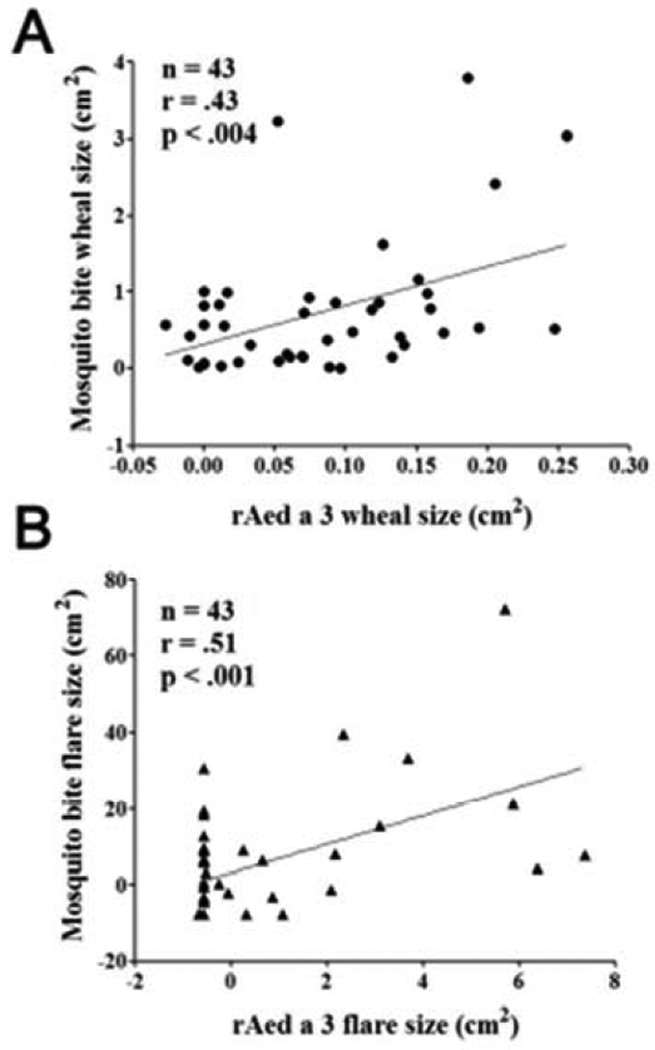
No systemic reactions were noted after the mosquito bite tests or skin tests. Twenty-eight out of the 43 participants (65%) had a positive immediate skin reaction in the bite test, while 15 (35%) had a negative bite test. Among the 28 bite-positive volunteers, 23 also had a positive delayed reaction. Similar to the bite reactions, skin prick tests with rAed a 3 also induced immediate wheals and flares and delayed indurations. Further, there were significant correlations between mosquito bite-induced and rAed a 3-induced skin immediate reactions (r= 0.51 for flares and r= 0.43 for wheals, p’s < 0.004) (Figure 5).
Figure 5.
rAed a 3-induced skin flare and wheal sizes correlate significantly with those induced by mosquito bite tests.
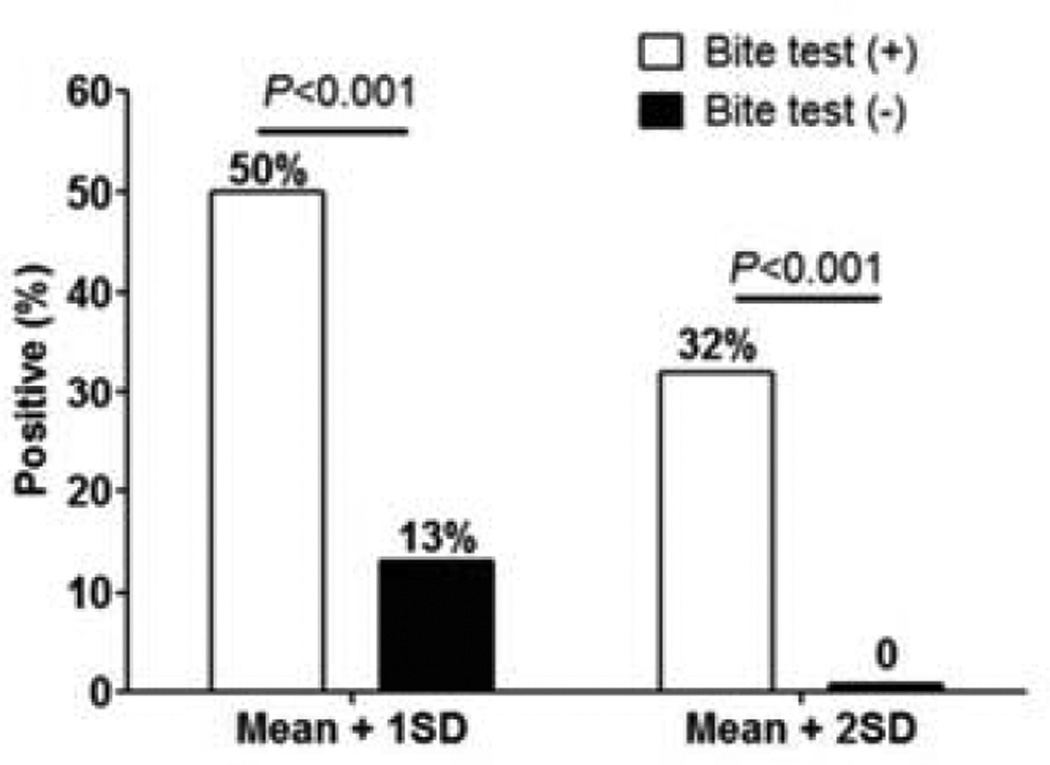
rAed a 3-induced skin reactions were found only in the mosquito bite-positive group and not in the bite-negative group. For immediate reactions, two different positive cut-off levels were compared: the mean of bite-negative individuals plus 1 standard deviation (SD) SD or 2SD. As shown in Figure 6, the positivity rate of rAed a 3 is significantly higher in the bite-positive group than in the bite-negative group for both cut-off levels: 50% vs 13% in 1SD cut-off and 32% vs 0% in 2SD cut-off. Using the method reported28 diagnostic values of rAed a 3-induced immediate reactions were calculated as “137” and “132”, respectively. Both cut-off levels had similar diagnostic values; the former is more sensitive and the latter is more specific (Table 1).
Figure 6.
The positivity rate of skin prick testing with rAed a 3 is significantly higher in the Ae. aegypti bite-positive group (n=28) than the negative group (n=15). Two positive cut-off levels: the mean of rAed 3-induced skin positive reactions in the bite-negative group plus 1SD or 2SD.
Table 1.
Diagnostic values* of skin testing with rAed a 3 at different cut-off levels (%)
| Sensitivity | Specificity | Diagnostic value* |
|
|---|---|---|---|
| Mean + 1SD | 50 | 87 | 137 |
| Mean + 2SD | 32 | 100 | 132 |
The reliability of a diagnostic test, its diagnostic value, is the sum of its sensitivity and its specificity. It ranges from “failure” at “0” to “perfect” at 200%.
Due to the small amount of rAed a 3 introduced to the skin by prick testing, delayed reactions were only found in 3 individuals in the bite-positive group.
Serum rAed a 3-specific IgE levels
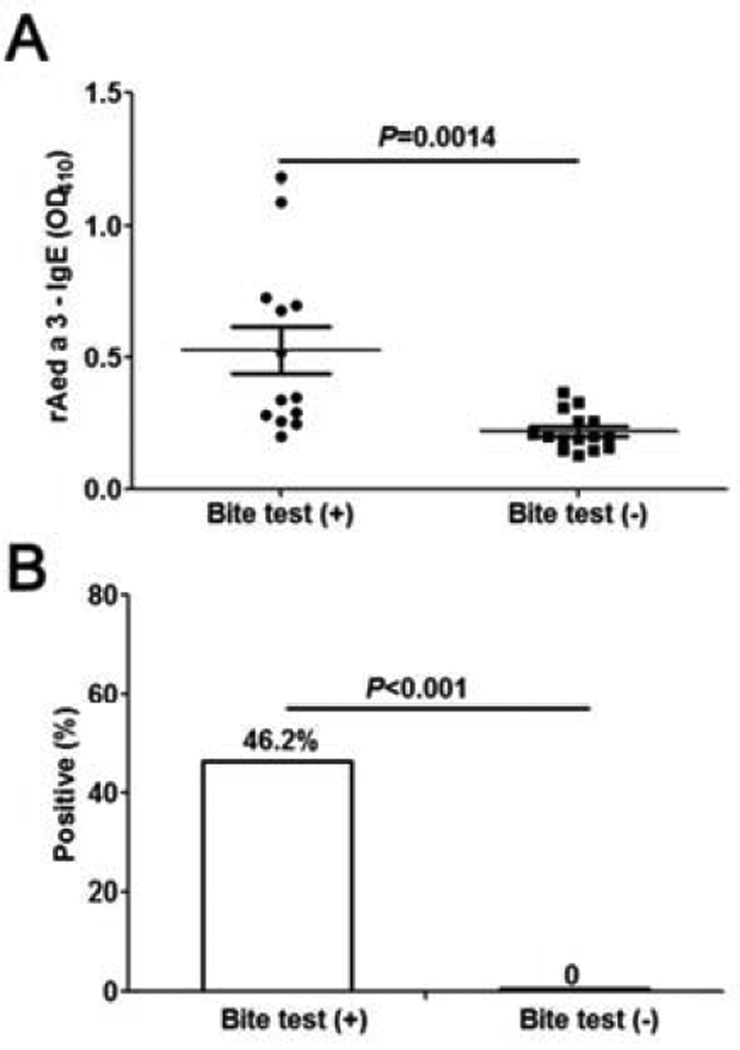
Serum rAed a 3-specific IgE levels were significantly higher in the mosquito bite test-positive group than in bite test-negative group (P=0.0014) (Figure 7). Using the geometric mean of the controls plus 2 SD as a cut-off level, ~46% (6/13) mosquito bite-positive individuals exhibited a positive serum rAed a 3-specific IgE, while none of the bite-negative individuals had a positive rAed a 3-IgE. This supports the conclusion that the assay is highly-specific. The diagnostic value of the IgE assay is “146”, which is higher than that of the skin tests (“137” or “132”) (Table I).
Figure 7.
Serum rAed a 3-specific IgE levels are elevated significantly in the Ae. aegypti-bite test-positive group (n=13) compared with the bite-negative group (n=15). The geometric mean of the negative controls plus 2 SD was used as a cut-off level.
Discussion
The saliva of an adult female Ae. aegypti was reported in 1987 to contains a complex of as many as 20 proteins seen by sodium dodecylsulphate polyacrylamide gel electrophoresis;29 in 2007 it was reported to contain 55 proteins coded by salivary gland-specific transcripts.30 Among them, at least 8 proteins have been identified as allergens that bind to the IgE of allergic individuals who have large local (skin) reactions to mosquito bites.18 In this study, we isolated the cDNA clone that encodes Aed a 3, a 30 kDa salivary allergen of Ae. aegypti.
Using molecular modeling, we identified an antigenic epitope region for IgE binding at the Aed a 3 C terminus region. This IgE-binding of the 30 kDa allergen (Aed a 3) was confirmed by several experiments. First, the fusion protein-selected mouse IgG and human IgE antibodies bound a 30 kDa protein present in Ae. aegypti saliva. Second, recombinant Aed a 3 directly bound to the IgE in mosquito-allergic serum that was induced by natural Aed a 3 in mosquito saliva in both immunoblotting and ELISA. More importantly, the binding of rAed a 3 to the IgE of a mosquito-allergic serum could be inhibited in a dose-dependent manner by addition of natural Aed a 3 present in mosquito head and thorax extract. Further, rAed a 3 was able to induce both skin immediate and delayed reactions in the bite test-positive volunteers and the sizes of skin test reactions to rAed a 3 significantly correlated with the sizes of skin reactions to Ae. aegypti bite.
Taken together, the above evidence indicates that Aed a 3 is an IgE-binding allergen and that rAed a 3 expressed using a baculovirus/insect cell system has identical antigenicity and biological activity to the natural form of Aed a 3 in Ae. aegypti saliva. Because rAed a 3 was expressed using a baculovirus/insect cell system that performs many of the post-translational modifications found in mammalian cells,31 it is not surprising that the rAed a 3 we produced has biological activity and the potential to be developed as a novel allergen.
Molecular cloning facilitates isolation and unlimited production of purified recombinant proteins. These recombinant allergens can not only serve as accurate diagnostic agents,32, 33 but also can facilitate safe and effective immunotherapy.15, 16 In this study recombinant Aed a 3 was proved to be an excellent candidate for use in in vivo and in vitro tests with 100% specificity in mosquito bite-negative individuals for the diagnosis of mosquito allergy. We have previously expressed and clinically evaluated 2 recombinant Ae. aegypti salivary allergens, rAed a 119 and rAed a 220 in addition to rAed a 3. Preparation of additional recombinant mosquito salivary allergens is in progress. Establishment of assays using a cocktail or combination of recombinant mosquito salivary proteins will eventually make it possible to diagnose mosquito allergy with accuracy and, if indicated, to provide immunotherapy.
In contrast to natural allergen extracts, recombinant allergens for immunotherapy can be produced in unlimited amounts with precise quality controls and modified to have more favorable characteristics including reduced IgE reactivity or enhanced immunogenicity.15–17 Currently, only mosquito whole body extracts are available for use in skin tests, serum specific IgE tests and immunotherapy which is effective in treating people who are sensitized to mosquito saliva;34–36 after an 18-month course of injections, skin reactions and respiratory and eye symptoms after mosquito bites were reduced.35 Application of recombinant allergens or their modified forms will potentially enhance the effectiveness and safety of this specific immunotherapy. As the binding sites of this molecule have been identified to locate at the C-terminal region (amino acids 187-253), it might be easier to modify this allergen for new immunotherapy approaches.
In our previous studies, in which we used a cut off level of the mean of controls plus 2SD, rAed a 1 induced a positive skin reaction in 29%19 or 43%37, 38 of the mosquito bite-test positive group and 0 in the bite-negative group, while rAed a 2 induces a positive reaction in 11% of the bite-positive group and 0 in the controls.37, 38 In another study of rAed a 2,20 using mean plus 1SD of control group as a cut-off level, although rAed a 2 induced 43% positivity in the bite-test positive group, it also caused 5% false positivity in control group. These variations occur because of different participants, allergen batches and cut-off levels selected. In the present study, even at a cut-off level of mean plus 2SD, rAed a 3 induced 32% positivity rate for skin tests and 46% positivity for serum specific IgE assay in the bite-positive group, and no positivity in the control group, confirming that rAed a 3 is a major allergen in Ae. aegypti saliva and that both skin prick and serum IgE tests using rArd a 3 are sensitive and specific in the assessment of mosquito allergy.
In addition to eliciting IgE responses, Aed a 3 plays an important role in mosquito feeding. After our isolation of the Aed a 3 cDNA (GenBank #AF001927, deposited in 1997),39 a study showed that the Aed a 3 coding protein, the Aegyptin protein (the biochemical name of Aed a 3, encoded by the same gene), secreted from the female Ae. aegypti salivary glands, plays a critical, non-redundant role in the feeding success of this mosquito on vertebrate hosts.40, 41 This protein binds collagen and inhibits platelet aggregation and adhesion.41 Future studies may identify additional roles for Aed a 3.
In conclusion, we have isolated and sequenced the cDNA coding for Aed a 3, a 30 kDa IgE-binding protein of mosquito Ae. aegypti saliva. Aed a 3 is a major allergen in Ae. aegypti saliva, eliciting positive reactions in 32% (skin tests) and 46% (IgE assay) of mosquito bite test-positive individuals. Recombinant Aed a 3 has identical biological activity to its native form in mosquito saliva, and can be used in in vivo and in vitro tests and in immunotherapy for people sensitized to mosquito saliva. In addition, Aed a 3 protein is involved in Ae. aegypti feeding success, an aspect that deserves to be investigated further, as Ae. aegypti is the main vector of dengue, chikungunya, and yellow fever viruses.42–44
Supplementary Material
Acknowledgments
We acknowledge Ms. Jieti Sun for her laboratory technical contributions to the study and the University of Minnesota Supercomputing Institute for providing all the necessary computational resources.
A declaration of all sources of funding: This work was supported by grants from the Children’s Hospital Research Institute of Manitoba (CHRIM) in Canada and conducted using facilities of the CHRIM. Z.P. received a Scholarship from the CHRIM. A.A.J. was supported in part by a grant from the National Institutes of Health in USA (AI29746).
Abbreviations
- apap
anti-platelet aggregation protein
- Ae.
Aedes
- ELISA
enzyme-linked immunosorbent assay
- PBS
phosphate-buffered saline
- PBST
PBS containing 0.05% tween 20
- PCR
polymerase chain reaction
- SDS-PAGE
SDS polyacrylamide gel electrophoresis
- Sf9
Spodoptera frugiperda f9 cells
Footnotes
The authors have declared that no competing interests exist.
Author Contributions
Z.P., W.W.W., Y.S., H.L., D.S., C.L., N.F.R., Q.G., and F.E.R.S. substantially contributing to conception and design of, or acquisition of data or analysis and interpretation of data. Z.P., W.W.W., A.A.J., and F.E.R.S. drafting the article or revising it critically for important intellectual content. Z.P. final approval of the version to be published.
References
- 1.Simons FER, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705–707. doi: 10.1016/s0091-6749(99)70348-9. [DOI] [PubMed] [Google Scholar]
- 2.Peng Z, Beckett AN, Engler RJ, Hoffman DR, Ott NL, Simons FER. Immune responses to mosquito saliva in 14 individuals with acute systemic allergic reactions to mosquito bites. J Allergy Clin Immunol. 2004;114:1189–1194. doi: 10.1016/j.jaci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Peng Z, Simons FE. Advances in mosquito allergy. Curr Opin Allergy Clin Immunol. 2007;7:350–354. doi: 10.1097/ACI.0b013e328259c313. [DOI] [PubMed] [Google Scholar]
- 4.Kulthanan K, Wongkamchai S, Triwongwaranat D. Mosquito allergy: clinical features and natural course. J Dermatol. 2010;37:1025–1031. doi: 10.1111/j.1346-8138.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- 5.Reiter N, Reiter M, Altrichter S, Becker S, Kristensen T, Broesby-Olsen S, et al. Anaphylaxis caused by mosquito allergy in systemic mastocytosis. Lancet. 2013;382:1380. doi: 10.1016/S0140-6736(13)61605-0. [DOI] [PubMed] [Google Scholar]
- 6.Brummer-Korvenkontio H, Reunala T. [Mosquito allergy] Duodecim. 2013;129:1362–1367. [PubMed] [Google Scholar]
- 7.Crisp HC, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65–69. doi: 10.1016/j.anai.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Seon HS, Roh JH, Lee SH, Kang EK. A case of hypersensitivity to mosquito bites without peripheral natural killer cell lymphocytosis in a 6-year-old Korean boy. J Korean Med Sci. 2013;28:164–166. doi: 10.3346/jkms.2013.28.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bemanian MH, Alizadeh Korkinejad N, Shirkhoda S, Nabavi M, Pourpak Z. Assessment of sensitization to insect aeroallergens among patients with allergic rhinitis in Yazd City, Iran. Iran J Allergy Asthma Immunol. 2012;11:253–258. [PubMed] [Google Scholar]
- 10.Cantillo JF, Fernandez-Caldas E, Puerta L. Immunological aspects of the immune response induced by mosquito allergens. Int Arch Allergy Immunol. 2014;165:271–282. doi: 10.1159/000371349. [DOI] [PubMed] [Google Scholar]
- 11.Miyake T, Yamamoto T, Hirai Y, Otsuka M, Hamada T, Tsuji K, et al. Survival rates and prognostic factors of Epstein-Barr virus-associated hydroa vacciniforme and hypersensitivity to mosquito bites. Br J Dermatol. 2015;172:56–63. doi: 10.1111/bjd.13411. [DOI] [PubMed] [Google Scholar]
- 12.Peng Z, Simons FER. Mosquito allergy: immune mechanisms and recombinant salivary allergens. Int Arch Allergy Immunol. 2004;113:198–209. doi: 10.1159/000076787. [DOI] [PubMed] [Google Scholar]
- 13.Peng Z, Simons FER. Comparison of proteins, IgE, and IgG binding antigens, and skin reactivity in commercial and laboratory-made mosquito extracts. Ann Allergy Asthma Immunol. 1996;77:371–376. doi: 10.1016/S1081-1206(10)63335-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Beckett A, Simons FE, Peng Z. Comparision of the mosquito saliva-capture enzyme-linked immunosorbent assay and the unicap test in the diagnosis of mosquito allergy. Ann Allergy Asthma Immunol. 2007;99:199–200. doi: 10.1016/S1081-1206(10)60650-3. [DOI] [PubMed] [Google Scholar]
- 15.Valenta R, Niespodziana K, Focke-Tejkl M, Marth K, Huber H, Neubauer A, et al. Recombinant allergens: what does the future hold? J Allergy Clin Immunol. 2011;127:860–864. doi: 10.1016/j.jaci.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Makatsori M, Pfaar O, Lleonart R, Calderon MA. Recombinant allergen immunotherapy: clinical evidence of efficacy—a review. Curr Allergy Asthma Rep. 2013;13:371–380. doi: 10.1007/s11882-013-0359-7. [DOI] [PubMed] [Google Scholar]
- 17.Marth K, Focke-Tejkl M, Lupinek C, Valenta R, Niederberger V. Allergen Peptides, Recombinant Allergens and Hypoallergens for Allergen-Specific Immunotherapy. Curr Treat Options Allergy. 2014;1:91–106. doi: 10.1007/s40521-013-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Z, Li H, Simons FER. Immunoblot analysis of salivary allergens in 10 mosquito species with worldwide distributions and the IgE responses to these allergens. J Allergy Clin Immunol. 1998;101:498–505. doi: 10.1016/S0091-6749(98)70357-4. [DOI] [PubMed] [Google Scholar]
- 19.Peng Z, Xu W, James AA, Lam H, Sun D, Cheng L, et al. Expression, purification, characterization, and clinical relevance of rAed a 1 - a 68 kDa recombinant mosquito Aedes aegypti salivary allergen. Inter Immunol. 2001;13:1445–1452. doi: 10.1093/intimm/13.12.1445. [DOI] [PubMed] [Google Scholar]
- 20.Peng Z, Xu W, Lam H, Cheng L, James AA, Simons FER. A new recombinant mosquito salivary allergen, rAed a 2: allergenicity, clinical relevance, and cross-reactivity. Allergy. 2006;61:485–490. doi: 10.1111/j.1398-9995.2006.00985.x. [DOI] [PubMed] [Google Scholar]
- 21.James AA, Blackmer K, Marinotti O, Ghosn CR, Racioppi JV. Isolation and characterization of the gene expressing the major salivary gland protein of the female mosquito, Aedes aegypti. Mol Biochem Parasitol. 1991;44:245–253. doi: 10.1016/0166-6851(91)90010-4. [DOI] [PubMed] [Google Scholar]
- 22.Champagne DE, Smartt CT, Ribeiro JM, James AA. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc Natl Acad Sci U S A. 1995;92:694–698. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Z, Li H, Simons FE. Immunoblot analysis of IgE and IgG binding antigens in extracts of mosquitos Aedes vexans, Culex tarsalis and Culiseta inornata. Int Arch Allergy Immunol. 1996;110:46–51. doi: 10.1159/000237309. [DOI] [PubMed] [Google Scholar]
- 24.Peng Z, Yang J, Wang H, Simons FER. Production and characterization of monoclonal antibodies to two new mosquito Aedes aegypti salivary proteins. Insect Biochem Mol Biol. 1999;29:909–914. doi: 10.1016/s0965-1748(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama K, Iyori M, Sawaguchi A, Akashi S, Tame JRH, Yoshida S, et al. Crystal structure of anopheline anti-platelet protein with Fab antibody. Protein data bank ( http://www.rcsb.ore/pdb/explore.do?structureId=4okv) 2015 (to be published). [Google Scholar]
- 26.Peng Z, Yang M, Simons FER. Measurement of mosquito Aedes vexans salivary gland-specific IgE and IgG antibodies and the distribution of these antibodies in human sera. Ann Allergy Asthma Immunol. 1995;74:259–264. [PubMed] [Google Scholar]
- 27.Peng Z, Yang M, Simons FER. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG antibodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238–244. doi: 10.1016/S1081-1206(10)63262-0. [DOI] [PubMed] [Google Scholar]
- 28.Armitage P, Berry G, editors. Statistical methods in medical research. Boston: Blackwell Scientific Publications; 1987. [Google Scholar]
- 29.Racioppi JV, Spielman A. Secretory proteins from the salivary glands of adult Aedes aegypti mosquitoes. Insect Biochem. 1987;17:503–511. [Google Scholar]
- 30.Ribeiro JM, Area B, Lombardo F, Calvo E, Phan VM, Chandra PK, et al. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics. 2007;8:6. doi: 10.1186/1471-2164-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aucoin MG, Mena JA, Kamen AA. Bioprocessing of baculovirus vectors: a review. Curr Gene Ther. 2010;10:174–186. doi: 10.2174/156652310791321288. [DOI] [PubMed] [Google Scholar]
- 32.Arruda LK, Barbosa MC, Santos AB, Moreno AS, Chapman MD, Pomes A. Recombinant allergens for diagnosis of cockroach allergy. Curr Allergy Asthma Rep. 2014;14:428. doi: 10.1007/s11882-014-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niederberger V, Eckl-Dorna J, Pauli G. Recombinant allergen-based provocation testing. Methods. 2014;66:96–105. doi: 10.1016/j.ymeth.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palosuo K, Brummer-Korvenkontio H, Mikkola J, Sahi T, Reunala T. Seasonal increase in human IgE and IgG4 antisaliva antibodies to Aedes mosquito bites. Int Arch Allergy Immunol. 1997;114:367–372. doi: 10.1159/000237696. [DOI] [PubMed] [Google Scholar]
- 35.Ariano R, Panzani RC. Efficacy and safety of specific immunotherapy to mosquito bites. Allerg Immunol (Paris) 2004;36:131–138. [PubMed] [Google Scholar]
- 36.Srivastava D, Singh BP, Sudha VT, Arora N, Gaur SN. Immunotherapy with mosquito (Culex quinquefasciatus) extract: a double-blind, placebo-controlled study. Ann Allergy Asthma Immunol. 2007;99:273–280. doi: 10.1016/S1081-1206(10)60664-3. [DOI] [PubMed] [Google Scholar]
- 37.Simons FER, Peng Z. Mosquito allergy: recombinant mosquito salivary allergens for new diagnostic tests. Int Arch Allergy Immunol. 2001;124:403–405. doi: 10.1159/000053771. [DOI] [PubMed] [Google Scholar]
- 38.Peng Z, Estelle F, Simons R. Mosquito allergy and mosquito salivary allergens. Protein Pept Lett. 2007;14:975–981. doi: 10.2174/092986607782541088. [DOI] [PubMed] [Google Scholar]
- 39.Xu W, Peng Z, Simons FER. Isolation of a cDNA encoding a 30 kDa IgE-binding protein of mosquito Aedes aegypti saliva. J Allergy Clin Immunol. 1998;101:S203. (abstract). [Google Scholar]
- 40.Chagas AC, Ramirez JL, Jasinskiene N, James AA, Ribeiro JM, Marinotti O, et al. Collagen-binding protein, Aegyptin, regulates probing time and blood feeding success in the dengue vector mosquito, Aedes aegypti. Proc Natl Acad Sci U S A. 2014;111:6946–6951. doi: 10.1073/pnas.1404179111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathur G, Sanchez-Vargas I, Alvarez D, Olson KE, Marinotti O, James AA. Transgene-mediated suppression of dengue viruses in the salivary glands of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2010;19:753–763. doi: 10.1111/j.1365-2583.2010.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future. Lancet Infect Dis. 2014;14:1271–1280. doi: 10.1016/S1473-3099(14)70834-5. [DOI] [PubMed] [Google Scholar]
- 44.Morens DM, Fauci AS. Chikungunya at the door—deja vu all over again? N Engl J Med. 2014;371:885–887. doi: 10.1056/NEJMp1408509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.