Abstract
Objective
To determine the association between antenatal steroids administration and intraventricular hemorrhage rates.
Methods
We used cross-sectional data from the California Perinatal Quality Care Collaborative during 2007-2013 for infants ≤ 32 weeks gestational age. Using multivariable logistic regression, we evaluated the effect of antenatal steroids on intraventricular hemorrhage, stratified by gestational age.
Results
In 25,979 very low birth weight infants, antenatal steroid use was associated with a reduction in incidence of any grade of intraventricular hemorrhage (odds ratio = 0.51, 95% confidence interval: 0.45, 0.58) and a reduction in incidence of severe intraventricular hemorrhage (odds ratio = 0.62, 95% confidence interval: 0.57, 0.67). This association was seen across gestational ages ranging from 22 to 29 weeks.
Conclusions
While current guidelines recommend coverage for preterm birth at 24 to 34 weeks gestation, our results suggest that treatment with antenatal steroids may be beneficial even before 24 weeks of gestational age.
Introduction
Intraventricular hemorrhage (IVH) is a significant cause of preterm morbidity and mortality, with approximately 12,000 infants developing IVH every year in the United States.1 Within the population of very low birth weight (VLBW; < 1500 g) infants, the incidence of IVH has been estimated at 20%. However, the incidence of IVH among VLBW infants has declined in the past few decades, from 40-50% in the early 1980s to approximately 20% in the late 1980s, then remaining relatively stable for the past two decades.2, 3, 4, 5 IVH occurs in nearly half of infants born at extremely low birth weight (500-750 g).2, 5 Infants who survive a severe IVH (Grade III or IV) may be at higher risk of significant long-term or permanent neurological injuries/deficits, including cerebral palsy, mental retardation, and post-hemorrhagic hydrocephalus.6
Previous studies have shown that treatment of pregnant women with steroids prior to delivery is associated with a reduced risk of adverse health outcomes, including IVH. Liggins et al., conducting the first randomized controlled trial of antenatal betamethasone, showed downward mortality and lower risks of RDS in infants of treated mothers compared to controls and the need for respiratory support.7 In that study, four of the infants of control mothers had IVH, compared to none of the infants of steroid-treated mothers. Although this difference was not statistically significant, this finding raised the possibility that antenatal steroid use might reduce the incidence of IVH.
A Cochrane review in 2006 consisting of a meta-analysis of randomized trials demonstrated a reduced risk of IVH (relative risk 0.54, 95% confidence interval 0.43 to 0.69) when treatment with antenatal steroids occurred.8 As most of the trials included occurred in the 1990’s or early 2000’s when IVH rates were higher, we were interested to see if this positive impact of antenatal steroids would still be present. Furthermore, quality improvement efforts to increase antenatal steroid use, as well as other independent efforts to reduce IVH may have weakened this beneficial link.
In the present study, we aimed to determine the magnitude of the effect of antenatal steroid treatment in the prevention of intraventricular hemorrhage in a modern population-based cohort, and to assess whether there is a difference in effect according to gestational age.
Subjects and Methods
Study Population and Data Source
Any hospital in California that provides neonatal intensive care is eligible for membership in the California Perinatal Quality Care Collaborative (CPQCC). Currently, 132 hospitals are members of the CPQCC, including 100% of all California Department of Public Health Children’s Services (CCS)-approved intermediate, community, and regional-level NICUs in California. Member hospitals submit data on newborn infants who require critical care to the CPQCC data center. Infants in the CPQCC database represent over 90% of VLBW infants treated in California NICUs.
During the seven-year study period January 1, 2007 - December 31, 2013, 42,904 VLBW births qualified for entry into the CPQCC database, i.e., a birth weight between 401-1500 grams or a gestational age between 22-32 weeks. Of these, 39,956 infants were born at ≤ 32 weeks gestational age. We limited our study cohort to infants who were VLBW who had a gestational age ≤ 32 weeks, as this population of neonates is most at risk for IVH. We limited our analyses to inborn infants as attributing the influence of more than one NICU’s care on IVH would not be possible, particularly in the time period immediately surrounding delivery.
Infants with missing data on IVH (n = 5,102) or antenatal steroids (n = 228) were excluded. Infants who died prior to having brain imaging performed would be considered as having missing data on IVH. Following the exclusions, 25,979 infants were included in the analysis.
Measures
Intraventricular hemorrhage: CPQCC members are instructed to classify intraventricular hemorrhage by neural imaging (cranial ultrasound, CT scan, or MRI) according to the criteria of Papile et al. with the most severe grade of hemorrhage recorded. (11). The outcome of IVH was recorded as (0) no IVH, (1) grade 1 IVH – subependymal germinal matrix hemorrhage only (2) grade 2 IVH – intraventricular blood with no ventricular dilation (3) grade 3 IVH – intraventricular blood with ventricular dilation, and (4) grade 4 IVH – intraparenchymal hemorrhage. For this analysis, we looked at IVH as a binary outcome in two ways: 1. Grades 3 and 4 were combined and defined as an infant having severe IVH and Grades 1 to 4 were combined into a category of having any IVH.
Variable of Interest: Exposure to antenatal steroids was classified as “yes / no” with any maternal receipt of betamethasone, dexamethasone, and hydrocortisone during pregnancy at any time point prior to delivery counting as “yes”. Other independent variables: birth weight, gestational age, small for gestational age, maternal age, race / ethnicity, prenatal care (any prenatal care visit vs none), multiple gestation, five-minute Apgar score, delivery mode, congenital malformations, and hospital level of neonatal care.
Hospital level of neonatal care was designated by the California Children’s Services (CCS) for NICUs according to their ability to provide varying levels of care. Regional NICUs are able to provide the most complex medical and surgical care. Community NICUs can provide unlimited ventilation, but in general, not major neonatal surgery. Intermediate NICUs provide care for those infants who do not need assisted ventilation. Hospitals with licensed NICU beds that do not choose to participate in the CCS program do not receive a CCS level designation and are classified as non-CCS hospitals.
Hispanic ethnicity with unknown, missing, or other race was considered to be Hispanic race / ethnicity. Infants below the 10th percentile of weight for their gestational age were classified as being small for gestational age while those above the 10th percentile were classified as normal for gestational age. Details for other variables are available in the CPQCC manual of definitions.9
Statistical Analysis
The outcomes of IVH were compared according to demographic and medical factors noted above. Because grades 3 and 4 are more commonly associated with adverse long-term neurological consequences, we examined two main outcomes: 1) severe IVH vs. lower grades of IVH / no IVH, and 2) any grade of IVH vs. no IVH (13). We conducted univariate analyses to compare infants exposed to antenatal steroids to infants not exposed to antenatal steroids and to compare infants who developed any or severe IVH to infants who did not develop any or severe IVH. We conducted a similar comparison for the outcome of in-hospital mortality, as death may be a competing outcome for severe IVH.
To test for differences amongst groups, we used the independent t-test for continuous variables and the Pearson chi-square test for categorical variables. Tests were two-sided. A p-value of < 0.05 was considered statistically significant.
Multivariable logistic regression was used to estimate the odds ratio with 95% confidence intervals as a measure of association between antenatal steroid use and intraventricular hemorrhage in VLBW infants. We included relevant covariates in our multivariable model to control for the potential confounding effects of maternal sociodemographic factors, obstetric factors, and comorbid conditions. These covariates were chosen a priori based on a review of the existing literature and included all of the variables listed above in the Measures section.
Although birth weight and gestational age of the infant are highly correlated with each other (r = 0.77), we employed both variables as covariates in our statistical models. We performed likelihood ratio tests to compare the fit of models that included only birth weight as a covariate to the fit of models that included both birth weight and gestational age as covariates. We found a statistically significant difference between the log likelihoods of the two models, indicating the models with both birth weight and gestational age included as covariates fit the data significantly better. We tested the goodness-of-fit of our final models with the Hosmer-Lemeshow test, which indicated that the final model fit the data optimally.
We did not include gestational age as a covariate in the analyses in which we stratified the cohort by gestational age. We stratified infants by one week gestational age intervals, ranging from 22 weeks to 32 weeks. There were six infants under 22 weeks gestational age that were included with 22 weeks and combined to form one gestational age group.
All data management and analysis was performed using SAS 9.4 for Windows (Cary, NC). Institutional review board approval was obtained from the Stanford School of Medicine and the University of California, Berkeley.
Results
Patient Characteristics
Sociodemographic and obstetric characteristics of infants and mothers are presented in Table 1. Of the infants in the study cohort, most were delivered through Cesarean section (n = 19,464, 74.9%) vs vaginal delivery (n = 6,514, 25.1%). Neonates were primarily cared for at regional (n = 7,145, 27.5%) and community (n = 16,651, 64.1%) NICUs rather than intermediate (n = 856, 3.3%) and non-CCS (n = 1,327, 5.1%) NICUs. The mean birth weight in the cohort was 1050.6 grams, and mean gestational age 27.9 weeks. A large majority of these infants’ mothers had received prenatal care (n = 25,119, 97.0%).
Table 1.
Maternal, infant, and delivery characteristics, California Perinatal Quality Care Collaborative, Stanford, CA, 2007-2013
| Mean | Standard deviation |
|
|---|---|---|
| Birth weight | 1050.6 | 282.6 |
| Gestational age | 27.9 | 2.4 |
| Maternal age | 29.5 | 6.8 |
|
| ||
| N | (%) | |
|
| ||
| Antenatal Steroids | ||
| Yes | 22645 | (87.2) |
| No | 3334 | (12.8) |
| Intraventricular Hemorrhage | ||
| None | 19814 | (76.3) |
| Grade 1 | 2898 | (11.2) |
| Grade 2 | 1317 | (5.1) |
| Grade 3 | 850 | (3.3) |
| Grade 4 | 1100 | (4.2) |
| Maternal race / ethnicity | ||
| Black | 3667 | (14.2) |
| Hispanic | 11278 | (43.8) |
| Non-Hispanic White | 6954 | (27.0) |
| Asian/Pacific Islander | 3175 | (12.3) |
| American Indian or Alaska Native | 160 | (0.6) |
| Other | 512 | (2.0) |
| Prenatal Care | ||
| Yes | 25119 | (97.0) |
| No | 778 | (3.0) |
| Multiple gestation | ||
| Singleton | 18777 | (72.3) |
| Multiple | 7201 | (27.7) |
| Mode of delivery | ||
| Vaginal | 6514 | (25.1) |
| Cesarean | 19464 | (74.9) |
| Neonatal unit level | ||
| Regional | 7145 | (27.5) |
| Community | 16651 | (64.1) |
| Intermediate | 856 | (3.3) |
| Non-CCS | 1327 | (5.1) |
| Congenital malformation | ||
| Yes | 2397 | (9.2) |
| No | 23571 | (90.8) |
| Small for gestational age | ||
| Yes | 3224 | (12.4) |
| No | 22752 | (87.6) |
| Apgar score – 5 minute | ||
| 0-3 | 1187 | (4.6) |
| 4-6 | 4330 | (16.7) |
| 7-10 | 20417 | (78.7) |
| Sex | ||
| Male | 13343 | (51.4) |
| Female | 12632 | (48.6) |
(N) number of records were missing for the following variables: gestational age (3), maternal age (14), maternal race / ethnicity (233), prenatal care (82), multiple gestation (1), mode of delivery (1), congenital malformation (11), small for gestational age (11), Apgar score (45), sex (4)
Trends of Antenatal Steroid Use throughout the Study Period
During the study interval, 87.2% (n = 22,645) of the women in the analytic cohort delivering a VLBW infant received antenatal steroids prior to delivery. During the seven year study period, the use of antenatal steroids gradually increased each year. In 2007, the first year of the study period, 84.6% of infants’ mothers received treatment with antenatal steroids. In 2013, 89.9% of mothers received antenatal steroids.
Trends in the Incidence of Intraventricular Hemorrhage During the Study Period
Of the VLBW infants, 23.7% (n = 6,165) developed any IVH. Among infants with any IVH, 31.6% had severe IVH. Overall, the incidence of any grade of IVH decreased from 25.2% to 22.4% over the seven year study period. The incidence of severe IVH decreased from 8.3% in 2007 to 7.0% in 2013.
Association Between Antenatal Steroid Use and Intraventricular Hemorrhage
Not receiving antenatal steroids was associated with both any IVH and severe IVH (Table 2). Infants with any IVH were more likely to have lower birthweight and younger gestational age. No prenatal care, singleton gestation (compared to multiple), vaginal delivery, low Apgar score, and male sex were associated with any IVH. Similarly, lower birthweight and younger gestational age were associated with higher likelihood of severe IVH (Table 2). No prenatal care, low Apgar score, and male sex were also associated with severe IVH.
Table 2.
Characteristics of infants with any intraventricular hemorrhage or severe intraventricular hemorrhage
| No intraventricular hemorrhage n = 19,814 (76.3%) |
Intraventricular Hemorrhage (Any Grade) n = 6,165 (23.7%) |
Grade 3, 4 Intraventricular Hemorrhage n = 1,950 (7.5%) |
|
|---|---|---|---|
| Antenatal steroids, n (%) | |||
| Yes | 17,548 (77.5%) | 5,097 (22.5%) | 1,473 (6.5%) |
| No | 2,266 (68.0%) | 1,068 (32.0%) | 477 (14.3%) |
|
| |||
| Birthweight (mean ± SD) | 1,087.8 ± 270.8 | 930.9 ± 286.3 | 821.0 ± 248.9 |
| Gestational age (mean ± SD) | 28.3 ± 2.3 | 26.6 ± 2.5 | 25.4 ± 2.0 |
| Maternal age (mean ± SD) | 29.7 ± 6.8 | 29.0 ± 6.9 | 28.6 ± 6.9 |
| Maternal Race, n (%) | |||
| Black | 2,708 (73.8%) | 959 (26.2%) | 327 (8.9%) |
| Hispanic | 8,514 (75.6%) | 2,754 (24.4%) | 944 (8.4%) |
| Non-Hispanic White | 5,420 (77.9%) | 1,534 (22.1%) | 454 (6.5%) |
| Asian/Pacific Islander | 2,459 (77.4%) | 716 (22.6%) | 161 (5.1%) |
| American Indian or Alaska Native | 133 (83.1%) | 27 (16.9%) | 7 (4.4%) |
| Other | 402 (78.5%) | 110 (21.5%) | 34 (6.6%) |
| Prenatal Care, n (%) | |||
| Yes | 19,206 (76.5%) | 5,913 (23.5%) | 1,842 (7.3%) |
| No | 546 (70.2%) | 232 (29.8%) | 101 (13.0%) |
| Multiple births or gestation, n (%) | |||
| Singleton | 14,193 (75.6%) | 4,584 (24.4%) | 1,417 (7.5%) |
| Multiple | 5,620 (78.0%) | 1,581 (22.0%) | 533 (7.4%) |
| Mode of delivery, n (%) | |||
| Vaginal | 4,442 (68.2%) | 2,072 (31.8%) | 657 (10.1%) |
| Cesarean | 15,372 (79.0%) | 4,092 (21.0%) | 1,293 (6.6%) |
| Neonatal unit level, n (%) | |||
| Regional | 5,220 (73.1%) | 1,925 (26.9%) | 570 (8.0%) |
| Community | 12,760 (76.6%) | 3,891 (23.4%) | 1,280 (7.7%) |
| Intermediate | 724 (84.6%) | 132 (15.4%) | 33 (3.9%) |
| Non-CCS | 1,110 (83.6%) | 217 (16.4%) | 67 (5.0%) |
| Congenital malformation, n (%) | |||
| Yes | 1,762 (73.5%) | 635 (26.5%) | 207 (8.6%) |
| No | 18,043 (76.5%) | 5,528 (23.5%) | 1,741 (7.4%) |
| Small for gestational age, n (%) | |||
| Yes | 2,704 (83.9%) | 520 (16.1%) | 98 (3.0%) |
| No | 17,107 (75.2%) | 5,645 (24.8%) | 1,852 (8.1%) |
| Apgar score – 5 minute, n (%) | |||
| 0-3 | 634 (53.4%) | 553 (46.6%) | 280 (23.6%) |
| 4-6 | 2,719 (62.8%) | 1,611 (37.2%) | 671 (15.5%) |
| 7-10 | 16,434 (80.5%) | 3,983 (19.5%) | 991 (4.9%) |
| Sex, n (%) | |||
| Male | 9,919 (74.3%) | 3,424 (25.7%) | 1,127 (8.4%) |
| Female | 9,891 (78.3%) | 2,741 (21.7%) | 823 (6.5%) |
Numbers in bold indicate increased risk, p < 0.05 – any intraventricular hemorrhage compared to no intraventricular hemorrhage, and grade 3, 4 intraventricular hemorrhage compared to no or grade 1, 2 intraventricular hemorrhage.
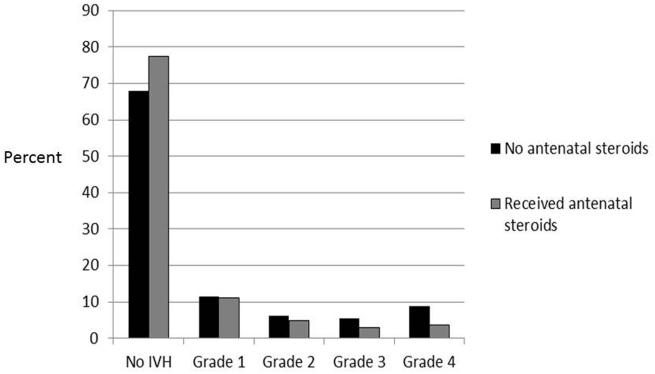
Figure 1 shows the distribution of IVH grade by receipt of antenatal steroids. The most prominent difference was the higher likelihood of grade 4 IVH in infants whose mothers did not receive antenatal steroids (8.9%) compared to those who did receive steroids (3.6%).
Figure 1.
Distribution of intraventricular hemorrhage grade by receipt of antenatal steroids
After adjusting for maternal sociodemographic and medical risk factors in multivariable logistic regression, receiving antenatal steroids was significantly associated with no IVH (adjusted odds ratio 0.68, 95% confidence interval 0.62, 0.75), and with not having severe IVH (adjusted odds ratio 0.51, 95% confidence interval 0.45, 0.58)
Stratified Analysis by Gestational Age
Adjusted odds ratios and 95% confidence intervals for the association between receipt of antenatal steroids and any IVH within gestational age subsets are shown in Table 3. Infants in gestational age groups encompassing 20 0/7 weeks to 29 6/7 weeks whose mothers received antenatal steroids had a significantly lower odds of developing any IVH, and groups encompassing 23 0/7 weeks to 29 6/7 weeks had lower odds of developing severe IVH. For infants above 30 weeks gestational age, there was not a significant reduction in IVH seen. As mortality may be a competing outcome for severe IVH, we examined mortality rates according to receipt of antenatal steroids and found that across all gestational age groups, those infants whose mothers received antenatal steroids had lower risk of in-hospital death.
Table 3.
Adjusted risk of any intraventricular hemorrhage stratified by gestational age according to antenatal steroid administration
| Gestational age (Weeks) |
Total n |
Any IVH n |
Adjusted OR for antenatal steroids (95% CI) Any IVH |
Severe IVH n |
Adjusted OR for antenatal steroids (95% CI) Severe IVH |
|---|---|---|---|---|---|
| <= 22 6/7 | 123 | 81 | 0.23 (0.09, 0.63)* | 50 | 0.54 (0.23, 1.30) |
| 23 0/7 – 23 6/7 | 809 | 483 | 0.66 (0.47, 0.92)* | 260 | 0.55 (0.39, 0.77)* |
| 24 0/7 – 24 6/7 | 1884 | 923 | 0.58 (0.43, 0.77)* | 461 | 0.47 (0.35, 0.63)* |
| 25 0/7 – 25 6/7 | 2256 | 884 | 0.58 (0.44, 0.76)* | 344 | 0.50 (0.36, 0.69)* |
| 26 0/7 – 26 6/7 | 2663 | 818 | 0.70 (0.54, 0.91)* | 281 | 0.52 (0.37, 0.74)* |
| 27 0/7 – 27 6/7 | 3073 | 787 | 0.73 (0.57, 0.94)* | 236 | 0.54 (0.38, 0.78)* |
| 28 0/7 – 28 6/7 | 3603 | 687 | 0.65 (0.51, 0.84)* | 156 | 0.47 (0.31, 0.72)* |
| 29 0/7 – 29 6/7 | 3903 | 639 | 0.67 (0.52, 0.86)* | 91 | 0.31 (0.19, 0.51)* |
| 30 0/7 – 30 6/7 | 3543 | 425 | 0.74 (0.55, 1.00) | 35 | 0.47 (0.22, 1.05) |
| 31 0/7 – 31 6/7 | 2494 | 273 | 1.14 (0.75, 1.72) | 25 | 0.86 (0.28, 2.66) |
| 32 0/7 – 32 6/7 | 1625 | 165 | 0.75 (0.48, 1.15) | 11 | 0.49 (0.11, 2.10) |
p < 0.05
Any intraventricular hemorrhage (IVH) indicates grades 1-4; severe IVH indicates grades 3 and 4. Odds ratios (ORs) are adjusted for maternal socio-demographic and medical risk factors as noted in Methods, with corresponding 95% confidence intervals (Cis).
Discussion
This large population-based study affirms current scientific knowledge showing a significant relationship between receipt of antenatal steroids and decreased risk of IVH. Both in crude assessment and risk adjusted analyses, infants exposed to antenatal steroids were less likely to develop any IVH or severe IVH. Adding to prior knowledge however, we found that the association between ANS use and IVH is limited to gestational age groups from 22 to 30 weeks.
Prior studies have shown that exposure to antenatal steroids was associated with a decrease in the risk of IVH in neonates born between 29-34 weeks gestational age when examined as an overall combined cohort.8 While our stratified analysis showed no significant differences in infants > 30 weeks gestational age, this is likely in part due to the overall lower risk of IVH in this group, which has continued to decrease over time.
Overall though, our findings are consistent with previous studies on historic cohorts. Shankaran et al. reported an unadjusted odds ratio of 0.39 (95% CI: 0.27, 0.57) for the association of a complete course of steroids with the development of grades 3 and 4 IVH in a large cohort of singleton VLBW infants (i.e. birthweight 501-1500 g).10 Wright et al. examined the association between partial, complete, and any course of antenatal steroids and the outcomes of any or severe IVH in 9,949 VLBW infants in 14 NICUs with diverse populations and management strategies. Receipt of complete or any antenatal steroid treatment was found to be associated with a statistically significant reduction in the risk of IVH (any IVH or severe IVH).10 A meta-analysis of placebo-controlled randomized trials by Crowley et al. found that steroid therapy reduces the odds of periventricular hemorrhage, with an odds ratio of 0.38 (95% CI: 0.23, 0.94).11 The evidence from these studies led the NIH Consensus Development Panel to publish a statement that antenatal steroids reduce mortality and the incidence of IVH in infants born at 29-34 weeks of gestation and recommended that mothers at risk for preterm delivery between 24-34 weeks gestation be candidates for treatment.12
Previous data concerning the association between use of antenatal steroids and the incidence of IVH in infants delivered at <29 weeks gestational age had been conflicting. A systematic review of 21 studies by Roberts et al. reported that antenatal steroids reduce the incidence of IVH in infants born before 28 weeks.8 In contrast, a systematic review of nine randomized trials by Onland et al. concluded that there was no evidence to support or refute recommending treatment with antenatal steroids to women at risk of preterm birth at <26 weeks of gestation.13 Since these reviews were published, Mori et al. found that exposure to antenatal steroids was associated with a significant decrease in the risk of IVH and severe IVH incidence in infants born between 24-29 weeks of gestational age.14 Similarly, a retrospective cohort study in Australia by Wong et al. found a significantly lower incidence of severe IVH in infants of 24-28 weeks of gestational age who received a complete course of antenatal steroids.15 Our findings are consistent with these more recent cohort studies.
Studies on this question in infants born at ≤24 weeks of gestational age are scarce. In a study of 117 neonates, Abbasi et al. reported that mothers of infants < 24 weeks of gestational age given antenatal steroids had a significantly lower incidence of grade 3 and 4 IVH (16.7% versus 36%, p < 0.05, relative risk = 0.46).16 However, Hayes et al. found that in neonates born at 23 weeks gestation, the occurrence of severe IVH in infants exposed to antenatal steroids was not significantly different compared to the occurrence of IVH in infants not exposed to antenatal steroids (23.1% compared with 57.1%, p = 0.17).17 The odds ratio for the association between antenatal steroids and the outcomes of IVH and severe IVH found by Mori et al. in infants 22-23 weeks gestational age was also found to be not significant.14 Due to our large sample size, we were able to detect significant differences even at these youngest gestational age groups.
Our study is limited in its observational nature, and therefore causal inference cannot be assumed. Although we attempted to account for confounding factors, there may be potential confounding from unobserved variables. A limitation of our study was lack of detailed information in our dataset on antenatal steroids, including full, partial, or repeated courses, and dosage or timing. Another limitation of our study was a lack of imaging data on 5,102 VLBW infants that were excluded from our analyses due to missing information on whether or not the infant developed IVH. This could result in selection bias that could influence the estimate in either direction. Some infants may have not received a head ultrasound because they were very healthy and were not assessed to have risk. Others may not have had a head ultrasound due to very severe clinical status and subsequent death prior to ability to receive an ultrasound. However, in either case, we would not expect antenatal steroids to cause bias in either direction. Characteristics of infants with missing data on antenatal steroids or IVH status are presented in Table S2. These patients tended to have lower birth weight and gestational age, and more likely to have been delivered vaginally.
Conclusion
We confirm the association between antenatal steroids and decreased risk of IVH. Our study goes further to demonstrate an association between antenatal steroids and decreased IVH in infants of gestational age ranging from 22-29 weeks. Currently the NIH recommends mothers at risk of preterm birth at 24-34 weeks gestational age be treated with antenatal steroids. Our analysis suggest that antenatal steroids could be beneficial in reduction of IVH incidence in infants < 24 weeks gestational age, and mothers at risk for preterm birth at 22 or 23 weeks gestational age may also be good candidates for treatment with antenatal steroids.
Supplementary Material
Acknowledgements
The project was supported by grant number K23HD068400, Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver NICHD, or NIH.
Financial Disclosure: This project was funded by grant number K23HD068400, Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD). Dr. Profit’s effort was supported in part by a grant from NICHD 1 R01 HD083368-01A1 (PI Profit).
Footnotes
Conflict of Interest: None
References
- 1.Guyer B, Hoyert DL, Martin JA, Ventura SJ, MacDorman MF, Strobino DM. Annual summary of vital statistics--1998. Pediatrics. 1999;104(6):1229–1246. doi: 10.1542/peds.104.6.1229. [DOI] [PubMed] [Google Scholar]
- 2.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatric research. 2010;67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics. 1989;84(5):797–801. [PubMed] [Google Scholar]
- 4.Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. The journal of maternal-fetal & neonatal medicine. 22(6):491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- 5.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clinics in perinatology. 2008;35(4):777–792. doi: 10.1016/j.clp.2008.07.014. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto-Martin JA, Whitaker AH, Feldman JF, Van Rossem R, Paneth N. Relation of cranial ultrasound abnormalities in low-birthweight infants to motor or cognitive performance at ages 2, 6, and 9 years. Developmental medicine and child neurology. 1999;41(12):826–833. doi: 10.1017/s0012162299001644. [DOI] [PubMed] [Google Scholar]
- 7.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525. [PubMed] [Google Scholar]
- 8.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 9.CPQCC Network Database Manual of Definitions for Infants Born in 2013. Available at: http://cpqcc.org/data/cpqcc_downloads Accessed February 22, 2014. [cited July 2, 2013]Available from: http://cpqcc.org/data/cpqcc_downloads.
- 10.Shankaran S, Bauer CR, Bain R, Wright LL, Zachary J. Relationship between antenatal steroid administration and grades III and IV intracranial hemorrhage in low birth weight infants. The NICHD Neonatal Research Network. American journal of obstetrics and gynecology. 1995;173(1):305–312. doi: 10.1016/0002-9378(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 11.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. American journal of obstetrics and gynecology. 1995;173(1):322–335. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 12.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273(5):413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 13.Onland W, de Laat MW, Mol BW, Offringa M. Effects of antenatal corticosteroids given prior to 26 weeks' gestation: a systematic review of randomized controlled trials. American journal of perinatology. 2011;28(1):33–44. doi: 10.1055/s-0030-1262509. [DOI] [PubMed] [Google Scholar]
- 14.Mori R, Kusuda S, Fujimura M. Antenatal corticosteroids promote survival of extremely preterm infants born at 22 to 23 weeks of gestation. J Pediatr. 2011;159(1):110–114. doi: 10.1016/j.jpeds.2010.12.039. e111. [DOI] [PubMed] [Google Scholar]
- 15.Wong D, Abdel-Latif M, Kent A. Antenatal steroid exposure and outcomes of very premature infants: a regional cohort study. Archives of disease in childhood Fetal and neonatal edition. 2014;99(1):F12–20. doi: 10.1136/archdischild-2013-304705. [DOI] [PubMed] [Google Scholar]
- 16.Abbasi S, Oxford C, Gerdes J, Sehdev H, Ludmir J. Antenatal corticosteroids prior to 24 weeks' gestation and neonatal outcome of extremely low birth weight infants. American journal of perinatology. 2010;27(1):61–66. doi: 10.1055/s-0029-1223269. [DOI] [PubMed] [Google Scholar]
- 17.Hayes EJ, Paul DA, Stahl GE, Seibel-Seamon J, Dysart K, Leiby BE, et al. Effect of antenatal corticosteroids on survival for neonates born at 23 weeks of gestation. Obstetrics and gynecology. 2008;111(4):921–926. doi: 10.1097/AOG.0b013e318169ce2d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.