Abstract
Coumarin is a phenolic compound that mainly affects the liver due to its metabolization into a toxic compound. The deterrent and ovicidal activities of coumarin in insect models such as Drosophila melanogaster have been reported. Here we explore the molecular mechanisms by which these insects protect themselves and their eggs from this toxic plant metabolite. Coumarin was fatal to the flies in a dosage-dependent manner. However, coumarin feeding could be inhibited through activation of the aversive gustatory receptor neurons (GRNs), but not the olfactory receptor neurons. Furthermore, three gustatory receptors, GR33a, GR66a, and GR93a, functioned together in coumarin detection by the proboscis. However, GR33a, but not GR66a and GR93a, was required to avoid coumarin during oviposition, with a choice of the same substrates provided as in binary food choice assay. Taken together, these findings suggest that anti-feeding activity and oviposition to avoid coumarin occur via separate mechanisms.
Keywords: bitter, chemosensation, feeding, oviposition, taste
INTRODUCTION
Every living being, from microbes to mammals, requires food for survival. Food consists of a number of complex compounds; some are beneficial, some are allergenic, while others may be harmful. The latter are commonly termed toxic compounds. There are numerous naturally occurring toxic metabolites in food. Phenol is one of these metabolites. A study found that phenol shows either carcinogenic or toxic effects in animal models (Smith et al., 1989). In fact, a phenolic metabolite, coumarin, is banned in food products (Dolan et al., 2010; Singleton, 1981). These toxic metabolites are normally produced by plants in order to protect themselves from insects and pests.
The taste sensory system normally functions in discriminating nutritious foods from non-nutritious ones. One part of the taste system controls acceptance behavior, while the other regulates avoidance behavior. The taste receptor in Drosophila consists of 68 gustatory receptors (GRs), which are encoded by 60 Grs genes by means of alternative splicing (Dunipace et al., 2001; Robertson et al., 2003; Scott et al. 2001). These GRs are distributed in various parts of the fly body, from mouth (proboscis) to leg, from wings to female genitalia (Stocker and Schorderet, 1981). The receptors in the legs and proboscis function in detection of food and ingestion. The legs detect chemicals and cause either proboscis extension or retention, and the proboscis functions to enable the ingestion of the chemicals into the body. Fly proboscis contains 31 hair sensilla in each lobe. These hair sensilla are categorized into short (S), intermediate (I), and long (L) according to their length (Hiroi et al., 2002). The L-type or S-type hair sensillum contains four gustatory receptor neurons (GRNs) that respond to sugar, water, and salt concentration. The L-type sensilla contain two GRNs; one responding to sugar and low salt concentrations, and the other to bitter compounds and high salt concentrations (Hiroi et al., 2004; Meunier et al., 2003). These bipolar GRNs extend their dendrites to the tip of the sensillum, and their axons to the subesophageal ganglion (SOG), thus signaling the taste message from the sensilla to the SOG (Montell, 2009). This taste message is transmitted to the higher order brain, which commands the fly to either ingest or to avoid the source of the taste (Lee and Poudel, 2014).
Previous studies of insect taste receptors have been performed on the sugar receptor. A common clade in the phylogenetic tree of 68 Grs, which includes the cluster of Gr64 and Gr5a, encodes sugar receptors. Among these genes, Gr5a encodes the receptor for trehalose, melezitose, and glucose, and Gr64a encodes the receptor for sucrose, maltose, and glucose. Gr64f encodes a receptor for all sugars except fructose (Chyb et al., 2003; Dahanukar et al., 2001; 2007; Jiao et al., 2007; 2008; Ueno et al., 2001). Additionally, Gr43a functions as a fructose receptor in the fly brain, which acts as a nutrient sensor by stimulating the hungry fly to eat, and the satiated fly to avoid (Miyamoto et al., 2012). The narrowly tuned L-type and broadly tuned S-type sensilla houses GRNs that mainly function in the detection of bitter compounds, although two types of sensilla also have a sugar-sensing GRN (Weiss et al., 2011). To date, mutant studies have reported that six GRs function in aversive behavior against various bitter compounds. Among them GR32a, GR33a and GR66a are broadly tuned GRs (Lee et al., 2010; Moon et al., 2009). While GR8a, GR47a, and GR93a are narrowly tuned GRs required for sensing L-canavaline, strychnine, and caffeine respectively (Lee et al., 2012; 2015).
The function of the GR receptor in detecting bitter compounds serves as an advantage to the fly, in terms of specifying the site for oviposition behavior (Joseph and Herberlein, 2012; Yang et al., 2008). In addition, the olfactory receptor (OR) also plays a role in substrate selection. The main aim of female flies when choosing substrate choice for oviposition, is to safeguard their progeny from parasites and the deleterious effects of toxic bitter compounds, as well as to provide a source of nourishment of their progeny. Similarly, the females choose to lay eggs in fermenting substrates containing ethanol and citrus fruits so as to protect their eggs and larvae from endoparasitoid wasps. The latter behavior is controlled by olfactory receptor neurons (ORNs) expressing Or19a+ (Dweck et al., 2013; Kacsoh et al., 2013).
Here, we show that coumarin has a toxic effect when fed in a dosage-dependent manner. Anti-feeding behavior to avoid coumarin-laced food is regulated by aversive GRNs, but not ORNs. Furthermore, we identified the possible coumarin receptor using six previously verified mutants, by binary food choice assay, assessment of proboscis extension response assay and electrophysiology measurement. Finally, we found that only Gr33a is required for oviposition to avoid coumarin-laced food.
MATERIALS AND METHODS
Fly stocks
Gr33a1, Gr33aGAL4, UAS-Gr33a, Gr66aex83, UAS-Gr66a, and Gr93a3 flies were previously deposited in the Bloomington Stock Center (Lee et al., 2009; Moon et al., 2006; 2009). H. Amrein provided the ΔGr32a (Miyamoto and Amerin, 2008) and the P[Gr66a-GAL4] flies (Thorne et al., 2004). We got the Orco2 flies from the Bloomington Stock Center. We described UAS-Gr93a in previous study (Poudel et al., 2015). We used w1118 as the “wild-type” control.
Chemical sources
Sucrose, coumarin, and sulforhodamine B were purchased from Sigma-Aldrich Co. Brilliant blue FCF was ordered from Wako Pure Chemical Industry Ltd.
Binary food choice assay
We performed binary food choice assays as described previously (Meunier et al., 2003; Moon et al., 2006). Firstly, 50–70 flies that were 3–6 days old were starved for 18 h in a humidified chamber. We prepared two different food substrates with 1% agarose: one containing 1 mM sucrose, and the other containing 5 mM sucrose with different concentration of coumarin. These food substrates were mixed with either one of two food coloring dyes, i.e. one was mixed with blue dye (brilliant blue FCF, 0.125 mg/ml) while the other was mixed with red dye (sulforhodamine B, 0.2 mg/ml). We distributed the mixture of two food sources in a 72-well microtiter dish, in alternative fashion, and then we introduced the starved flies into the dish. The flies in the microtiter dish were kept in a dark, humidified chamber, and allowed to feed for 90 min at room temperature. To sacrifice the flies, we kept them at −20°C and then analyzed the color of their abdomens by microscopy. Blue (NB), red (NR), or purple (NP) flies were counted. The preference index (P.I.) was calculated according to the following equation: (NB+0.5NP)/(NR+NB+NP) or (NR+0.5NP)/(NR+NB+NP), depending on the dye/tastant combinations. P.I.s = 1.0 and 0 indicated complete preferences for either 1 mM or 5 mM sucrose, with or without coumarin, respectively. A P.I. = 0.5 indicated no bias between the two food choices.
Proboscis extension response (PER) assay
PER assay was performed as previously described (Lee et al., 2015), with slight modification. The concentration of sucrose used for the initial stimuli was 2%, and then 10 mM coumarin was applied with 2% sucrose. The flies that did not respond to sucrose as a positive stimulant were discarded. Kim-wipe paper wicks were used as media to provide flies with tastant stimuli. Wet wicks were gently brought in contact with the proboscis. Prolonged contact with the stimulating agent may give a negative result. Water, which acts as a negative stimulant, was given to the flies as described above. Flies showing proboscis extension in response to this negative stimulant were discarded. Next, the test solution, i.e. 10 mM coumarin in 2% sucrose stimuli, was given, and positive PER was calculated. The test was performed for 10 flies at a time, and the positive PER for each fly was calculated as 10% proboscis extension. The test was repeated four times for each fly strain, i.e. mutant, control, and rescue fly strains.
Tip recordings
We performed tip recordings as previously described (Moon et al., 2006). We immobilized freshly enclosed flies by keeping them on ice and then inserted reference glass electrodes filled with Ringer’s solution into the thorax of the flies, extending the electrode towards their proboscis. We stimulated the sensilla with tastants dissolved in buffer solution in recording pipettes (10–20 μm tip diameter). We used 1 mM KCl or 30 mM tricholine citrate as the electrolyte for recording. The recording electrode was connected to a preamplifier (TastePROBE, Syntech, Hilversum, The Netherlands), and the signals were collected and amplified 10x, using a signal connection interface box (Syntech) in conjunction with a 100–3000 Hz band-pass filter. Recordings of action potentials were acquired using a 12-kHz sampling rate, and analyzed using Autospike 3.1 software (Syntech). First, we performed recordings on S6 sensilla with different concentrations of coumarin i.e. 0 mM, 0.1 mM and 1 mM for control flies. Finally, we performed recordings with the mutant and rescue flies from S5, S6 and S9 sensilla with 1 mM coumarin.
Oviposition assay
We developed our own protocol for egg laying (Poudel et al., 2015). Fifteen male and 15 female flies, all 2–3 days-old, were kept in a fresh food source and incubated at 25°C for 2 days. The experiment was divided into two parts: 6 h adaptation (starvation), and 18 h egg laying period, which completes 24 h circadian rhythm. For starvation, we kept the flies on 1% agarose on the egg laying apparatus, which was a 5 cm diameter petri dish divided by 4 mm spacer. We started the experiment exactly at 12 PM for starvation, and then at 6 PM we transferred the flies to the petri dish containing 1% agarose, with either 1 mM sucrose, or 5 mM sucrose plus indicated concentrations for coumarin. The flies were then allowed to lay eggs for 18 h. The flies were kept in a dark and humidified chamber for both starvation and oviposition. The numbers of eggs laid over 18 h were counted. The oviposition index was calculated, as previously described (Yang et al., 2008).
Survival assay
We performed survival assays with the control flies. We prepared three different combinations of food sources: one with 1% sucrose, and the other two with 1% sucrose plus 1 mM and 10 mM coumarin. We placed 10 male and 10 female flies, 3–4 days-old, on each of these food sources. The flies were observed every 12 h, and then transferred to new vials containing the same food source. The assay was performed for 72 h, by which time all the flies feeding on coumarin containing media were dead. The test was repeated 10 times.
Statistical analyses
All error bars represent SEMs. Single factor ANOVA with Scheffe’s analysis was used as a post hoc test to compare multiple sets of data. Asterisks indicate statistical significance (*P < 0.05, **P < 0.01).
RESULTS AND DISCUSSION
Coumarin, a fragrant organic chemical compound, is considered a phenylpropanoid (Fig. 1A). It is produced by plants as a defense mechanism to repel predators. Coumarin is moderately toxic to the liver and kidneys in mammals (Lake, 1999); therefore, we decided to test its toxicity on insect models. The toxicity of coumarin was investigated by performing survival assay with control flies. Flies maintained in 1% sucrose lived for more than 72 h (Fig. 1B). However, 1 mM coumarin was sufficient to kill 50% of the flies in 45 h (LT50). This was reduced to 30 h (LT50) for 10 mM coumarin (Fig. 1B). All coumarin-feeding flies were dead before 72 h. This indicates that coumarin is also toxic to insects.
Fig. 1.
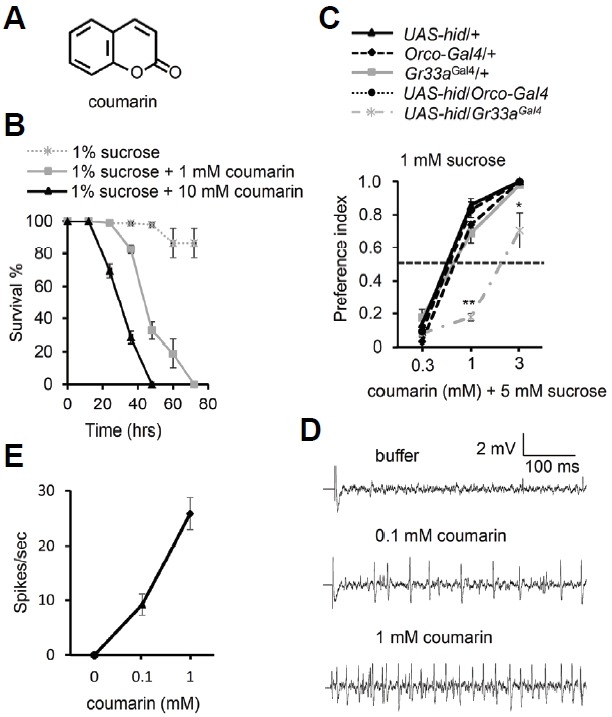
Coumarin is a toxic compound, and bitter-sensing GRNs are required for behavioral avoidance to coumarin. (A) Chemical structure of coumarin. (B) The survival rate of wild-type flies consuming 1% sucrose combined with the indicated concentrations of coumarin. N = 10. (C) Binary food choice assays performed after either ORNs or GRNs were ablated by expressing a pro-apoptotic gene (hid), under the control of Gr33aGAL4 or Orco-GAL4. N = 5. All heterozygote controls (Gr33aGAL4/+, Orco-GAL4/+, and UAS-hid/+) are also shown. (D) Sample traces after inducing action potentials by the indicated concentrations of coumarin from S6 sensillum. (E) Dose responsive curve of action potentials for the indicated concentrations of coumarin for controls. n = 7−10. The error bars represent SEMs. The asterisks indicate significant differences from control (**P < 0.01, *P < 0.05), using a single factor ANOVA with Scheffe’s analysis as a post hoc test to compare two sets of data.
Coumarin is known to have a pleasant smell to humans. However, it also has a somewhat bitter-tasting anti-feedant effect. To determine whether the repellent behavior is actually mediated by its bitterness or by its odor, we expressed a proapoptosis gene (hid) under the control of Gr33a-GAL4 (aversive GRN reporter) or Orco-GAL4 (ORN reporter), using GAL4/UAS system (Fig. 1C) (Larsson et al., 2004; Moon et al., 2009) . We tested these flies along with their controls (+/Orco-Gal4, +/Gr33aGal4, and +/UAS-hid) for feeding behavior, using 0.3 mM, 1 mM and 3 mM coumarin (Fig. 1C). We found that 0.3 mM coumarin was not sufficient to repel the flies. However, 1 mM coumarin was enough to repel not only controls (+/Orco-Gal4, +/Gr33aGal4, and +/UAS-hid), but also the ORN-ablated flies (Orco-Gal4/UAS-hid; P.I. = 0.82 ± 0.04), although the aversive GRN-ablated flies (Gr33aGal4/UAS-hid; P.I. = 0.18 ± 0.02) were found to show a greatly reduced repellent behavior (Fig. 1C). Furthermore, while all the controls, as well as the ORN-ablated flies, completely avoided 3 mM coumarin, the aversive GRN-ablated flies still showed relatively low avoidance (Fig. 1C). The increase in P.I. is explained by the sugar-inhibition effect of bitter chemicals (Meunier et al., 2003). To further investigate the activation of the GRNs in the fly’s proboscis by coumarin, we performed tip recordings on S6 sensilla (Figs. 1D and 1E). We found that coumarin induced action potentials from bitter-sensing S6 sensilla, in a dose-dependent manner. Our results suggest that GRNs, but not ORNs, are physiologically required for avoidance behavior towards coumarin in Drosophila.
To find the Grs required for sensing coumarin, we compared the six previously described Gr-mutants with the controls. First, we tested the broadly tuned and required Gr-mutants, ΔGr32a, Gr33a1 and Gr66aex83, using binary food choice assay, including Orco2 mutant (Lee et al., 2010; Moon et al., 2009) (Fig. 2A). We found that Gr33a1 and Gr66aex83 show defects in coumarin-avoidance behavior (Fig. 2A). However, ΔGr32a and Orco2 showed normal avoidance to coumarin (Fig. 2A). This provides further conviction that ORNs are not required for avoidance behavior to coumarin. Next, we tested the narrowly tuned Grmutants, Gr8a1, Gr47a1 and Gr93a3 (Fig. 2B), which are deficient in sensing L-canavaline, strychnine, and caffeine, umbelliferone, respectively (Lee et al., 2009; 2012; 2015; Poudel et al., 2015). Only the Gr93a3 mutant showed a decrease in avoidance behavior to 1 mM coumarin. In order to confirm our results, we decided to restore the behavior of the Gr33a1, Gr66aex83 and Gr93a3 mutants using the GAL4/UAS system. Three Grs are already reported to be expressed in the same all bitter-sensing GRNs (Lee et al., 2009; Moon et al., 2009). Gr33aGal4 obtained by homologous recombination is expressed in nearly 20 cells of bitter-sensing GRNs (Moon et al., 2009). Similarly, Gr66a-Gal4 is expressed in one of the GRNs in all L-type and S-type sensilla (Wang et al., 2004). Both Gr66a and Gr93a are expressed in the same GRNs (Lee et al., 2009). The colabelling studies using reporters and antibodies provide that all three Grs are expressed in all l-type and S-type sensilla in labellum. Therefore, we recovered the behavior of both Gr66aex83 and Gr93a3 by using Gr66a-Gal4, and that of Gr33a1 by using Gr33aGAL4, which introduces wild-type Gr66a+, Gr93a+, and Gr33a+ transgenes, respectively, into GRNs mediating aversion in each mutant background (Figs. 2C–2E). This indicates that at least three Grs are essential for the avoidance of the toxic phenol compound, coumarin.
Fig. 2.
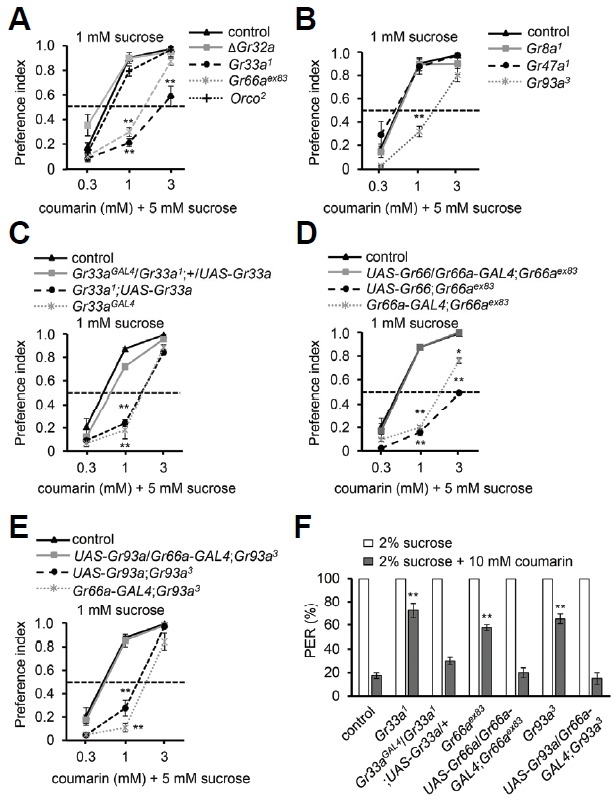
Binary food choice assay and PER for coumarin. (A–E) Concentration-dependent avoidance of the indicated concentrations of coumarin. The flies were given a choice to feed between 1 mM sucrose alone and 5 mM sucrose combined with 0.3 mM, 1 mM, or 3 mM concentrations of coumarin. n = 5. (A) Screening with broadly required Grs: ΔGr32a, Gr33a1, Gr66aex83, Orco2 and control. (B) Screening with narrowly required Grs: Gr8a1, Gr47a1, Gr93a3, and control. (C) Rescue of the coumarin sensation defect in Gr33a1 after expression of UAS-Gr33a under control of Gr33aGAL4. (D) Rescue of the coumarin sensation defect in Gr66aex83 after expression of UAS-Gr66a under control of Gr66a-GAL4. (E) Rescue of the coumarin sensation defect in Gr93a3 after expression of UAS-Gr93a under control of Gr66a-GAL4. (F) PER assay for the indicated mutants and rescue flies. The flies were initially given 2% sucrose, and then 2% sucrose in combination with 10 mM coumarin. n = 4. The asterisks indicate significant differences from control (**P < 0.01, *P < 0.05), using a single factor ANOVA with Scheffe’s analysis as a post hoc test.
The fly has multiple contact-chemosensory organs including a proboscis, legs, wings and genitalia. The main contact-chemosensory organs are the proboscis and legs. To further verify the roles of GRs in the labellum, we performed the proboscis extension response (PER) assay for coumarin with defective Gr-mutants (Fig. 2F). To specifically address the role of each GR in the labellum, we applied sucrose alone, or sucrose plus coumarin, to the labellum. We first selected the flies showed positive extension responses to sucrose alone. Control flies showed only 17.5% extension to sucrose plus coumarin, while Gr33a1, Gr66aex83 and Gr93a3 showed 72.5%, 57.5%, and 65% PER, respectively (Fig. 2F). This indicates that suppression by 10 mM coumarin was strongly impaired in these mutants. We restored this defect by expressing each wild type transgene in Gr33a1, Gr66aex83 and Gr93a3 mutant background (Fig. 2F). This suggests that the expression of the Grs in the labellum is necessary to induce coumarin-induced avoidance.
Next, we performed tip recordings to elicit coumarin-induced action potentials from S5, S6 and S9 sensilla, which are known to be highly activated by coumarin (Weiss et al., 2011). Consistent with the behavioral defects of Gr33a1, Gr66aex83 and Gr93a3, we found that these mutants did not display normal action potentials in response to coumarin (Figs. 3A–3D). Furthermore, we rescued this physiological defect by expressing the wild-type gene in each mutant background (Figs. 3A–3D).
Fig. 3.
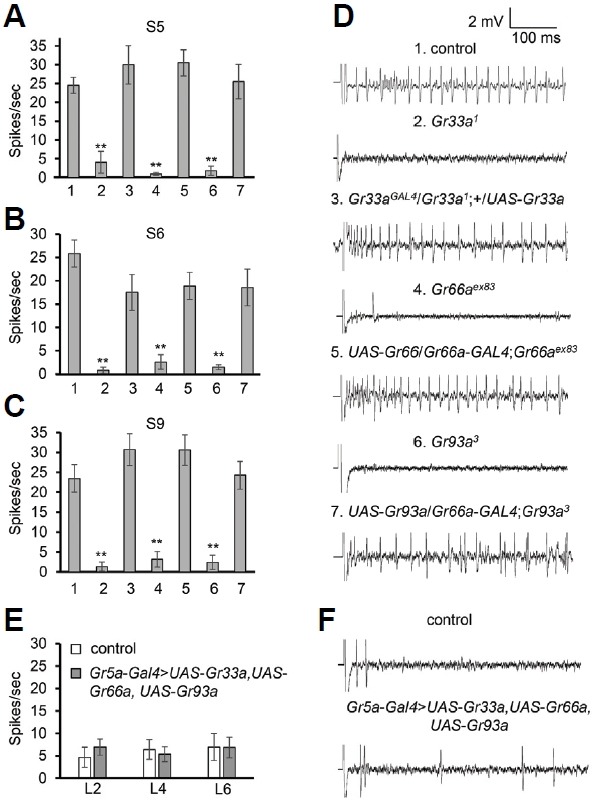
Gr33a, Gr66a, and Gr93a were indispensable for coumarin-induced nerve firing. (A–C) Average frequencies of action potentials (spikes/s) to 1 mM coumarin are shown for the indicated genotypes: control, mutants (Gr33a1, Gr66aex83, Gr93a3) and rescue flies. n ≥ 10. The number 1, 2, 3, 4, 5, 6, and 7 indicate control, Gr33a1, Gr33aGAL4/Gr33a1;+/UAS-Gr33a, Gr66aex83, UAS-Gr66/Gr66a-GAL4; Gr66aex83, Gr93a3 and UAS-Gr93a/Gr66a-GAL4;Gr93a3, respectively. The asterisks indicate significant differences from wild-type (**P < 0.01) using a single factor ANOVA with Scheffe’s analysis as a post hoc test to compare two sets of data. The error bars represent SEMs. Tip recordings were performed on S5 (A), S6 (B), and S9 (C) bristles, based on Carlson’s nomenclature. (D) Representative traces of coumarin-evoked nerve response on S6 bristles from the indicated genotypes. (E–F) Ecotopic expression of Gr33a, Gr66a and Gr93a in sugar sensing neuron. (E) Tip recordings were carried out on L2, L4, and L6 with the indicated flies. n ≥ 15. (F) Sample traces of coumarin-induced action potentials on L2 bristles from the indicated genotypes. The error bars represent SEMs.
These data support the hypothesis that the activity of GRs in the labellum is necessary to discriminate between nutritious food sources and toxic foods.
Recent study provides the evidence that three bitter-sensing GRs are enough to recapitulate L-canavanine receptor (GR33a, GR66a, and GR98b) in sweet neurons (Shim et al., 2015). To recapitulate a coumarin receptor in sweet neurons ectopically, we generated the flies expressing all the three Grs (UAS-Gr33a, UAS-Gr66a and UAS-Gr93a) under the control of Gr5a-GAl4. We did the tip recording from L-type sensilla (L2, L4 and L6) with 1 mM coumarin for both control and UAS-Gr93a/UAS-Gr66a;Gr5a-GAl4/UAS-Gr33a (Figs. 3E and 3F). However, ectopic expression of three Grs did not induce further action potentials compared with control. This suggests that at least one more Gr is required for recapitulating a coumarin receptor.
The site selection for the deposition of the egg in fruit fly is crucial for the safety of the developing larvae. The studies done till date support the idea that flies sense media using smell and taste for the oviposition in Drosophila (Dweck et al., 2013; Joseph and Herberlein, 2012; Yang et al., 2008).
Coumarin is an ovicidal compound (Nakajima and Kawazu, 1980). So our assumption was that female flies should avoid a coumarin-containing substrate for egg laying. We carried out egg laying assay with the same condition as binary food choice assay. 0.3 mM coumarin was not enough for the female flies to avoid egg laying as binary food choice assay (Figs. 1C, 4A, and 4B). Interestingly, only Gr33a1, but not Gr66aex83 and Gr93a3 showed a decrease in avoidance for 1 mM coumarin. For 3 mM coumarin, control, Gr66aex83 and Gr93a3 showed a complete avoidance while Gr33a1 still showed mild avoidance (Fig. 4A). The oviposition defect in Gr33a1 was fully rescued by the expression of wild-type Gr33a+ using knock-in Gr33aGAL4 (Fig. 4B). This indicates that Gr33a+ is required for proper oviposition to avoid ovicidal coumarin. It is possible that GR66a and GR93a are less sensitive to coumarin than GR33a with the oviposition behavior. However, recent study suggests that GR66a in the ventral cibarial sensory organ (VCSO) is required for oviposition on lobeline-laced food (Joseph and Heberlein, 2012). This indicates that other internal and external sensory organs might be required for different kinds of behavior. In addition, other study also suggests that Drosophila prefer laying eggs on a nutritious food depending on the egg-laying apparatus and the substrate type. If the apparatus is large and the food is not diffusive, females prefer the nutritious food to save their progenies. However, if the apparatus is small, and the food is diffusive, females prefer laying eggs on the non-nutritious food (Schwartz et al., 2012). This indicates that egg-laying behavior can be modulated in a context-dependent manner. It is also possible explanation that the different GR combination required for feeding or egg-laying on the same compound activates different sensory organs which have independent roles to make decision.
Fig. 4.
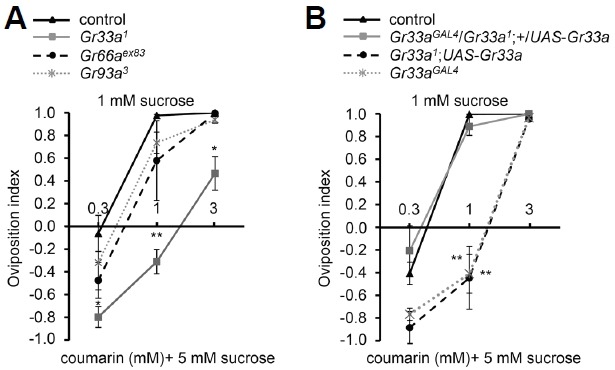
Gr33a is necessary for oviposition to avoid coumarin. (A) Concentration-dependent avoidance for oviposition for control, Gr33a1, Gr66aex83, and Gr93a3 in either 1 mM sucrose alone, or 5 mM sucrose combined with the indicated concentrations of coumarin. n = 4−6 (B) The oviposition defect in Gr33a1 can be recovered by the expression of wild-type Gr33a+ under control of Gr33aGAL4. n = 4−9. The error bar represents SEMs. The asterisks indicate significant differences from wild-type (*P < 0.05, **P < 0.01), using single factor ANOVA with Scheffe’s analysis as a post hoc test to compare two sets of data.
The olfactory receptor is composed of Orco and specific OR, which resides in specific ORNs. However, GRs are distributed on overlapped sensilla. For example, Gr93a is known to be expressed in all bitter-sensing GRNs, but it has been reported to be required for sensing only caffeine and umbelliferone but not other aversive compounds such as quinine, berberine, denatonium, and so on (Lee et al., 2009; Poudel et al., 2015). In addition, the same GRN can respond to several different compounds. For instance, S6 sensillum shows action potentials by most bitter compounds tested (Lee et al., 2010; Weiss et al., 2011). We previously categorized GRs as broadly tuned or narrowly tuned on the study of GR mutants (Lee et al., 2015). Here we identified multiple roles of GR93a in sensing coumarin as well as umbelliferone and caffeine as a middle class between narrowly and broadly tuned GRs. In addition, we found that GRs may play additional roles in finding food or laying eggs. In future, it would be interesting to find the different circuits that control feeding or egg-laying, as it is important to control pests at the adult as well as larval stages.
Acknowledgments
This work was supported by a grant to Y.L. from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2012M3A9B2052525), and the Basic Science Research Program of the NRF of Korea funded by the Ministry of Education (2012R1A1A2003727).
REFERENCES
- Chyb S., Dahanukar A., Wickens A., Carlson J.R. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl. Acad. Sci. USA. 2003;100:14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A., Foster K., Carlson J.R. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- Dahanukar A., Lei Y.T., Kwon J.Y., Carlson J.R. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L. C., Matulka R.A., Burdock G.A. Naturally occurring food toxins. Toxins. 2010;2:2289–2332. doi: 10.3390/toxins2092289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunipace L., Meister S., McNealy C., Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Dweck H.K., Ebrahim S.A., Kromann S., Bown D., Hillbur Y., Sachse S., Hansson B.S., Stensmyr M.C. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr. Biol. 2013;23:2472–2480. doi: 10.1016/j.cub.2013.10.047. [DOI] [PubMed] [Google Scholar]
- Hiroi M., Marion-Poll F., Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zool. Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Hiroi M., Meunier N., Marion-Poll F., Tanimura T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J. Neurobiol. 2004;61:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- Jiao Y., Moon S.J., Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc. Natl. Acad. Sci. USA. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Moon S.J., Wang X., Ren Q., Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. M., Heberlein U. Tissue-specific activation of a single gustatory receptor produces opposing behavioral responses in Drosophila. Genetics. 2012;192:521–532. doi: 10.1534/genetics.112.142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacsoh B.Z., Lynch Z.R., Mortimer N.T., Schlenke T.A. Fruit flies medicate offspring after seeing parasites. Science. 2013;339:947–950. doi: 10.1126/science.1229625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake B. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem. Toxicol. 1999;37:423–453. doi: 10.1016/s0278-6915(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Larsson M.C., Domingos A.I., Jones W.D., Chiappe M.E., Amrein H., Vosshall L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lee Y., Moon S.J., Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. USA. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim S.H., Montell C. Avoiding DEET through Insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kang M.J., Shim J., Cheong C.U., Moon S.J., Montell C. Gustatory receptors required for avoiding the insecticide L-canavanine. J. Neurosci. 2012;32:1429–1435. doi: 10.1523/JNEUROSCI.4630-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Poudel S. Taste sensation in Drosophila melanoganster. Hanyang Med. Rev. 2014;34:130–136. [Google Scholar]
- Lee Y., Moon S.J., Wang Y., Montell C. A Dro-sophila gustatory receptor required for strychnine sensation. Chem. Senses. 2015;40:525–533. doi: 10.1093/chemse/bjv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier N., Marion-Poll F., Rospars J. P., Tanimura T. Peripheral coding of bitter taste in Drosophila. J. Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Miyamoto T., Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Slone J., Song X., Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.J., Köttgen M., Jiao Y., Xu H., Montell C. A taste receptor required for the caffeine response in vivo. Curr. Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Moon S.J., Lee Y., Jiao Y., Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kawazu K. Coumarin and euponin, two inhibitors for insect development from leaves of Eupatorium japonicum. Agr. Biol. Chem. 1980;44:2893–2899. [Google Scholar]
- Poudel S., Kim Y., Kim Y.T., Lee Y. Gustatory receptors required for sensing umbelliferone in Drosophila melanogaster. Insect Biochem. Mol. 2015;66:110–118. doi: 10.1016/j.ibmb.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Robertson H.M., Warr C.G., Carlson J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2003;100:14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N.U., Zhong L., Bellemer A., Tracey W.D. Egg laying decisions in Drosophila are consistent with foraging costs of larval progeny. PLoS One. 2012;7:e37910. doi: 10.1371/journal.pone.0037910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K., Brady R., Jr, Cravchik A., Morozov P., Rzhetsky A., Zuker C., Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Shim J., Lee Y., Jeong Y.T., Kim Y., Lee M.G., Montell C., Moon S.J. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat. Commun. 2015;6:8867. doi: 10.1038/ncomms9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V.L. Naturally occurring food toxicants: phenolic substances of plant origin common in foods. Adv. Food Res. 1981;27:149–242. doi: 10.1016/s0065-2628(08)60299-2. [DOI] [PubMed] [Google Scholar]
- Smith M.T., Yager J.W., Steinmetz K.L., Eastmond D.A. Peroxidase-dependent metabolism of Benzene’s phenolic metabolites and its potential role in Benzene toxicity and carcinogenicity. Environ. Health Persp. 1989;82:23–29. doi: 10.1289/ehp.898223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Schorderet M. Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res. 1981;216:513–523. doi: 10.1007/BF00238648. [DOI] [PubMed] [Google Scholar]
- Thorne N., Chromey C., Bray S., Amrein H. Taste perception and coding in Drosophila. Curr. Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Ueno K., Ohta M., Morita H., Mikuni Y., Nakajima S., Yamamoto K., Isono K. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr. Biol. 2001;11:1451–1455. doi: 10.1016/s0960-9822(01)00450-x. [DOI] [PubMed] [Google Scholar]
- Wang Z., Singhvi A., Kong P., Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Weiss L.A., Dahanukar A., Kwon J.Y., Banerjee D., Carlson J.R. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.H., Belawat P., Hafen E., Jan L.Y., Jan Y.N. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]


