Abstract
Cephalotaxus oliveri is an endangered tertiary relict conifer endemic to China. The species survives in a wide range from west to east with heterogeneous climatic conditions. Precipitation and temperature are main restrictive factors for distribution of C. oliveri. In order to comprehend the mechanism of adaptive evolution to climate variation, we employed ISSR markers to detect adaptive evolution loci, to identify the association between variation in temperature and precipitation and adaptive loci, and to investigate the genetic structure for 22 C. oliveri natural populations. In total, 14 outlier loci were identified, of which five were associated with temperature and precipitation. Among outlier loci, linkage disequilibrium (LD) was high (42.86%), which also provided strong evidence for selection. In addition, C. oliveri possessed high genetic variation (93.31%) and population differentiation, which may provide raw material to evolution and accelerate local adaptation, respectively. Ecological niche modeling showed that global warming will cause a shift for populations of C. oliveri from south to north with a shrinkage of southern areas. Our results contribute to understand the potential response of conifers to climatic changes, and provide new insights for conifer resource management and conservation strategies.
Global climates including temperatures and precipitation are rapidly changing. The changes of temperature mainly embody in variations in the daily, regional, seasonal, and annual mean temperatures as well as the increase of the intensity, frequency, and duration of abnormally low and high temperatures1. The alterations in precipitation primarily reflect in seasonal and temporal variability with the number of precipitation days and the length of dry spells2. It is noted that rapid changes in temperature and precipitation exert new selection pressures on plant populations3, which strongly influences their physiology, abundance, and distribution4. For instance, when plants encounter temperature extremes and extreme events including droughts and storms, flowering which is highly relevant for plant genetic adaptation to climate change is significantly ahead of time5. In this context, changes in temperature and precipitation provide an opportunity to investigate plant evolutionary adaptation.
Temperature and precipitation have been revealed as important drivers for local adaptation for conifers6. Temperature can profoundly influence seed germination, seedling growth, productivity and distribution of conifers, whereas precipitation has a determinative impact on variables such as soil moisture and the length of a wet or dry season7,8. Temporal and spatial variation in temperature and precipitation can also strongly influence the survival of conifers9. In extreme situations, high or low temperature stresses and precipitation events will ultimately cause mortality. Thus how to cope with temperature and precipitation becomes particularly important for conifers10. Due to their immobility and limited gene flow among populations, conifers locally have to adapt to fluctuations of temperature and precipitation by change of genotypes11,12 and inevitably leaves adaptive imprints at the genomic level. Indeed adaptive fingerprints have been widely detected in conifers. For instance, six SNPs in five climate-related candidate genes have been shown under divergent selection between two closely related species Pinus massoniana and Pinus hwangshanensis6. Twenty-three candidate SNPs related to temperature and precipitation have been identified in black spruce (Picea mariana)13. The prime candidate genes for adaptation to climatic variation involves in a variety of putative functions, including phenology, growth, reproduction, wood formation, lignin metabolism, and stress response4. Moreover, conifers also possess specific characteristics such as low domestication, large open-pollinated native populations, and high levels of genetic variation14, which makes them particularly valuable for examining local adaptation evoked by temperature and precipitation14. Investigating the adaptability of conifers to the two factors may also help to predict their response to future climate change15.
Cephalotaxus oliveri is an endangered tertiary relict conifer endemic to China with important economical and medical values16,17. The plant is ascribed to the Genus Cephalotaxus sect. Pectinate in Cephalotaxaceae17. Cephalotaxus oliveri is a wind-pollinated dioecious woody shrubs or small trees up to 4 m tall with yellow to grayish brown and scaly bark. Its distinguishing features embody in leaves densely arranged on leafy shoots and stomatal bands on abaxial surface17. Due to deforestation and overexploitation, it has been regarded as a vulnerable species by IUCN17. This species is essentially undomesticated and has large geographic ranges including montane regions of northern Guangdong, Guizhou, western Hubei, Hunan, eastern Jiangxi, southern and western Sichuan, and eastern Yunnan in China. Its natural populations have long been disjunctly distributed in subtropical evergreen and deciduous broad-leaved forests, where they occupy humid, shady niches at elevations of 300–1800 m with significant climate heterogeneity17. Precipitation and temperature are restrictive factors for the distribution of C. oliveri18. For instance, precipitation, extremely highest temperature, annual average precipitation, monthly highest average temperature, and annual average temperature have been documented to limit the horizontal and vertical distribution of Cephalotaxus in Yunnan19. Moreover, temperature can also influence the seed and seedling physiology and morphology20,21 as well as the speed of seed germination in Cephalotaxus22. Cephalotaxus oliveri was hypothesized to originate in the Oligocene and its population diversification was associated with the rapid uplift of the Qinghai-Tibetan Plateau23. The plant has experienced severe changes of temperature and precipitation over the past millions of years and well adapts to cold and arid environment. Hence, C. oliveri is suitable for investigating the adaptive evolution mechanism to climate variation. More importantly, in the light of global climate change, it is conducive to understanding the adaptive potential of C. oliveri and formulating protection strategy.
Due to the lack of genomic resources for nonmodel species, molecular marker-based genomic scans have been widely used to explore adaptive loci. All loci across the genome are generally expected to share the same demographic history13. However, when selective pressures result in strong differentiation of allele frequencies at some loci in the genome24, these loci will deviate from the equilibrium model and are considered to be potentially adaptive25. Currently, two approaches have been frequently applied to detect adaptive loci. One is Bayescan, which uses Bayesian estimation of the coancestry coefficient FST to decide whether a particular locus is adaptive26,27. The other is Dfdist, which is mainly a frequentist method based on summary statistics of a symmetrical island model to identify the loci under selection26,28. If adaptive loci are further combined with climatic variables, it may be possible to estimate which climatic factors are responsible for adaptive evolution. The association between allele frequency variation and environmental variables can be evaluated by the Spatial Analysis Method (SAM) through logistic regressions29,30. The advantages of SAM lie in that it does not depend on genetic models and works at the individual level29.
In this study, we employed ISSR markers to detect adaptive loci under selection and utilize SAM to identify the association between climatic variations and genetic data. In addition, we also investigated the genetic structure among natural populations of C. oliveri. Because populations sampled represented over a local scale, our results may precisely detect the genetic signatures for adaptation. The goals of the study were (1) to examine adaptive loci in the genome of C. oliveri; (2) to analyze the correlation between climatic variables and adaptive loci; (3) to evaluate the population genetic structure of C. oliveri, and (4) to explore the genetic basis of the adaptation of C. oliveri to climate.
Results
Genetic Structure
Twenty-one ISSR primers were selected to investigate the genetic structure in C. oliveri populations. Overall, 310 reliable loci were identified with 100% polymorphic ranging in size from 200 to 2000 bp. A high level of genetic variation was observed with 93.31% polymorphic loci at the species level (Table 1). The highest number of polymorphic loci (PPB = 78.8%, Hs = 0.2769, I = 0.4125) was exhibited in HNym population and the lowest (PPB = 18.44%, Hs = 0.0798, I = 0.1141) in JXxs population. At the regional scale, Hunan (PPB = 87.88%, Hs = 0.2435, I = 0.377) maintained the highest level of variation and Guangdong (PPB = 44.67%, Hs = 0.1792, I = 0.2607) the lowest. The results indicated that C. oliveri possesses a high level of genetic variation.
Table 1. Origin locations of sampled populations and parameters of genetic diversity revealed by ISSR in Cephalotaxus oliveri.
| Province | Population | Population abbreviation | Geographical coordinate | Altitude (m) | Sample Size | Number of loci | Number of polymorphic loci | Percentage of polymorphic loci | Nei’s gene diversity | Shannon’s index |
|---|---|---|---|---|---|---|---|---|---|---|
| Chongqing | Jin Fo Shan | CQjfs | 29°01′N 107°05′E | 650 | 15 | 185 | 102 | 0.5514 | 0.2007 | 0.2973 |
| Liang Ping Zhu Shan | CQzs | 30°39′N 107°32′E | 530 | 15 | 193 | 133 | 0.6891 | 0.2156 | 0.3291 | |
| Yunnan | Da Wei Shan | YNdws | 27°37′N 113°52′E | 2109 | 15 | 185 | 118 | 0.6378 | 0.2249 | 0.334 |
| Sichuan | E Mei Shan | SCems | 29°33′N 103°23′E | 980 | 15 | 173 | 97 | 0.5607 | 0.2089 | 0.3082 |
| Jiangxi | An Fu | JXaf | 27°13′N 114°11′E | 420 | 15 | 180 | 131 | 0.7278 | 0.2477 | 0.3712 |
| Yi Feng | JXyf | 28°37′N 114°54′E | 741 | 8 | 155 | 62 | 0.4 | 0.1477 | 0.2186 | |
| Xiu Shui | JXxs | 28°46′N 114°46′E | 307 | 5 | 141 | 26 | 0.1844 | 0.0798 | 0.1141 | |
| Hunan | De Hang | HNdh | 28°21′N 109°35′E | 429 | 15 | 190 | 101 | 0.5316 | 0.1782 | 0.2672 |
| Hu Ping Shan | HNhps | 29°57′N 110°38′E | 428–561 | 15 | 177 | 106 | 0.5989 | 0.2302 | 0.3373 | |
| Hui Long | HNhl | 28°54′N 110°10′E | 399 | 15 | 199 | 136 | 0.6834 | 0.2346 | 0.3514 | |
| Yong Mao | HNym | 28°58′N 110°18′E | 534 | 15 | 184 | 145 | 0.788 | 0.2769 | 0.4125 | |
| Ha Ni Gong | HNhng | 28°56′N 109°57′E | 305 | 15 | 187 | 115 | 0.615 | 0.2098 | 0.3145 | |
| Guizhou | Wu Yang He | GZwyh | 27°03′N 108°18′E | 550 | 15 | 184 | 115 | 0.625 | 0.2235 | 0.3324 |
| Da Sha He | GZdsh | 29°04′N 107°24′E | 700 | 15 | 174 | 117 | 0.6724 | 0.2414 | 0.359 | |
| Fan Jing Shan | GZfjs | 27°49′N 108°36′E | 860 | 15 | 166 | 80 | 0.4819 | 0.1643 | 0.246 | |
| Guangdong | Dan Xia Shan | GDdxs | 25°03′N 113°45′E | 800 | 8 | 150 | 67 | 0.4467 | 0.1792 | 0.2607 |
| Hubei | Chang Yang | HBcy | 30°43′N 110°54′E | 420 | 15 | 185 | 117 | 0.6324 | 0.2203 | 0.3293 |
| Long Dong | HBld | 34°40′N 111°02′E | 342 | 15 | 176 | 91 | 0.517 | 0.1731 | 0.2615 | |
| La Mei Xia | HBlmx | 30°39′N 111°03′E | 307 | 15 | 179 | 104 | 0.581 | 0.2067 | 0.3072 | |
| Chai Bu Xi | HBcbx | 30°11′N 111°01′E | 248 | 15 | 187 | 113 | 0.6043 | 0.2076 | 0.3108 | |
| Hou He | HBhh | 30°05′N 110°40′E | 440 | 15 | 174 | 86 | 0.4943 | 0.1772 | 0.2629 | |
| Zi Gui Si Xi | Hbzg | 30°43′N 111°54′E | 248 | 15 | 182 | 120 | 0.6593 | 0.2141 | 0.3234 | |
| Total | 306 | 269 | 251 | 0.9331 | 0.2214 | 0.3527 |
Populations were significantly structured as revealed by overall FST (0.39565) and GST (0.3862). AMOVA results further showed that most genetic variation was occurred within populations (60.44%, FST = 0.39565, P < 0.001), whereas the proportion of genetic variation among populations within regions was 29.87% (FSC=0.33076, P < 0.001) (Table 2). Only 9.7% genetic variation occurred among regions (FCT=0.09696, P < 0.001). In addition, significant patterns of isolation by distance were revealed by comparing FST values with geographical distances (r = 0.571069, P < 0.001).
Table 2. AMOVA results for Cephalotaxus oliveri populations.
| Source of variation | d.f. | Sum of squares | Variance components | Percentage of total variation | P-value | F statistics |
|---|---|---|---|---|---|---|
| Among regions | 7 | 1657.683 | 2.84409 | 9.7 | <0.001 | FCT = 0.09696 |
| within regions | 14 | 1972.364 | 8.76169 | 29.87 | <0.001 | FSC = 0.33076 |
| Within populations | 284 | 5034.692 | 17.72779 | 60.44 | <0.001 | FST = 0.39565 |
Dendrogram was constructed by the UPGMA method. The results showed that 22 populations of C. oliveri were clustered into two groups (Fig. 1). YNdws, the most geographically distant population, was clustered into the separated group. Other 21 populations were clustered into the other group.
Figure 1. UPGMA dendrogram among 22 populations of C. oliveri was constructed based on Nei’s unbiased genetic distances.
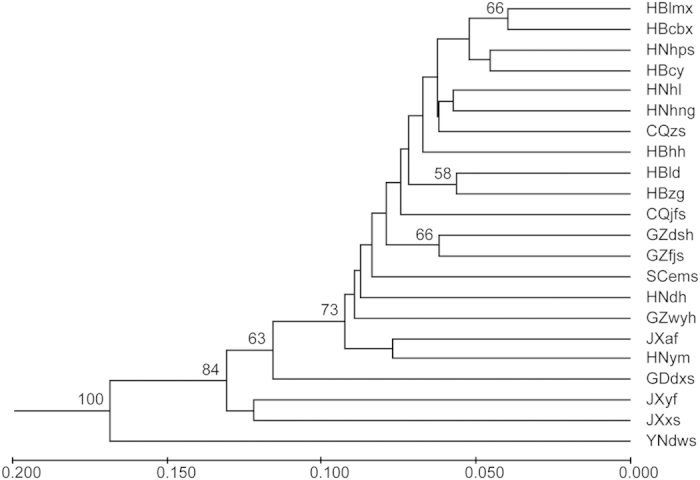
Bootstrap values larger than 50% were displayed above branches (% of 1000 replicates). Scale between branch lengths and genetic distances was shown at the bottom of figure.
ΔK clearly demonstrated that the uppermost K equaled 21 (Fig. 2a). The most likely number of genetic clusters K from STRUCTURE was also 21 (mean LnP(D) = −39912.5, Var[LnP(D)] = 4877) (Fig. 2b). Populations HBzg and HBld, GZdsh, GZfjs, JXyf, and JXxs were clearly evident, with high proportions of individual assignment to the correct region (Fig. 2c). Only a few populations appeared admixed with others. The results suggested that a high differentiation level existed in C.oliveri populations.
Figure 2. Bayesian structure analyses of C. oliveri populations based on ISSR loci.

(a) Estimates of an ad hoc quantity ΔK with respect to K. The result for K = 21 supported by a high ΔK value was presented. (b) A plot of the posterior probability of the data (LnP(D)) values for a given K (1–26). The 21 represented the value of K with the highest likelihood. (c) Genetic structure of C. oliveri in eight regions for K = 21 clusters.
Outlier detection
Bayescan identified 32 loci as outliers with a log10PO above 2, which is a threshold for decisive evidence for accepting a model under selection, corresponding to a posterior probability greater than 0.9931 (Fig. 3a). Using the Dfdist, we detected 61 adaptive loci at the 99.5% confidence level (Fig. 3b). Based on two complementary analyses, 14 outlier loci (1, 7, 8, 13, 40, 63, 102, 161, 165, 196, 206, 259, 262, and 300) were identified, which represented truly adaptive loci (Fig. 4a). The very stringent significance criteria in the two approaches also ensured the robustness of 14 outlier loci.
Figure 3. Outlier loci identified by Bayescan and Dfdist.
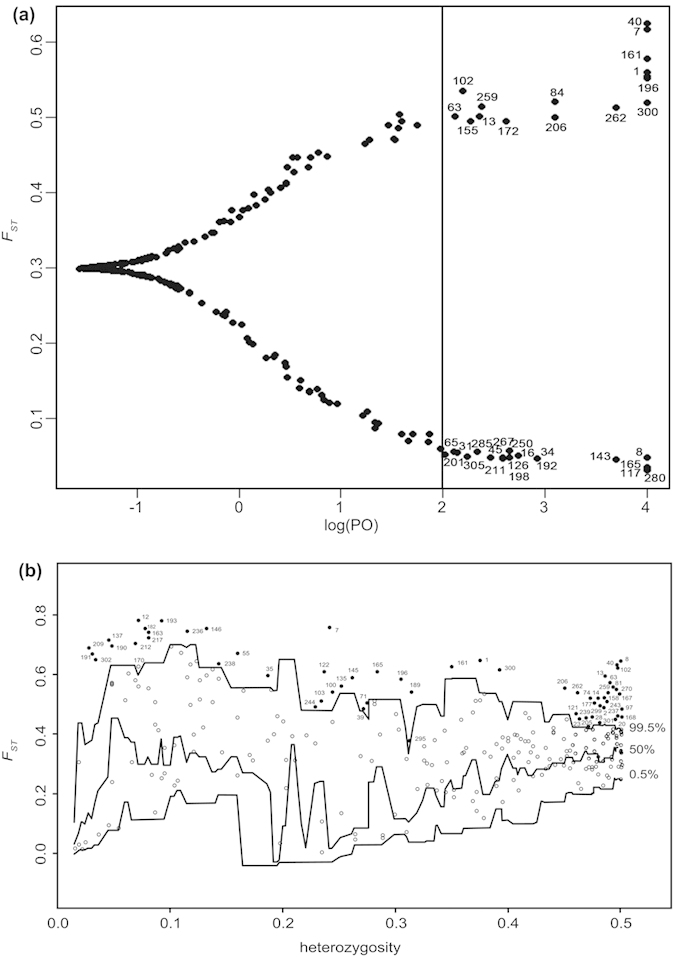
(a) Plot of FST values and log10PO for 310 loci identified using Bayescan. Lines log10PO = 2 indicate “decisive” evidence for selection corresponding to a posterior probability of 0.99. Solid black dots greater than log10PO 2 represented outlier loci. (b) Outlier detection performed with Dfdist. Plot of FST values of 310 loci in C. oliveri populations was against heterozygosity. The 0.5%, 50%, and 99.5% represented confidence intervals, respectively. Loci above the 99.5% line were designated as outlier loci.
Figure 4. Number summary of outlier loci and significant association between outliers and climatic variables.
(a) Thirty-two, 61, and 14 outlier loci were detected to be subject selection in C. oliveri using Bayescan, Dfdist, and both with Dfdist and Bayescan, respectively. (b) The number association between outliers and climatic variables in SAM analysis. Locus 7 and 8 were significantly associated with three and two temperature variables, respectively. Locus 206 was simultaneously linked to two variables of temperature and precipitation. Locus 102 exhibited 5 significant allele–climate variables. Locus 259 was only linked to one climatic factor.
Linkage disequilibrium
Linkage disequilibrium (LD) was detected for 28 of the 14225 combinations of all 310 loci with the false discovery rate of 0.1. Twenty of the 310 loci (6.45%) were involved in the detected combinations in LD. In contrast, six (42.86%) of the 14 outlier loci were involved in LD; they were Locus 40, 63, 102, 161, 165, and 206. When two or more linked loci were in LD within a chromosomal region, this region was defined as an LD block32. We found that Locus 165 formed LD blocks with nine loci (Locus 130, 135, 144, 149, 150, 151, 152, 154, and 156) (Table 3).
Table 3. The results of linkage disequilibrium analyzed by TASSAL.
| Locus | D’ | r2 | p |
|---|---|---|---|
| 40 and 34 | 0.46113208 | 0.14939114 | 0.00000002 |
| 63 and 53 | 0.36157921 | 0.10927404 | 0.00000002 |
| 102 and 79 | 1.0000 | 0.10018378 | 0.00000035 |
| 161 and 143 | 0.78518778 | 0.12668338 | 0.00000102 |
| 165 and 130 | 0.82413793 | 0.2462112 | 0.00000117 |
| 165 and 135 | 0.50758618 | 0.24071099 | 0.00000004 |
| 165 and 144 | 0.91574889 | 0.13294548 | 0.00000001 |
| 165and 149 | 0.46627906 | 0.02834072 | 0.00837694 |
| 165 and 150 | 1.00 | 0.01317884 | 0.04848577 |
| 165 and 151 | 0.56534094 | 0.11084024 | 0.00000369 |
| 165 and 152 | 0.35944977 | 0.01535935 | 0.0493768 |
| 165 and 154 | 0.89827126 | 0.0709272 | 0.00000371 |
| 165 and 156 | 0.7589286 | 0.11122229 | 0.00000039 |
| 206 and 180 | 1.0000 | 0.16000 | 0.00000124 |
Association with climatic variables
The logistic regressions of 310 ISSR markers and 19 climatic variables were calculated using the SAM program. With 99.9999% confidence level, on a total of 6510 models computed, SAM identified 20 significant associations (0.31%). Of those, ten loci were significantly related with temperature, and 11 were significantly related to precipitation. Furthermore, five of the 20 loci related with climatic factors were also outlier loci detected by Dfdist and Bayescan (Fig. 4b). Locus 7 was significantly associated with annual mean temperature (Bio 1), max temperature of warmest month (Bio 5), and mean temperature of warmest quarter (Bio 10). Locus 8 showed significant association with mean diurnal range (Bio 2) and isothermality (Bio 3). Locus 102 exhibited 5 significant allele–climate variable associations including annual precipitation (Bio 12), precipitation of driest month (Bio 14), precipitation seasonality (Bio 15), precipitation of driest quarter (Bio 17), and precipitation of coldest quarter (Bio 19). Locus 206 was significantly associated with max temperature of warmest month (Bio 5) and precipitation of coldest quarter (Bio 19). Locus 259 was linked to isothermality (Bio 3). Loci 7 and 206 were linked to max temperature of warmest month (Bio 5); Loci 8 and 259 were associated with isothermality (Bio 3); Loci 102 and 206 were correlated to precipitation of coldest quarter (Bio 19). Locus 206 was simultaneously linked to temperature and precipitation.
Potential current and future geographic distribution
Present and future ecological niche models for C. oliveri were estimated by MAXENT (Maximum Entropy Model). The average AUC test for replicate runs and the standard deviation were 0.955 and 0.019, respectively, which indicated good predictive model performance. Minimum training presence logistic threshold was 0.1270. The predicted current geographical distribution of C. oliveri was generally similar to its actual distribution including Jiangxi, Hunan, Guizhou, Guangxi, and Hubei, even though some predicted areas do not have any records at present (Fig. 5a). These resulting potential distributions are climatically suitable for C. oliveri.
Figure 5. Potential current and future distributions inferred from ecological niche modeling (Program MAXENT 3.3.3k, URL http://www.cs.princeton.edu/~schapire/maxent/.) for C. oliveri in China based on WorldClim variables.
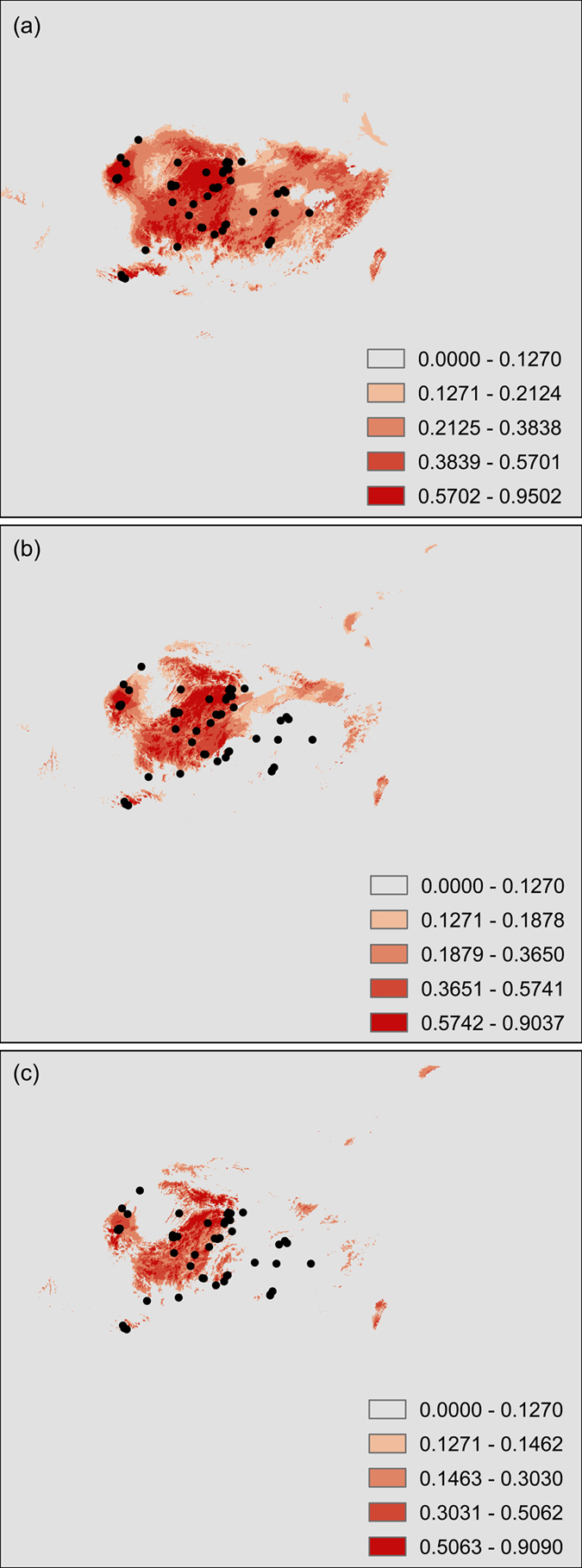
Two climate scenarios IPCC-CMIP5 RCP 4.5 and RCP 8.5 under HadGEM2-ES model were used to ensure the accuracy of evaluation on future distribution. (a) Predicted current distribution of C. oliveri based on climate grids for the periods 1950–2000. (b) Possible future distribution of C. oliveri in 2070 based on RCP 4.5 climate scenario. (c) Projected future distribution of C. oliveri in 2070 based on RCP 8.5 climate scenario.
The predicted future and current distribution of C. oliveri was considerably different in range. The main difference was that the predicted future suitable area showed a significant reduction in comparison with the current one with a general northward range shift (Fig. 5b,c); the present southern and southeastern regions such as Jiangxi, Hunan, Hubei, and Guangxi were predicted to become significantly unfavorable. Loss of suitable habitats indicated a drastically range contraction (Fig. 5b,c). Populations of C. oliveri may become more patchily distributed than at present.
Discussion
This study has analyzed the adaptive evolution of C. oliveri to temperature and precipitation through ISSR markers (Inter-Simple Sequence Repeats). ISSRs are highly variable and have been widely applied to the assessment of population genetic diversity and structure in plants33,34. However, ISSRs were seldom used to detect adaptive loci in genome. Currently, the most efficient approach to identify candidate genomic regions under selection is AFLP (Amplified Fragment Length Polymorphism) based on PCR (Polymerase Chain Reaction) to obtain amplified polymorphisms35. Similar to AFLP, ISSR can also generate a large number of polymorphic loci in genome without prior sequence information33,36,37. Although ISSR are assumed to be neutral markers, its primers also can match to microsatellite regions and genes encoding specific proteins33,38,39. Hence ISSR is suitable for detecting candidate loci under selection.
We first applied ISSRs to identify adaptive evolution in C. oliveri and ascertain the relationships between candidate loci and climatic factors. In order to ensure unbiased analysis, only distinct, reproducible ISSR loci were scored in this study. Three hundred and ten loci produced by 21 primers have good genome coverage in C. oliveri. Fourteen outlier loci were identified by both Dfdist and Bayescan. The two complementary and exhaustive methods guaranteed strong confidence of the 14 loci with very stringent significance criteria. The proportion of outlier loci detected in C. oliveri was 4.22%, which conformed to the percentage between 2% to 15% in AFLP genome scan or other molecular markers40. For instance, 2.9% in Mikania micrantha from AFLP35, 3% in Norway spruce (P. abies) from RAPD markers41, and 3.7% in white spruce from SNPs42.
Temperature and precipitation have been identified to be the major selective pressure driving plant adaptation8,9,43,44,45. The two climatic factors are very important for plant growth, development, survival, reproduction and defense8. Currently, adaptive loci associated with temperature and precipitation have been detected in plants9. For instance, the close relationship between AFLP allele frequencies and temperature and precipitation have been found in P. monticola and Keteleeria davidiana var. formosana9,43,46. Nine SNPs associated with climate-related complex trait variation have also been identified in Sitka spruce (P. sitchensis)47. SNPs associated with seasonal minimum temperatures are detected in four conifers, Abies alba, Larix decidua, P. cembra, and P. mugo, whereas SNPs in L. decidua and P. cembra are found to be related to seasonal maximum temperature and winter and autumn precipitations48. The local polymorphism patterns of candidate genes linked to drought tolerance has also been detected in a widespread Mediterranean conifer (P. halepensis)49. As restrictive factors, temperature and precipitation were suggested to strongly influence the geographical distribution of C. oliveri18. In this study, five among 14 outlier loci were also revealed to be associated with temperature or precipitation. Local climatic conditions impacted 1.6% of ISSR loci in C. oliveri, suggesting that a relatively small number of loci govern climatic adaptation in this species. The result was very similar to previous studies13,50. Twenty-two adaptive loci associated with climatic variables was identified in Loblolly pine (P. taeda)50, whereas ten outlier loci were detected in black spruce (P. mariana)13. Their proportions of detected loci associated with the climatic variables were 1.3% and 1.7%, respectively.
Furthermore, we found that one adaptive locus simultaneously was linked to temperature and precipitation. The result indicated that the candidate locus might have undergone the same selective pressures51. The same phenomenon was also demonstrated in black spruce (P. mariana), whose four of 26 outlier SNPs were common to both the temperature and the precipitation13. It has been noted that physiological processes involved in adaptation to temperature and precipitation may be related in conifers52,53. In P. abies, drought tolerance was found to be genetically correlated with tolerance to freezing temperature53. In P. mariana, a gene coding for the DnaJ heat shock protein was detected to carry an adaptive SNP related to both temperature and precipitation, and the protein was produced under stresses involving temperature and moisture10. In whitebark pine (P. albicaulis) and loblolly pine (P. taeda), annual, seasonal mean temperatures, and rainfall patterns also appear to drive local adaptation, which primarily involved response to both temperature and drought50,54. Cephalotaxus oliveri extends from the west to the east in China, with an environmental gradient showing significant precipitation and temperature differences55. The finding of adaptive loci implies that C. oliveri has successfully responded and adapted to historic climate changes. The species was inferred to originate in the Oligocene [ca. 28.32 million years ago (Ma)] and diversified in the early Miocene (ca. 17.73 Ma)23. In the long evolutionary process, the high genetic variation possessed by C. oliveri may have provided raw material for its adaptation to changing climatic conditions. Meanwhile, its high population differentiation possibly also accelerated local adaptation. Our results indicated that C. oliveri populations well adapt to temperature and precipitation factors including annual mean temperature (Bio 1), mean diurnal range (Bio 2), isothermality (Bio 3), max temperature of warmest month (Bio 5), mean temperature of warmest quarter (Bio 10), annual precipitation (Bio 12), precipitation of driest month (Bio 14), precipitation seasonality (Bio 15), precipitation of driest quarter (Bio 17), and precipitation of coldest quarter (Bio 19) (Fig. 4b).
The geographic pattern also provided a useful indication of adaptive variation56,57. The natural populations of C. oliveri have a wide geographic range, from south to north is from 25°3′N, 130°45′E to 34°40′N, 111°02′E and east to west from 28°37′N, 114°54′E to 27°37′N, 113°52′E (Table 1). Within the range, Cephalotaxus oliveri has to face with a great climate variation, including subtropical monsoon climate, East Asian humid monsoon climate, subtropical mountain monsoon humid climate, and eastern humid mountain monsoon climate, respectively55, which results in seasonal difference of precipitation. Water availability is one of the major abiotic stressors that can lead to adaptive variation in conifers50. Specifically, lack of precipitation during winter period represents a great threat to conifers58. In line with this, precipitation of coldest quarter becomes the most often climate variable that was detected in the significant allele-environment associations57. In this study, SAM analysis further showed that precipitation of coldest quarter was one of the factors driving the adaptive evolution of C. oliveri, which was consistent to the previous ecological hypothesis55.
Linkage disequilibrium (LD) in the genomic region can reflect the genetic signature associated with local adaptation, especially for long-lived plants51,59. Here we observed that of all the 310 loci examined for C. oliveri, only twenty (6.45%) were found to be involved in LD. Our result lends further support to the theory that LD in forest trees decays rapidly60. However, if only considering outlier loci, the proportion (42.86%) of loci involved in LD was relatively high. This is not unexpected as strong positive selection may increase the frequency of an advantageous allele, causing linked loci remain in strong LD with that allele (genetic hitch-hiking)59. More importantly, Locus 165 was identified to form LD blocks with nine loci. The significant LD among the loci reflect that they may not only have experienced the same selection pressure, but also have been acted upon by evolutionary mechanisms like co-adaptation of gene complexes51,61. The LD blocks implies potential genomic regions that are associated with adaptations.
It is also worthy of note that for the pairwise LD analysis between Locus 102 and 79, 165 and 150, and 206 and 180, the two statistics D’ and r2 acted quite differently; namely, D’ had a value of 1, but r2 were much smaller (Table 3). This performance difference is due to the fact that D’ and r2 reflect different aspects of LD62. D’ measured only recombinational history, whereas r2 summarized both recombinational and mutational history. The results of C. oliveri indicated that the polymorphisms between the three pairs of locus were not completely correlated, but there was no evidence of recombination.
We used MAXENT to project the distribution of C. oliveri under current and future climate conditions. MAXENT captured well a major portion of current distribution of C. oliveri in China and also deduced its future range under a climatic warming scenario. With a rate of rising of 0.1 oC–0.4 oC per decade63, future temperature was assumed to increase by 2.3 oC–2.7 oC in 2070. As a result, the projection predicted that C. oliveri will lose considerable suitable areas with climate warming. Similar predictions have been made for other tree species54,64,65. More specifically, the southern and southeastern populations of C. oliveri were projected to be more sensitive to climate warming than others. This information is quite helpful for formulating a protection strategy when considering future climate conditions. Although C. oliveri possesses high levels of population genetic variation, its long generation time and limited seed dispersal will constrain adaptations to rapid climate change54,64,66,67. On the other hand, at the regional scale Hunan had the highest ISSR variation, whereas the lowest was found in Guangdong. In our previous research, the similar variation pattern has also been revealed for C. oliveri populations based on trnL-F, atpB-rbcL and trnD-trnT sequences23. Population HNhps was recognized as the refugium during the Pleistocene ice ages, and populations in Guangdong were speculated to expand from Hunan23. In conjunction with the MAXENT predictions, the population genetic data will be used to develop an ex situ conservation action plan for C. oliveri.
In summary, this is the first study examining the adaptive loci, relationship between outliers and climatic factors, and the underlying mechanisms of local adaptation in C. oliveri. Our results indicated that C. oliveris exhibits remarkable adaptations to temperature and precipitation. Global warming may profoundly affect its viability and distribution. In the next steps we will dissect the adaptive value of the identified loci by sequencing and gene analysis in order to further understand the adaptation of C. oliveri to climatic factors.
Methods
Sample collection
Twenty-two naturally fragmented populations of Cephalotaxus oliveri were collected from the whole distribution range of the species (Table 1). Population samples contained five to fifteen individuals (Table 1). All selected populations except JXxs, JXyf, and GDdxs had 15 individuals, which were randomly sampled with 10–20 m interval. If the population size was less than 15, all individuals were collected. Young, healthy leaves were collected and dried in silica gel in zip-lock plastic bags until DNA extraction. Vouchers were deposited at the Herbarium of Sun Yat-sen University (SYSU), Guangzhou, China.
Climatic data collection
Climatic layers at 2.5′ resolution for current conditions were obtained from the WorldClim database (http://www.worldclim.org/)68. The 19 bioclimatic variables were extracted by DIVA-GISv7.5 software (http://www.diva-gis.org/) in terms of informatic characterization of C. oliveri. The bioclimatic variables included annual mean temperature (Bio 1), mean diurnal range (Bio 2), Isothermality (Bio 3), temperature seasonality (Bio 4), max temperature of warmest month (Bio 5), min temperature of coldest month (Bio 6), temperature annual range (Bio 7), mean temperature of wettest quarter (Bio 8), mean temperature of driest quarter (Bio 9), mean temperature of warmest month (Bio 10), mean temperature of coldest month (Bio 11), annual precipitation (Bio 12), precipitation of wettest month (Bio 13), precipitation of driest month (Bio 14), precipitation seasonality (Bio 15), precipitation of wettest quarter (Bio 16), precipitation of driest quarter (Bio 17), precipitation of warmest quarter (Bio 18), and precipitation of coldest quarter (Bio 19) (Supplementary Table S1).
DNA extraction
We extracted genomic DNA from tissues using a modified cetyltrimethyl ammonium bromide (CTAB) method with −20 °C propanone pretreatment to eliminate polysaccharides, which was successful for conifers34,69. The DNA was stored at −20 °C until further use.
ISSR amplification
To cover the widest genomic region and ensure high-quality reproducible bands, an initial screening was developed using one individuals randomly obtained from HNhng population. Twenty-one primers were screened from 100 primers (UBC primer set #9) of the Biotechnology Laboratory, University of British Columbia. PCR amplification was carried out in a total volume of 20 μl consisting of 20 ng of template DNA, 10 mM Tris-HCl (pH 8.3) reaction buffer, 50 mM KCl, 2.0 mM MgCl2, 0.25 mM dNTPs, 0.24 μM primer, 1.5 units of Taq polymerase, and DNA-free water. In an ABI veriti thermocycler, PCR started with an initial denaturation at 94 °C for 5 min followed by 40 cycles with 94 °C for 30 s, 53 °C–54 °C for 45 s and 72 °C for 90 s, and ended with a final extension of 7 min at 72 °C. DNA quality and quantity were estimated using 1.7% agarose gel in TAE 1X buffer, stained with Ethidium Bromide. Additionally, 100 bp ladder and negative and positive controls were loaded and run at constant voltage (135 V) for 95 min. After running, the gels were UV visualized and recorded using a Gel Doc 2000 Camera.
Data Analysis
Unambiguous ISSR fragments were transformed into 01 character matrix (1 = presence, 0 = absence).
POPGENE ver 1.31 was used to calculate genetic parameters. The estimates included the percentage of polymorphic loci (P), Nei’s (1973) gene diversity (H), Shannon’s information index (I), total gene diversity (Ht), and gene differentiation (GST)70.
The variation among and within 22 populations were performed by analysis of molecular variance (AMOVA) with 1000 permutations using ARLEQUIN version 3.071,72. Using the same software, a Mantel test72 was conducted to analyze the relationship between pairwise population genetic and geographic distances. Genetic distances were computed as pairwise FST values between all pairs of populations73. The unweighted pair group method (UPGMA) based on Nei’s unbiased (1978) genetic distance74 was performed using the TFPGA 1.3 program for constructing a dendrogram to reveal the genetic relationship among populations75. Bootstrap values for nodes were estimated based on 1000 replications.
To infer population structure, we assigned individuals and populations to clusters by using the model-based program STRUCTURE 2.376. Thirty independent runs for each value of K = 1–27 were conducted to estimate the number of clusters (K) with maximum likelihood with the following settings: admixture model, correlated allele frequencies, burn-in length of 100 000, MCMC repetitions of 100077, and ten times of iterations. A best-estimated K was defined both using log probabilities [Pr (X|K)] and hoc statistic ΔK78.
To identify loci under selection, software Dfdist and Bayescan were used to ensure truly adaptive regions of the genome. The software Dfdist was applied to simulate a null distribution of FST values under an island model, which was insensitive to population structure, demographic structure and mutation level. Simulations were computed with a mean FST similar to the trimmed mean FST, which was calculated by excluding 30% of the most extreme FST values observed in the empirical dataset. We compared the distributions of the FST values over all loci to null hypothesis of neutral evolution. Loci with a high or low FST value were considered as potentially under selection. In this study, we simulated the neutral distribution of FST with 50 000 iterations at the 99.5% confidence level. Bayescan developed by Foll and Gaggiotti27 is an FST based model, which uses reversible jump MCMC and estimation of the Bayesian posterior probability26. It searches for loci with extreme FST values. Large FST is then interpreted as signature of local adaptation. We calculated outliers using a burn-in of 50 000 iterations, a thinning interval of 20, and a sample size of 5 000.
Linkage disequilibrium (LD) between all pairs of ISSR loci was calculated by the squared allele frequency correlation coefficient (r2) implemented in TASSEL 2.2 (Trait Analysis by aSSociation, Evolution, and Linkage)79. The pair-wise significance was computed by 1,000 permutations after removal of loci with rare alleles (f < 0.05).
The evaluated estimates included the standardized disequilibrium coefficient (D’), as well as the squared correlation coefficient (r2) and p values based on Fisher’s exact test. D’ determined whether recombination had occurred between a pair of alleles80. The critical value of r2 was the conventional 0.181. Statistical tests for each r2 were provided by the p value calculated in TASSEL.
To investigate the association between ISSR genetic data and climatic variables, SAM was employed (available at http://www.econogene.eu/software/sam). The likelihood ratio (G) and Wald tests were used to determine the significance of the models. The null hypothesis was designed so that the above two statistical parameters conformed to a normal distribution. A model was considered significant only if the null hypothesis was rejected by both statistical tests at the 95 and 99.999% confidence level. The 310 ISSR markers in all 22 C. oliveri populations were examined against 19 climatic variables in the SAM analysis.
We used MAXENT 3.3.3k82 to predict distribution changes for C. oliveri as a result of climate warming. MAXENT is a program for maximum entropy modelling of the geographical distributions of species; it combines presence-only data with ecological-climatic layers to predict suitable areas. For current distribution, we downscaled climate grids for the periods 1950–2000. In addition to sample locations in this study, we also collected the distribution records of C. oliveri from the Chinese Virtual Herbarium (http://www.cvh.org.cn/). After removing duplicate records, it remained a total of 57 records of C. oliveri that were used to generate the distribution model by using 19 bioclimatic data layers from the WorldClim database (http://www.worldclim.org) at 2.5-arcmin resolution (Supplementary Table S1).
We selected the Hadley Global Environment Model 2 (HadGEM2-ES) as a general circulation model under two climate scenarios (IPCC-CMIP5 RCP 4.5/8.5) to ensure the accuracy of evaluation. The RCP 8.5 scenario represents a higher predicted greenhouse gas emission than RCP 4.583 (Supplementary Tables S2–S3).
MAXENT was run according to the following settings: random test percentage = 25; regularization multiplier = 1; convergence threshold = 0.00001; maximum iterations = 1000 and averaged across 10 cross-validation runs. Ultimately, variable importance was determined by a jackknife test. We evaluated the accuracy of each model prediction by calculating the area under the curve (AUC) values. AUC is an efficient indicator of model performance84. AUC values > 0.9 indicates high reliability of the model85.
Additional Information
How to cite this article: Wang, T. et al. Local adaptation to temperature and precipitation in naturally fragmented populations of Cephalotaxus oliveri, an endangered conifer endemic to China. Sci. Rep. 6, 25031; doi: 10.1038/srep25031 (2016).
Supplementary Material
Acknowledgments
We thank Huawei Pan and Jianjun Liu of School of Life Sciences, Sun Yat-sen University, for assistance with the collection of plant materials. This work was supported by the National Natural Science Foundation of China (31070594, 31370364, and 31570652), and Project of Department of Science and Technology of Zhuhai City, China (2012D0401990031).
Footnotes
Author Contributions T.W. designed and performed the experiments, and wrote the manuscript; Z.W. conducted data analysis and checked English grammar; F.X. performed the ISSR experiment; Y.S. contributed to the supervision of the work and wrote the manuscript. All authors read and approved the final version of the manuscript.
References
- Yamori W., Hikosaka K. & Way D. A. Temperature response of photosynthesis in C-3, C-4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth. Res. 119, 101–117 (2014). [DOI] [PubMed] [Google Scholar]
- Jongen M. et al. Resilience of montado understorey to experimental precipitation variability fails under severe natural drought. Agr. Ecosyst. Environ. 178, 18–30 (2013). [Google Scholar]
- Hoffmann A. A. & Sgrò C. M. Climate change and evolutionary adaptation. Nature 470, 479–85 (2011). [DOI] [PubMed] [Google Scholar]
- Franks J. S. & Hoffmann A. A. Genetics of climate change adaptation. Annu. Rev. Genet. 46, 185–208 (2012). [DOI] [PubMed] [Google Scholar]
- Parmesan C. & Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003). [DOI] [PubMed] [Google Scholar]
- Zhou Y. F., Zhang L. R., Liu J. Q., Wu G. L. & Savolainen O. Climatic adaptation and ecological divergence between two closely related pine species in Southeast China. Mol. Ecol. 23, 3504–3522 (2014). [DOI] [PubMed] [Google Scholar]
- Concilio A., Chen J. Q., Ma S. & North M. Precipitation drives interannual variation in summer soil respiration in a Mediterranean-climate, mixed-conifer forest. Climatic Change 92, 109–122 (2009). [Google Scholar]
- Poncet B. N. et al. Tracking genes of ecological relevance using a genome scan in two independent regional population samples of Arabis alpina. Mol. Ecol. 19, 2896–2907 (2010). [DOI] [PubMed] [Google Scholar]
- Manel S. et al. Broad-scale adaptive genetic variation in alpine plants is driven by temperature and precipitation. Mol. Ecol. 21, 3729–3738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier J., Gérardi S., Laroche J., Beaulieu J. & Bousquet J. Parallel and lineage-specific molecular adaptation to climate in boreal black spruce. Mol. Ecol. 21, 4270–4286 (2012). [DOI] [PubMed] [Google Scholar]
- Kiani S. P. et al. Allelic heterogeneity and trade-off shape natural variation for response to soil micronutrient. PLos Genet. 8, e1002814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberto F. J. et al. Potential for evolutionary responses to climate change evidence from tree populations. Global Change Biol. 19, 1645–1661 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier J., Laroche J., Beaulieu J. & Bousquet J. Scanning the genome for gene SNPs related to climate adaptation and estimating selection at the molecular level in boreal black spruce. Mol. Ecol. 20, 1702–1716 (2011). [DOI] [PubMed] [Google Scholar]
- González-Martínez S. C., Krutovsky K. V. & Neale D. B. Forest-tree population genomics and adaptive evolution. New Phytol. 170, 227–238 (2006). [DOI] [PubMed] [Google Scholar]
- de Luis M. et al. Plasticity in dendroclimatic response across the distribution range of Aleppo Pine (Pinus halepensis). PLos One 8, e83550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L. G. & Jin J. M. Red list of endangered plants in China. (Science Press, 1992). [Google Scholar]
- Fu L. G., Li N. & Mill R. R. Cephalotaxaceae. In Flora of China (eds Wu Z. Y. & Raven P. H.) 85–88 (Science Press, Beijing and Missouri Botanical Garden Press, 1999). [Google Scholar]
- Ai Q. F., Chen M. H. & Liang X. Research progress on Cephalotaxus oliveri. Guizhou Agric. Sci. 3, 55 (2010). [Google Scholar]
- Sima Y. K., Yu H., Yang G. Y. & Zhao W. S. The relation between Yunnan geographic distribution of Cephalotaxus and environment. For. Invent. Plann. 29, 83–87 (2004). [Google Scholar]
- Jiao Y. L., Zhou Z. C., Jin G. Q. & Li Y. G. Cephalotaxus fortunei seed-physiological changes and differences among three seed sources during low temperature priming. J. Zhejiang For. Coll. 24, 173–178 (2007). [Google Scholar]
- Jiao Y. L. et al. Provenance differences for seedling morphology and growth of Cephalotaxus fortunei. For. Res. 19, 452–456 (2006). [Google Scholar]
- Yang C. J. et al. Deep simple morphophysiological dormancy in seeds of the basal taxad Cephalotaxus. Seed Sci. Res. 21, 215–226 (2011). [Google Scholar]
- Wang C. B., Wang T. & Su Y. J. Phylogeography of Cephalotaxus oliveri (Cephalotaxaceae) in relation to habitat heterogeneity, physical barriers and the uplift of the Yungui Plateau. Mol. Phylogen. Evol. 80, 205–216 (2014). [DOI] [PubMed] [Google Scholar]
- Coop G., Witonsky D., Di Rienzo A. & Pritchard J. K. Using environmental correlations to identify loci underlying local adaptation. Genetics 185, 1411–1423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyhäjärvi T., Hufford M. B., Mezmouk S. & Ross-Ibarra J. Complex patterns of local adaptation in teosinte. Genome Biol. Evol. 5, 1594–1609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont M. A. & Balding D. J. Identifying adaptive genetic divergence among populations from genome scans. Mol. Ecol. 13, 969–980 (2004). [DOI] [PubMed] [Google Scholar]
- Foll M. & Gaggiotti O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A bayesian perspective. Genetics 180, 977–993 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont M. A. & Nichols R. A. Evaluating loci for use in the genetic analysis of population structure. Proc. R. Soc. Lond. B-Biol. Sci. 263, 1619–1626 (1996). [Google Scholar]
- Joost S. et al. A spatial analysis method (SAM) to detect candidate loci for selection: towards a landscape genomics approach to adaptation. Mol. Ecol. 16, 3955–3969 (2007). [DOI] [PubMed] [Google Scholar]
- Joost S., Kalbermatten M. & Bonin A. Spatial analysis method(SAM): a software tool combining molecular and environmental data to identify candidate loci for selection. Mol. Ecol. Resour. 8, 957–960 (2008). [DOI] [PubMed] [Google Scholar]
- Fischer M. C., Foll M., Excoffier L. & Heckel G. Enhanced AFLP genome scans detect local adaptation in high-altitude populations of a small rodent (Microtus arvalis). Mol. Ecol. 20, 1450–1462 (2011). [DOI] [PubMed] [Google Scholar]
- Xie C. X. et al. An analysis of population structure and linkage disequilibrium using multilocus data in 187 maize inbred lines. Mol. Breed. 21, 407–418 (2008). [Google Scholar]
- Thorogood C. J., Rumsey F. J., Harris S. A. & Hiscock S. J. Host-driven divergence in the parasitic plant Orobanche minor Sm. (Orobanchaceae). Mol. Ecol. 17, 4289–4303 (2008). [DOI] [PubMed] [Google Scholar]
- Su Y. J., Wang T. & Ouyang P. Y. High genetic differentiation and variation as revealed by ISSR marker in Pseudotaxus chienii (Taxaceae), an old rare conifer endemic to China. Biochem. Syst. Ecol. 37, 579–588 (2009). [Google Scholar]
- Wang T., Chen G. P., Zan Q. J., Wang C. B. & Su Y. J. AFLP genome scan to detect genetic structure and candidate loci under selection for local adaptation of the invasive weed Mikania micrantha. PLos One 7, e41310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J. Plant genotyping: the DNA fingerprinting of plants. (CABI Publishing, 2001). [Google Scholar]
- Mort M. E. et al. Relationships among the Macaronesian members of Tolpis (Asteraceae: Lactuceae) based upon analyses of inter simple sequence repeat (ISSR) markers. Taxon 52, 511–518 (2003). [Google Scholar]
- Storz J. F., Payseur B. A. & Nachman M. W. Genome scans of DNA variability in humans reveal evidence for selective sweeps outside of Africa. Mol. Biol. Evol. 21, 1800–1811 (2004). [DOI] [PubMed] [Google Scholar]
- Minder A. M. & Widmer A. A population genomic analysis of species boundaries: neutral processes, adaptive divergence and introgression between two hybridizing plant species. Mol. Ecol. 17, 1552–1563 (2008). [DOI] [PubMed] [Google Scholar]
- Meyer C. L., Vitalis R., Saumitou-Laprade P. & Castric V. Genomic pattern of adaptive divergence in Arabidopsis halleri, a model species for tolerance to heavy metal. Mol. Ecol. 18, 2050–2062 (2009). [DOI] [PubMed] [Google Scholar]
- Acheré V., Favre J. M., Besnard G. & Jeandroz S. Genomic organization of molecular differentiation in Norway spruce (Picea abies). Mol. Ecol. 14, 3191–3201 (2005). [DOI] [PubMed] [Google Scholar]
- Namroud M. C., Beaulieu J., Juge N., Laroche J. & Bousquet J. Scanning the genome for gene single nucleotide polymorphisms involved in adaptive population differentiation in white spruce. Mol. Ecol. 17, 3599–3613 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. A., Rehfeldt G. E. & Kim M. S. Congruent climate-related genecological responses from molecular markers and quantitative traits for western white pine (Pinus Monticola). Int. J. Plant Sci. 170, 1120–1131 (2009). [Google Scholar]
- Manel S., Poncet B. N., Legendre P., Gugerli F. & Holderegger R. Common factors drive adaptive genetic variation at different spatial scales in Arabis alpina. Mol. Ecol. 19, 3824–3835 (2010). [DOI] [PubMed] [Google Scholar]
- Zulliger D., Schnyder E. & Gugerli F. Are adaptive loci transferable across genomes of related species? Outlier and environmental association analyses in Alpine Brassicaceae species. Mol. Ecol. 22, 1626–1639 (2013). [DOI] [PubMed] [Google Scholar]
- Fang J. Y. et al. Divergent selection and local adaptation in disjunct populations of an endangered conifer, Keteleeria davidiana var. formosana (Pinaceae). PLos One 8, e70162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday J. A., Ritland K. & Aitken S. N. Widespread, ecologically relevant genetic markers developed from association mapping of climate-related traits in Sitka spruce (Picea sitchensis). New Phytol. 188, 501–514 (2010). [DOI] [PubMed] [Google Scholar]
- Mosca E. et al. The geographical and environmental determinants of genetic diversity for four alpine conifers of the European Alps. Mol. Ecol. 21, 5530–5545 (2012). [DOI] [PubMed] [Google Scholar]
- Grivet D., Sebastiani F., Gonzalez-Martinez S. C. & Vendramin G. G. Patterns of polymorphism resulting from long-range colonization in the Mediterranean conifer Aleppo pine. New Phytol. 184, 1016–1028 (2009). [DOI] [PubMed] [Google Scholar]
- Eckert A. J. et al. Back to nature: ecological genomics of loblolly pine (Pinus taeda, Pinaceae). Mol. Ecol. 19, 3789–3805 (2010). [DOI] [PubMed] [Google Scholar]
- Tsumura Y., Uchiyama K., Moriguchi Y., Ueno S. & Ihara-Ujino T. Genome scanning for detecting adaptive genes along environmental gradients in the Japanese conifer. Cryptomeria japonica. Heredity 109, 349–360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigras F. J. & Colombo S. Conifer cold hardiness. (Springer Science & Business Media, 2001). [Google Scholar]
- Blödner C., Skroppa T., Johnsen O. & Polle A. Freezing tolerance in two Norway spruce (Picea abies [L.] Karst.) progenies is physiologically correlated with drought tolerance. J. Plant Physiol. 162, 549–558 (2005). [DOI] [PubMed] [Google Scholar]
- Bower A. D. & Aitken S. N. Ecological genetics and seed transfer guidelines for Pinus albicaulis (Pinaceae). Am. J. Bot. 95, 66–76 (2008). [DOI] [PubMed] [Google Scholar]
- Chen W. et al. The east-west zonal distribution of gymnosperm floras in China and the relationship with the main climatic factors. Acta Sci. Natur. Univ. Sunyatseni 52, 130–139 (2013). [Google Scholar]
- Sork V. L. et al. Gene movement and genetic association with regional climate gradients in California valley oak (Quercus lobata Nee) in the face of climate change. Mol. Ecol. 19, 3806–3823 (2010). [DOI] [PubMed] [Google Scholar]
- Li C., Sun Y., Huang H. W. & Cannon C. H. Footprints of divergent selection in natural populations of Castanopsis fargesii (Fagaceae). Heredity 113, 533–541 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañas R. A. et al. Understanding developmental and adaptive cues in pine through metabolite profiling and co-expression network analysis. J. Exp. Bot. 66, 3113–3127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin M. et al. Linkage disequilibrium-understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 9, 477–485 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale D. B. & Savolainen O. Association genetics of complex traits in conifers. Trends Plant Sci. 9, 325–330 (2004). [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Genetics of the evolutionary process. (Columbia University Press, 1970). [Google Scholar]
- Flint-Garcia A. S., Thornsberry M. J. & Buckler S. E. IV Structure of linkage disequilibrium in plants. Annu. Rev. Plant Biol. 54, 357–74 (2003). [DOI] [PubMed] [Google Scholar]
- Solomon S. et al. IPCC fourth assessment report: the physical science basis. (2007) Available at: http://www.ipcc.ch/publications_and_data/ar4/wg1/en/spmsspm-projections-of.html. (Accessed: 4th May 2015).
- Davis M. B. & Shaw R. G. Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679 (2001). [DOI] [PubMed] [Google Scholar]
- Hamann A. & Wang T. L. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 87, 2773–2786 (2006). [DOI] [PubMed] [Google Scholar]
- Hamrick J. L. et al. Response of forest trees to global environmental changes. For. Ecol. Manage. 197, 323–335 (2004). [Google Scholar]
- Burger R. & Lynch M. Evolution and extinction in a changing environment—a quantitative genetic analysis. Evolution 49, 151–163 (1995). [DOI] [PubMed] [Google Scholar]
- Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G. & Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- Pan H. W., Guo Y. R., Su Y. J. & Wang T. Development of microsatellite loci for Cephalotaxus Oliveri (Cephalotaxaceae) and cross-amplification in Cephalotaxus. Am. J. Bot. 98, e229–e232 (2011). [DOI] [PubMed] [Google Scholar]
- Yeh F. C., Yang R. & Boyle T. POPGENE (version 1.31): Microsoft window-based freeware for population genetic analysis. Department of Renewable Resources, University of Alberta, Edmonton, Canada. URL https://www.ualberta.ca/~fyeh/popgene_download.html/(1999).
- Excoffier L., Laval G. & Schneider S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 1, 47–50 (2005). [PMC free article] [PubMed] [Google Scholar]
- Mantel N. et al. The detection of disease clustering and a generalized regression approach. Cancer Res. 27, 209–220 (1967). [PubMed] [Google Scholar]
- Rousset F. et al. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145, 1219–1228 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89, 583–590 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. P. Tools for Populations Genetic Analyses (TFPGA) (version 1.3): A windows program for the analysis of allozyme and molecular population genetic data. Department of Biological Sciences, Northern Arizona University, Flagstaff, United States of America. URL http://www.marksgeneticsoftware.net/tfpga.htm/ (1997).
- Pritchard J. K., Stephens M. & Donnelly P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Compton S. G. & Chen X. Y. Fragmentation can increase spatial genetic structure without decreasing pollen-mediated gene flow in a wind-pollinated tree. Mol. Ecol. 20, 4421–4432 (2011). [DOI] [PubMed] [Google Scholar]
- Evanno G., Regnaut S. & Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14, 2611–2620 (2005). [DOI] [PubMed] [Google Scholar]
- Bradbury P. J. et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635 (2007). [DOI] [PubMed] [Google Scholar]
- Buckler E., Kroon D., Casstevens T., Bradbury P. & Zhang Z. W. Trait analysis by association, evolution and linkage (TASSEL): user manual. (USDA-ARS and the National Science Foundation, 2009). [Google Scholar]
- Wang Y. H. et al. Genetic structure and linkage disequilibrium in a diverse, representative collection of the C4 model plant, Sorghum bicolor. G3-Genes Genom. Genet. 3, 783–793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. J., Anderson R. P. & Schapire R. E. Maximum entropy modelling of species’ geographic distributions. Ecol. Model. 190, 231–259 (2006). [Google Scholar]
- Ortega-Andrade H. M., Prieto-Torres D. A., Gomez-Lora I. & Lizcano D. J. Ecological and geographical analysis of the distribution of the mountain tapir (Tapirus pinchaque) in Ecuador: importance of protected areas in future scenarios of global warming. PLos One 10, e0121137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel S., Williams H. C. & Ormerod S. J. Evaluating presence-absence models in ecology: the need to account for prevalence. J. Appl. Ecol. 38, 921–931 (2001). [Google Scholar]
- Swets J. A. et al. Measuring the accuracy of diagnostic systems. Science 240, 1285–1293 (1988). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



