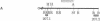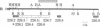Abstract
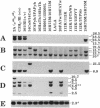
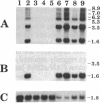
Homozygous deletion of the hepatocyte-specific developmental regulation 1 (hsdr-1) locus in mouse chromosome 7 results in perinatal death and a pleiotropic syndrome characterized by ultrastructural abnormalities of the liver and kidney, failure of induction of a number of specific transcription units in the liver and kidney during late gestation, and marked overexpression of an enzyme that defends against oxidative stress. Previously, the breakpoints of two albino (c) deletions (c14CoS and c1FAFyh) that genetically define hsdr-1 were localized, on a long-range map, in the vicinity of the distal breakpoint of a viable albino deletion (c24R75M) that breaks proximally within the c locus. Here we report the use of a probe derived from a deletion breakpoint fusion fragment cloned from c24R75M/c24R75M DNA to clone a breakpoint fusion fragment caused by the c14CoS deletion. The proximal breakpoint of the c14CoS deletion was discovered to disrupt a gene (Fah) encoding fumarylacetoacetate hydrolase, the last enzyme in the tyrosine degradation pathway. All of the extant c deletions eliciting the hsdr-1 phenotype prevent expression of the Fah gene in the liver, and all but one disrupt the coding segment of the gene. Therefore, the Fah gene maps within or proximal to the hsdr-1 locus, as defined by deletion breakpoints, and disruption of this gene may be partially or completely responsible for the phenotypes associated with the hsdr-1 deletion syndrome. These mouse mutants may also provide models for the human genetic disorder hereditary tyrosinemia, which is associated with fumarylacetoacetate hydrolase deficiency and liver and kidney dysfunction.
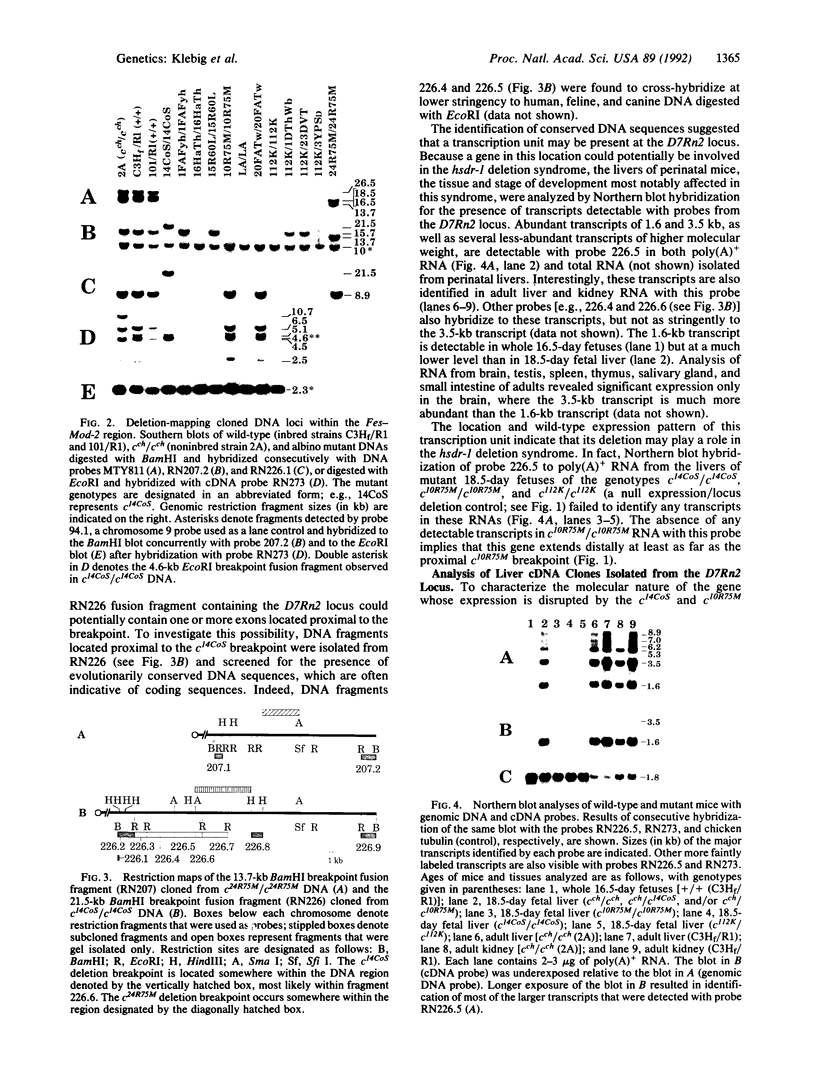
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agsteribbe E., van Faassen H., Hartog M. V., Reversma T., Taanman J. W., Pannekoek H., Evers R. F., Welling G. M., Berger R. Nucleotide sequence of cDNA encoding human fumarylacetoacetase. Nucleic Acids Res. 1990 Apr 11;18(7):1887–1887. doi: 10.1093/nar/18.7.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier L. J., Hanash S. M., Erickson R. P. Mice homozygous for chromosomal deletions at the albino locus region lack specific polypeptides in two-dimensional gels. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2132–2136. doi: 10.1073/pnas.81.7.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P. F., Swift R. A. Double cos site vectors: simplified cosmid cloning. Gene. 1983 Dec;26(2-3):137–146. doi: 10.1016/0378-1119(83)90183-x. [DOI] [PubMed] [Google Scholar]
- DeFranco D., Morris S. M., Jr, Leonard C. M., Gluecksohn-Waelsch S. Metallothionein mRNA expression in mice homozygous for chromosomal deletions around the albino locus. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1161–1164. doi: 10.1073/pnas.85.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner M. E., Leonard C. M., Gluecksohn-Waelsch S. Developmental regulation of constitutive and inducible expression of hepatocyte-specific genes in the mouse. Proc Natl Acad Sci U S A. 1988 May;85(9):3049–3051. doi: 10.1073/pnas.85.9.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. P., Gluecksohn-Waelsch S., Cori C. F. Glucose-6-phosphatase deficiency caused by radiation-induced alleles at the albino locus in the mouse. Proc Natl Acad Sci U S A. 1968 Feb;59(2):437–444. doi: 10.1073/pnas.59.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatmaitan Z., Lewis S., Turchin H., Arias I. M. Premature development of ligandin (GSH transferase B) in mice with an inherited defect in endoplasmic reticulum-Golgi structure and function. Biochem Biophys Res Commun. 1977 Mar 21;75(2):337–341. doi: 10.1016/0006-291x(77)91047-6. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979 Feb;16(2):225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Herrmann B. G., Barlow D. P., Lehrach H. A large inverted duplication allows homologous recombination between chromosomes heterozygous for the proximal t complex inversion. Cell. 1987 Mar 13;48(5):813–825. doi: 10.1016/0092-8674(87)90078-x. [DOI] [PubMed] [Google Scholar]
- Klebig M. L., Kwon B. S., Rinchik E. M. Physical analysis of murine albino deletions that disrupt liver-specific gene regulation or mesoderm development. Mamm Genome. 1992;2(1):51–63. doi: 10.1007/BF00570440. [DOI] [PubMed] [Google Scholar]
- Kvittingen E. A. Hereditary tyrosinemia type I--an overview. Scand J Clin Lab Invest Suppl. 1986;184:27–34. [PubMed] [Google Scholar]
- Lindblad B., Lindstedt S., Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe S., Sassa S., Kappas A. Hereditary tyrosinemia. Formation of succinylacetone-amino acid adducts. J Exp Med. 1985 Sep 1;162(3):1060–1074. doi: 10.1084/jem.162.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Lane M. D., Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989 Dec;3(12B):2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- Mitchell G., Larochelle J., Lambert M., Michaud J., Grenier A., Ogier H., Gauthier M., Lacroix J., Vanasse M., Larbrisseau A. Neurologic crises in hereditary tyrosinemia. N Engl J Med. 1990 Feb 15;322(7):432–437. doi: 10.1056/NEJM199002153220704. [DOI] [PubMed] [Google Scholar]
- Morris S. M., Jr, Moncman C. L., Kepka D. M., Nebes V. L., Diven W. F., Dizikes G. J., Cederbaum S. D., DeFranco D. Effects of deletions in mouse chromosome 7 on expression of genes encoding the urea-cycle enzymes and phosphoenolpyruvate carboxykinase (GTP) in liver, kidney, and intestine. Biochem Genet. 1988 Dec;26(11-12):769–781. doi: 10.1007/BF02395522. [DOI] [PubMed] [Google Scholar]
- Petersen D. D., Gonzalez F. J., Rapic V., Kozak C. A., Lee J. Y., Jones J. E., Nebert D. W. Marked increases in hepatic NAD(P)H:oxidoreductase gene transcription and mRNA levels correlated with a mouse chromosome 7 deletion. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6699–6703. doi: 10.1073/pnas.86.17.6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaneuf D., Labelle Y., Bérubé D., Arden K., Cavenee W., Gagné R., Tanguay R. M. Cloning and expression of the cDNA encoding human fumarylacetoacetate hydrolase, the enzyme deficient in hereditary tyrosinemia: assignment of the gene to chromosome 15. Am J Hum Genet. 1991 Mar;48(3):525–535. [PMC free article] [PubMed] [Google Scholar]
- Ruppert S., Boshart M., Bosch F. X., Schmid W., Fournier R. E., Schütz G. Two genetically defined trans-acting loci coordinately regulate overlapping sets of liver-specific genes. Cell. 1990 Jun 1;61(5):895–904. doi: 10.1016/0092-8674(90)90200-x. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Müller G., Kwon B., Schütz G. Multiple transcripts of the mouse tyrosinase gene are generated by alternative splicing. EMBO J. 1988 Sep;7(9):2715–2722. doi: 10.1002/j.1460-2075.1988.tb03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B., Montgomery C. S., Raymer G. D. Analysis of the albino-locus region of the mouse: IV. Characterization of 34 deficiencies. Genetics. 1982 Mar;100(3):427–453. doi: 10.1093/genetics/100.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharan S. K., Holdener-Kenny B., Ruppert S., Schedl A., Kelsey G., Rinchik E. M., Magnuson T. The albino-deletion complex of the mouse: molecular mapping of deletion breakpoints that define regions necessary for development of the embryonic and extraembryonic ectoderm. Genetics. 1991 Nov;129(3):825–832. doi: 10.1093/genetics/129.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay R. M., Valet J. P., Lescault A., Duband J. L., Laberge C., Lettre F., Plante M. Different molecular basis for fumarylacetoacetate hydrolase deficiency in the two clinical forms of hereditary tyrosinemia (type I). Am J Hum Genet. 1990 Aug;47(2):308–316. [PMC free article] [PubMed] [Google Scholar]
- Thaler M. M., Erickson R. P., Pelger A. Genetically determined abnormalities of microsomal enzymes in liver of mutant newborn mice. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1244–1250. doi: 10.1016/s0006-291x(76)80148-9. [DOI] [PubMed] [Google Scholar]
- Trigg M. J., Gluecksohn-Waelsch S. Ultrastructural basis of biochemical effects in a series of lethal alleles in the mouse. Neonatal and developmental studies. J Cell Biol. 1973 Sep;58(3):549–563. doi: 10.1083/jcb.58.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]