Abstract
Epidemiologic evidence suggested chronic obstructive pulmonary disease (COPD) might increase risk for abdominal aortic aneurysm (AAA). However, the association between COPD and AAA remains inconclusive. We searched PubMed and Cochrane databases until June 2015. Forty-eight articles were included for meta-analysis. COPD was found to be positively associated with AAA, regardless of study design and smoking status. AAA mortality is higher among COPD patients compared with non-COPD patients (postoperative [adjusted OR 2.11; 95% CI 1.33–3.34]; long-term [adjusted OR 1.70; 95% CI 1.37–2.12]). But the association between postoperative mortality and COPD was not found to be significant in patients underwent endovascular aneurysm repair (mixed OR 2.53; 95% CI 0.70–9.18). Rupture AAA may increase the postoperative mortality in COPD patients (rupture [adjusted OR 4.75; 95% CI 2.07–10.89]; non-rupture [adjusted OR 1.97; 95% CI 1.11–3.49]). The AAA postoperative morbidity was found to be positively associated with COPD (adjusted OR 1.59; 95% CI 1.14–2.21). Increased COPD severity may increase the long-term mortality (medical versus oxygen dependent: [OR 1.26; 95% CI 1.07–1.49] versus [OR 2.79; 95% CI 2.24–3.49]). In conclusion, COPD may increase the risk of AAA, morbidity and mortality of AAA patients underwent endovascular aortic repair.
Epidemiological data revealed that chronic obstructive pulmonary disease (COPD) affects 34–200 out of 1000 patients older than 65 years1. According to the Global Burden of Disease (GBD) 2010, COPD affects approximately 329 million people (4.8% of the population) in the world as of 20102, and is the fourth leading cause of morbidity and mortality in the United States3. Some population-based screenings (PBS) indicated that the prevalence of abdominal aortic aneurysm (AAA) among COPD patients was 4% to 6%4,5,6,7,8. Other studies found even higher prevalence varied from 7% to 11%9,10,11. The above evidence suggested that COPD might be a risk factor for AAA. Smoking and increased elastolytic activity in lung and aorta are found to be strongly associated with both COPD and AAA10,12,13,14,15,16. The causal relationship between smoking and COPD has been reported in previous studies; however, smoking was only mentioned as a risk factor of AAA. The precise mechanism about how COPD can influence the development and prognosis of AAA remains to be unclear. Previous studies revealed possible association between COPD and AAA4,6,17,18 adjusting for smoking or not. To our knowledge no study up to date has focused on the comprehensive and systematic analysis of this issue and no conclusion has been drawn. Moreover, there is also limited data on AAA postoperative clinical outcome among COPD patients. To better understand this issue, we performed a meta-analysis to investigate the influence of COPD on prevalence of AAA, as well as the AAA postoperative mortality and morbidity among COPD patients.
Methods
Data Sources and Search Strategy
PubMed and Cochrane databases were searched to identify studies published from January 1982 to June 2015 (ultrasound technology was not available before 198219). A combination of the following search terms was used: “abdominal aortic aneurysm”, “chronic obstructive pulmonary disease”, “chronic obstructive lung disease”, “chronic obstructive airway disease” and “emphysema”. We restricted our research to human studies and published in English language. The review articles were initially involved for identifying potential eligible data, and were not included for meta-analysis. Two authors independently reviewed each potential study for eligibility. The reference list of eligible studies was screened to further identify relevant studies by a manual search. Type of study design was not restricted.
Inclusion Criteria
Studies were included if they: (1) reported odd ratio (OR), relative risk (RR) or hazard rate (HR) of AAA, or if the original data was available for calculating prevalence or incidence of AAA (i.e., for assessing prevalence of AAA in COPD patients); (2) focused on infrarenal AAA patients who received endovascular aneurysm repair (EVAR) or open repair (i.e., for examination of AAA postoperative outcomes among COPD patients).
Exclusion Criteria
Studies were excluded if they reported on suprarenal AAA or thoracoabdominal aneurysm, or thoracoabdominal approaches. Additionally, the following were excluded: nonhuman studies, studies published prior to 1982, studies with data duplicated, editorials, case reports and letters.
Data Extraction and Quality Assessment
The following data were independently extracted by two researchers (J.X., Z.W.): study design, AAA screening method, country of origin, age, gender, intervention strategy, duration of follow-up, number of defined AAA and COPD patients, outcomes (e.g., 30-day mortality, long-term mortality, 30-day morbidity, long-term morbidity), and type of analysis (univariate or multivariate calculations). Discrepancies in data extraction were resolved through discussion. Study quality was quantified based on the Newcastle-Ottawa Scale20. According to this assessment scale, all the selected cohort and case-control studies were evaluated on three groups of items, including: (1) the selection of study; (2) the comparability of the groups; and (3) the ascertainment of outcomes of interest. The scale uses a star system for assessment. Stars were awarded for each quality item, with a maximum of 9 stars. Studies with five stars or more were considered to be “good” in quality. Studies with four stars or less were considered to be “fair” in quality.
Statistical Analysis
Studies were separately analyzed according to whether they reported on prevalence/incidence or clinical outcomes using RevMan v.5.0 (The Cochrance Collaboration, http://ims.cochrane.org/revman) and STATA 12 software (StataCorp, College Station, Texas, USA). The overall estimates, such as RR, HR or OR with corresponding 95% confidence intervals (CIs), were combined in a meta-analysis using the generic inverse variance option in RevMan to obtain a pooled result. Because the prevalence of AAA in COPD patients was low (less than 10%), we assumed similarity between the OR, RR and HR. The adjusted estimates of risk were employed when available. When data had been presented in original form, we input total and number of events into RevMan 5.0 for calculating estimates and then used the generic inverse variance method. The meta-analysis was performed using the Mantel-Haenszel method. We used I2 values and χ2 statistic to assess heterogeneity. If I2 > 50% significant heterogeneity between-study were indicated, and random-effects models were used to estimate pooled effect sizes; otherwise, fixed-effect models were applied. All pooled estimates of risk were reported with 95% CIs. If meta-analysis included 10 studies or more, potential publication bias was evaluated by using the Egger test, and the results were represented graphically with Begg funnel plots of the natural log of the OR versus its standard error.
Sensitivity Analysis
Prespecified sensitivity analysis was conducted for studies reporting different treatment (open repair or EVAR), different hemodynamic status (ruptured or non-ruptured) and different grades of COPD (untreated, medical or oxygen dependent) where they were available.
Results
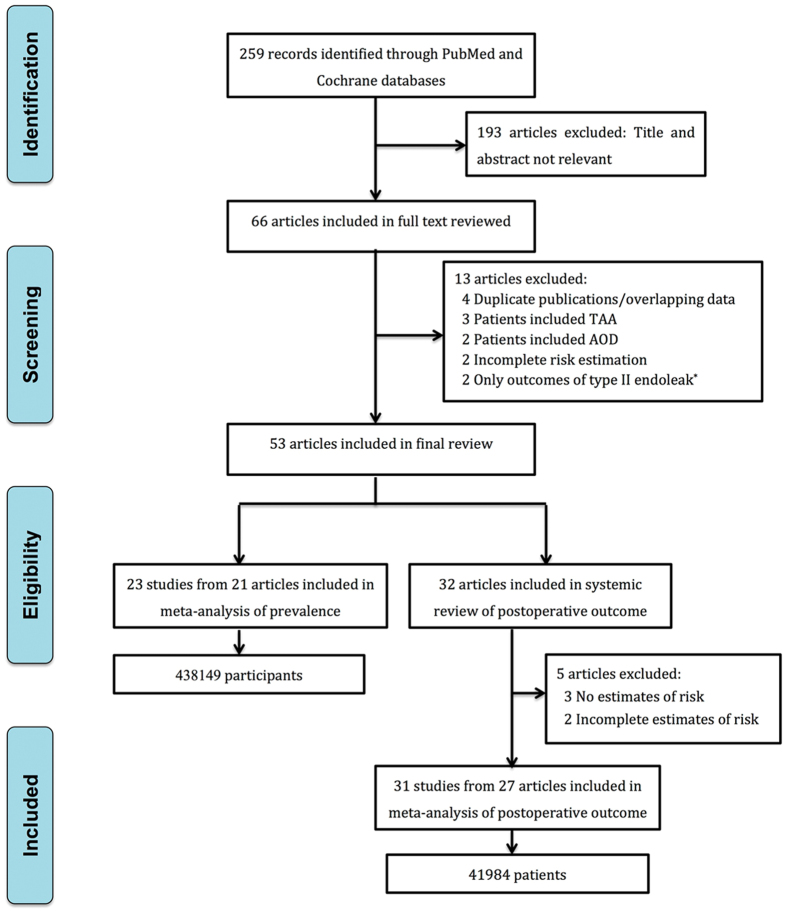
A total of 259 records were identified by the initial search strategy (Fig. 1). After screening the titles and abstracts, 66 articles were selected to be further reviewed. Among those 66 articles, 13 articles were excluded (Fig. 1). Of the selected 53 articles, 21 reported on the prevalence of AAA in COPD patients and included 438149 participants, and 32 articles provided data on clinical outcomes of endovascular aortic repair (EVAR) or open surgery among COPD patients. Majority included articles (n = 43) were ranked as “good” in methodological quality, and the other ten articles were considered to have fair methodological quality (Online Table 1).
Figure 1. Flow chart of articles included in the meta-analysis.
TAA: thoracic aortic aneurysm; AOD: aortic occlusive disease. *The type II endoleak was not considered as the complication.
Prevalence of AAA in COPD
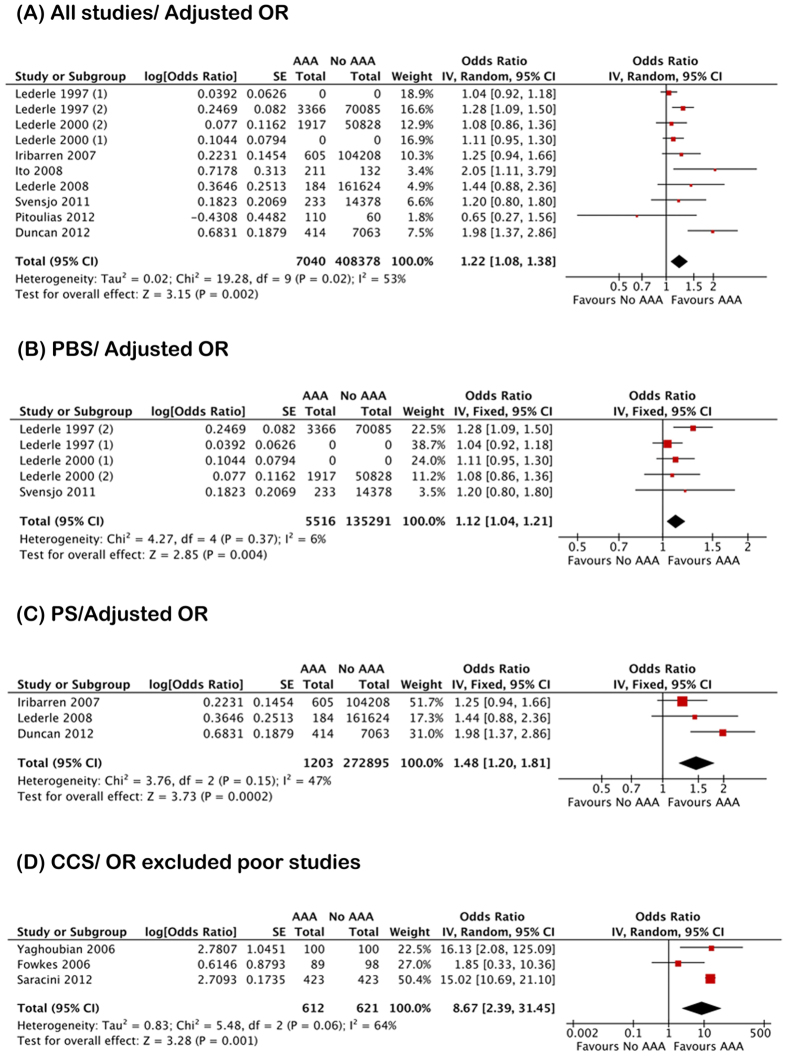
Twenty-three studies from 21 articles reported the prevalence of AAA in COPD. According to the categorization of studies based on AAA diameter (3.0–3.9 cm vs. ≥4.0 cm), four studies were extracted from two of Lederle’s articles. The main characteristics of the 23 studies were listed in Table 1. Based on those 23 studies (AAA/Non-AAA: 8869/429280), when combining the adjusted and unadjusted data, the pooled estimates indicated a significant positive association between COPD and the prevalence of AAA (OR 1.78; 95% CI 1.35–2.35; I2 93%) (Online Fig. 1A). The possibility of publication bias was low (P = 0.143) (Online Fig. 2A). Based on the ten studies which reported adjusted OR (AAA/Non-AAA: 7040/408378), a pooled adjusted estimate indicated a slight but significant positive association between COPD and prevalence of AAA (OR 1.22; 95% CI 1.08–1.38; I2 53%) (Fig. 2A). The possibility of publication bias was low (P = 0.193) (Online Fig. 2B).
Table 1. Abdominal aortic aneurysms (AAA) and chronic obstructive pulmonary disease (COPD): Prevalence and incidence studies.
| Study (published year) | Study type | Male (%) | Age | Region | AAA (case/total) | No AAA (case/control) | Reported OR (95% CI) | P | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Lederle et al. (1988) | PBS | 100 | ≥60 | US | 1/18 | 7/183 | NA | NA | |
| Smith et al. (1993) | PBS | 100 | ≥65 | UK | 28/219 | 205/2378 | NA | <0.05 | |
| Simoni et al. (1995) | PBS | 46 | ≥65 | Italy | 22/70 | 126/1504 | NA | <0.001 | |
| Lederle et al. (1997) (1)¶ | PBS | 97 | ≥50 | US | NA | NA | 1.04 (0.92–1.16)* | NA | AAA (3.0–3.9cm) |
| Lederle et al. (1997) (2)¶ | PBS | 97 | ≥50 | US | NA | NA | 1.28 (1.09–1.50)* | NA | AAA (≥4.0cm) |
| Lindholt et al. (1998) | PBS | 100 | ≥65 | Denmark | 16/139 | 264/4265 | 2.05 (1.14–3.62) | 0.01 | |
| Lederle et al. (2000) (1)§ | PBS | 97 | ≥50 | US | NA | NA | 1.11 (0.95–1.30)* | NA | AAA (3.0–3.9cm) |
| Lederle et al. (2000) (2)§ | PBS | 97 | ≥50 | US | NA | NA | 1.08 (0.86–1.36)* | NA | AAA (≥4.0cm) |
| Shteinberg et al. (2000) | CCS | 88 | ≥43 | Israel | 28/82 | 19/73 | NA | NS | AAA vs. PAOD |
| Petersen et al. (2002) | CCS | 70 | ≥50 | Sweden | 2/10 | 3/30 | NA | NA | AAA vs (AOD + SCS + NAM) |
| Barba et al. (2005) | CCS | 93.7 | NA | Spain | 40/151 | 184/1015 | NA | 0.015 | AAA vs. PAOD |
| Fowkes et al. (2006) | CCS | 72 | 74m | UK | 87/89 | 94/98 | NA | NA | AAA vs. No |
| Yaghoubian et al. (2006) | CCS | 91 | 74m | US | 14/100 | 1/100 | 16.1 (2.08–125.1) | 0.0005 | AAA vs. No |
| Iribarren et al. (2007) | PS | 45 | >18 | US | NA | NA | 1.25 (0.94–1.65)* | NA | AAA/No AAA: 605/104208 |
| Koksal et al. (2007) | CCS | NA | ≥47 | Turkey | 7/40 | 9/40 | NA | NA | AAA vs. AOD |
| Ito et al. (2008) | CCS | 78.7 | 73m | Japan | 63/211 | 20/132 | 2.38 (1.28–4.26); 2.05 (1.11–3.89)* | 0.002; 0.025* | AAA vs. TAA |
| Lederle et al. (2008) | PS | 0 | ≥50 | US | 22/184 | 5657/161624 | 1.44 (0.88–2.37)* | NA | |
| Svensjo et al. (2011) | PBS | 100 | 65 | Sweden | 21/233 | 920/14378 | 1.2 (0.8–1.9)* | 0.44* | |
| Duncan et al. (2012) | PS | 100 | ≥65 | UK | 73/414 | 623/7063 | 3.13 (2.28–4.30);1.98 (1.37–2.86)* | NA | |
| Pitoulias et al. (2012) | CCS | 88.2 | ≥45 | Greece | 35/110 | 10/60 | 0.43 (0.20–0.94);0.65 (0.27–1.58)* | 0.035 0.34* | AAA vs. AOD |
| Saracini et al. (2012) | CCS | 87.7 | ≥40 | Italy | 311/423 | 66/423 | NA | NA | AAA vs. No |
| Svensjo et al. (2013) | PBS | 0 | 70 | Sweden | 3/19 | 447/5120 | 1.96 (0.57–6.75) | 0.277 | |
| Chun et al. (2014) | PBS | 99.6 | ≥50 | US | 122/469 | 647/5673 | 1.75 (1.41–2.18) | <0.001 |
PBS: population based screening; CCS: case control study; PS: prospective study; NA: not available, NS: not significant; AOD: aortic occlusive disease; SCS: plaque ulceration; NAM: no similar specific manifestations of atherosclerotic disease; PAOD: peripheral artery occlusive disease; TAA: thoracic aorta aneurysm; ¶: accumulated data in the two studies (AAA/No AAA: 3366/70085); §: accumulated data in the two studies (AAA/No AAA: 1917/50828); m mean; *adjusted data available.
Reference in supplemental files.
Figure 2. The association between chronic obstructive pulmonary disease and abdominal aortic aneurysm: Pooled adjusted odd ratio.
(A) All studies. (B) Population based screenings. (C) Prospective studies. (D) Case-control studies excluded poor studies.
According to the study type, AAA prevalence data were separately extracted from 11 PBS, three prospective studies (PS) and 9 case-control studies (CCS). If adjusted data were employed when available, the pooled analysis from the 11 PBS (AAA/Non-AAA: 6450/154414) revealed a significant positive association between COPD and AAA (OR 1.56; 95% CI 1.21–2.00; I2 89%) (Online Fig.1B). The possibility of publication bias was low (P = 0.151) (Online Fig. 2C). Based on five PBS with adjusted estimates (AAA/Non-AAA: 5516/135291), the pooled result indicated a slight positive association between COPD and the prevalence of AAA (OR 1.12; 95% CI 1.04–1.21; I2 6%) (Fig. 2B). A strong positive association (OR 1.48; 95% CI 1.20–1.81; I2 47%) was found in the pooled adjusted estimates based on 3 PS (AAA/No AAA: 1203/272895) (Fig. 2C). However, the pooled result from the 9 CCS (AAA/No AAA: 1216/1971) demonstrated no significant association between COPD and AAA (OR 2.27; 95% CI 0.90–5.76; I2 93%) (Online Fig.1C). After excluding 6 studies with poor control group (5 studies with arteriosclerosis and 1 study with TAA) from the analysis, the pooled result based on the remaining 3 studies (AAA/Non-AAA: 612/621) suggested a strongly positive association between COPD and AAA (OR 8.66; 95% CI 2.39–31.44; I2 64%) (Fig. 2D).
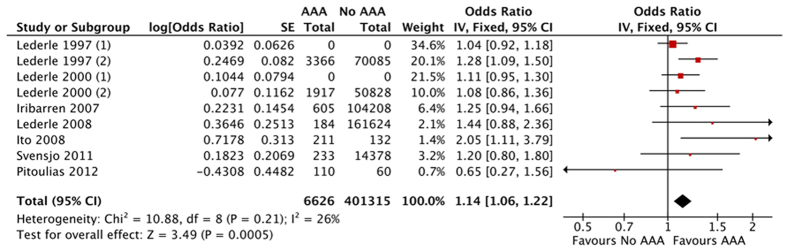
Nine studies (AAA/Non-AAA: 6626/401315) reported data adjusted for smoking status. The pooled result showed that COPD was positive associated with AAA with adjustment of smoking status (OR 1.14, 95% CI 1.06–1.22, I2 26%) (Fig. 3).
Figure 3. The association between abdominal aortic aneurysm and chronic obstructive pulmonary disease: Pooled odd ratio adjusted smoking status.
Clinical outcomes in AAA patients with COPD
Twenty-seven articles were included for estimation of postoperative clinical outcome of AAA in COPD patients. Based on the purpose of this meta-analysis, we identified 9 studies from 4 of the articles according to disease characteristics and type of surgery. Finally, 31 studies from 27 articles were identified for meta-analysis of postoperative outcome, and a total of 41984 patients were included (Table 2).
Table 2. Postoperative outcome in chronic obstructive pulmonary disease (COPD) and non-COPD patients with abdominal aortic aneurysms (AAA).
| Study (published year) | Study type | Male (%) | Age | Region | AAA | 30-d mortality | Long term mortality | 30-d morbidity | Long-term morbidity | Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Crawford et al. (1986) | PBS | 85.1 | 28–85 | US | 101 elective open COPD: 34 Non-COPD: 67 | 30-d 1 (2.9%) 7 (10.4%) (P = 0.2611) | 30-d Dialysis 2 (5.9%) 5 (7.5%) (P = 1.000) | |||
| Katz et al. (1994) | PBS | 82 | ≥50 | US | 8185 open COPD: 1792 Non-COPD: 6393 | In-hospital 142 (7.9%) 473 (7.4%) (P = 0.6) | Elective: 8185 Rupture: 1829 | |||
| Koskas et al. (1997) | PS | 89.9 | 42–92 | France | 158 elective open COPD: 51 Non-COPD: 107 | 5.3-y (P < 0.01) | ||||
| Eskandari et al. (oxygen) | CCS | 76.9 | 72m | US | 65 elective open COPD: 14 Non-COPD: 51 | In-hospital 4 (28.6%) 18 (35.3%) | Oxygen dependent COPD | |||
| Cuypers et al. (2000) | PBS | 91.8 | 70m | Netherland | 1871 elective EVAR COPD: 683 Non-COPD: 1188 | 1.5-y Conversion 27 (4.0%) 22 (1.9%) OR 2.22 (1.12–4.37)* | ||||
| Axelrod et al. (2001) | PBS | 100 | 69m | US | ||||||
| Rupture | 52 open rupture COPD: 20 Non-COPD: 32 | 30-d 8 (40.0%) 2 (6.3%) | ||||||||
| No-rupture | 1001 elective open COPD: 244 Non-COPD: 757 | 30-d 9 (3.7%) 28 (3.7%) OR 1.1(P = 0.81)* | 30-d OR 2.3 (P = 0.07)*In ventilation >96 h | |||||||
| Huber et al. (2001) | PBS | 79.7 | 72m | US | 16450 elective open | In-hospital OR 1.0; 95%CI 0.9–1.3 (P = 0.67)* | In-hospital OR 1.3; 95% CI 1.1–1.4 (P < 0.0001)* | |||
| Biancari et al. (2002) | PBS | 90.4 | 66m | Finland | 208 EVAR COPD: 54 Non-COPD: 154 | 15-y (P = 0.001) RR 1.76, 95% CI 1.22–2.44 (P = 0.002)* | Elective: 167; Emergency for no rupture: 9; Emergency for rupture: 32 | |||
| Piper et al. (2003) | PBS | 68.4 | 72m | US | 147 open rupture COPD: 35 Non-COPD: 112 | In-hospital 9 (25.7%) 42 (37.5%) | ||||
| Tassiopoulos et al. (2004) | PBS | 81.7 | 71m | US | 115 elective open COPD: 22 Non-COPD: 93 | In-hospital Ventilator time (P = 0.12) ICU stay (P = 0.015) Postoperative ileus (NS) Hospital stay (P = 0.03) | ||||
| Hertzer et al. (2005) | PBS | 86 | ≥47 | US | 855 elective open COPD: 53 Non-COPD: 802 | 30-d OR 3.8; 95% CI 1.2–11.6 (P = 0.036) OR 5.1; 95% CI 1.6–16.5 (P = 0.0006)* | 15-y HR 1.55; 95% CI 1.11–2.17 (P = 0.016) HR 1.6 95% CI 1.1–2.2 (P = 0.012)* | HR: any death (postoperative and late death) | ||
| Hua et al. (2005) | PS | 81.8 | 72m | US | 1042 elective EVAR and open COPD: 221 Non-COPD: 821 | 30-d OR 1.67; 95%CI 0.81–3.44 (P = 0.17) | 30-d OR 1.42; 95CI% 1.03–1.94 (P = 0.03) OR 1.32; 95%CI 0.91–1.91 (P = 0.15)* | EVAR: 460 Open: 582 | ||
| Schouten et al. (2006) | PBS | 86 | 70m | Netherland | 500 elective open COPD: 31 Non-COPD: 469 | 30-d OR 1.83; 95% CI 0.86–3.87 (P = 0.12) OR 1.50; 95% CI 0.63–3.58 (P = 0.36)* | 30-d OR 1.91; 95% CI 0.93–3.93(P = 0.08) OR 1.81; 0.85–3.89 (P = 0.13)* | |||
| Zarins et al. (2006) | PS | 88.3 | 74m | US | 923 elective EVAR COPD: 90 Non-COPD: 833 | 5-y HR 1.84 (P < 0.0001)*AAA related death HR 1.75 (P = 0.18)* | 5-y AAA rupture HR 0.987 (P = 0.98)*Conversion HR 1.07 (P = 0.18)* | |||
| Anain et al. (2007) | PBS | 75 | 57–89 | US | 40 EVAR and open rupture COPD: 17 Non-COPD: 23 | 30-d 5 (29.4%) 4 (17.4%) | EVAR: 30 Open: 10 | |||
| Bonardelli et al. (2007) | PBS | 93.5 | NA | Italy | 1111 elective open COPD: 428 Non-COPD: 683 | 30-d 14 (3.3%) 16 (2.3%) (P = 0.35) | 5-y (P < 0.001) (P = 0.014)* | |||
| Berge et al. (2008) | PBS | 82.9 | 71m | US | 1041 EVAR and open COPD: 144 Non-COPD: 897 | 30-d Open OR 1.2; 95% CI 0.7–2.1 (P = 0.48)* | 20-y OR 1.5; 95% CI 1.17–1.92 (P = 0.001)* | EVAR: 136 Open rupture: 299; Open no rupture: 606 | ||
| Botha et al. (2008) | PBS | 80.6 | 76m | Australia | 62 open rupture COPD: 17 Non-COPD: 45 | In-hospital 8 (47.1%) 12 (26.7%) OR 6.7; 95% CI 1.1–41.3 (P = 0.04)* | ||||
| Park et al. (2008) | CCS | 88.6 | 76m | US | 342 elective EVAR COPD: 137 Non-COPD: 205 | In-hospital 3 (2.2%) 4 (2.0%) (P = 1.0) | In-hospital 18 (40.1%) 18 (43.9%) | Long-term mortality: COPD (CHF: 3; MI:1;; RC: 14); Non-COPD (CHF: 2; MI: 2; RC: 14) | ||
| Abedi et al. (2009) | PBS | 82.3 | 74m | US | 3662 elective EVAR COPD: 700 Non-COPD: 2962 | 30-d OR 1.31 (P < 0.05)*in postoperative transfusion 4u. w/in 72 h of procedure OR 2.28 (P < 0.05)* | ||||
| Antonello et al. (2009) | PBS | 79.6 | 47–91 | Italy | 103 open rupture COPD: 35 Non-COPD: 68 | 30-d 15 (42.9%) 15 (22.1%) (P = 0.028) | ||||
| Beck et al. (2009) | PS | 71 | 74m | US | ||||||
| Open | 748 elective open COPD: 269 Non-COPD: 479 | 1-y P = 0.002 HR 3.6; 95% CI 1.9–7.0* | ||||||||
| EVAR | 639 elective EVAR COPD: 249 Non-COPD: 390 | 1-y P = 0.007 | ||||||||
| Holst et al. (2009) | PBS | 86 | 76m | Sweden | 90 EVAR rupture COPD: 28 Non-COPD: 62 | 30-d RR 4.3; 95%CI 1.7–11.0 (P = 0.003)* | ||||
| Mastracci et al. (2010) | PS | 88 | 75m | US | 412 elective EVAR COPD: 155 Non-COPD: 257 | 6-y 106 (68.4%) 136 (52.9%) HR1.6; 95% CI 1.3–2.1 (P = 0.002) HR 1.6; 95% CI 1.2–2.2 (P = 0.001)* | ||||
| Geisbüsch et al. (2011) | PBS | 85 | >80 | US, Germany | 299 elective EVAR COPD: 90 Non-COPD: 209 | 6.8-y HR 1.56; 95% CI 1.03–2.35 (P = 0.032)* | ||||
| Twine et al. (2011) | PBS | 92.7 | 55–88 | UK | 178 elective EVAR COPD: 33 Non-COPD: 145 | 3-y HR 0.42; 95% CI 0.25–0.72 (P = 0.002) | ||||
| Wisniowski et al. (2011) | PBS | 87.3 | 73m | Australia | 197 elective EVAR COPD: 39 Non-COPD: 158 | 3-y (P = 0.027) (P = 0.035)* | ||||
| Gupta et al. (2012) | PBS | 72.5 | 66–78 | US | 598 elective open COPD: 122 Non-COPD: 476 | 30-d 11 (9.0%) 16 (3.4%) OR 3.17; 95% CI 1.39–7.25* | 30-d Major complication: 48 (39.3%) 132 (27.7%) (P = 0.01) | |||
| Ohrlander et al. (2012) | PS | 86 | 53–89 | Sweden | 233 elective EVAR COPD: 95 Non-COPD: 138 | 5-y 38 (40.0%) 39 (28.3%) (P = 0.058) | Long-term mortality COPD (grade ≥2) (P = 0.040) COPD (grade ≥3) (P = 0.016) PaO2 < 8.0 kPa or COPD, grade ≥3 (P = 0.005) HR 2.06; 95% CI 1.24–3.42 (P = 0.005)* | |||
| De Martino et al. (2013) | PBS | 79.3 | 67–82 | US | 2367 elective EVAR and open | |||||
| Untreated | 1897 elective EVAR and open COPD: 336 Non-COPD: 1562 | 5-y OR 1; 95% CI 0.7–1.3* | EVAR: 1303 Open: 594 Untreated COPD | |||||||
| Medical | 1936 elective EVAR and open COPD: 374 Non-COPD: 1562 | 5-y OR 1.3; 95% CI 1.0–1.8* | EVAR: 1355 Open: 579 Medication COPD | |||||||
| Oxygen | 1659 elective EVAR and open COPD: 97 Non-COPD: 1562 | 5-y OR 3.0; 95% CI 2.0–4.5* | EVAR: 1165 Open: 493 Oxygen dependent COPD | |||||||
| Stone et al. (2013) | PS | 78.1 | 72m | US | ||||||
| Medical | 3320 elective EVAR and open COPD: 1082 Non-COPD: 2238 | 5-y HR 1.22; 95% CI 1–1.5 (P = 0.02)* | EVAR: 2043 Open: 1412 Medical COPD | |||||||
| Oxygen | 2373 elective EVAR and open COPD: 135 Non-COPD: 2238 | In-hospital OR 2.02; 95% CI 1.0–4.0 (P = 0.04)* | 5-y HR 3.02; 95%C 2.2–4.1 (P < 0.001)* | Oxygen dependent COPD | ||||||
| Raux et al. (2014) | CCS | 76.6% | 73m | US and France | 563 elective FEVAR and open | 30-d OR 3.3; 95%CI 1.7–6.7 (P = 0.0008)* |
PBS: population based screening; CCS: case control study; PS: prospective study; COPD: chronic obstructive pulmonary disease; EVAR: endovascular aortic repair; OR: odd ratio; RR: relative risk; HR: hazard rate; CI: confidence interval; SDD: same day discharge; POD: postoperative discharge; CHF: congestive heart failure; MI: myocardial infarction; RC: respiratory complications; ICU: intensive care unit; NS: not significant; NA: not available; O2: oxygen; m mean; *adjusted data available.
Reference in supplemental files.
30-day/in-hospital mortality
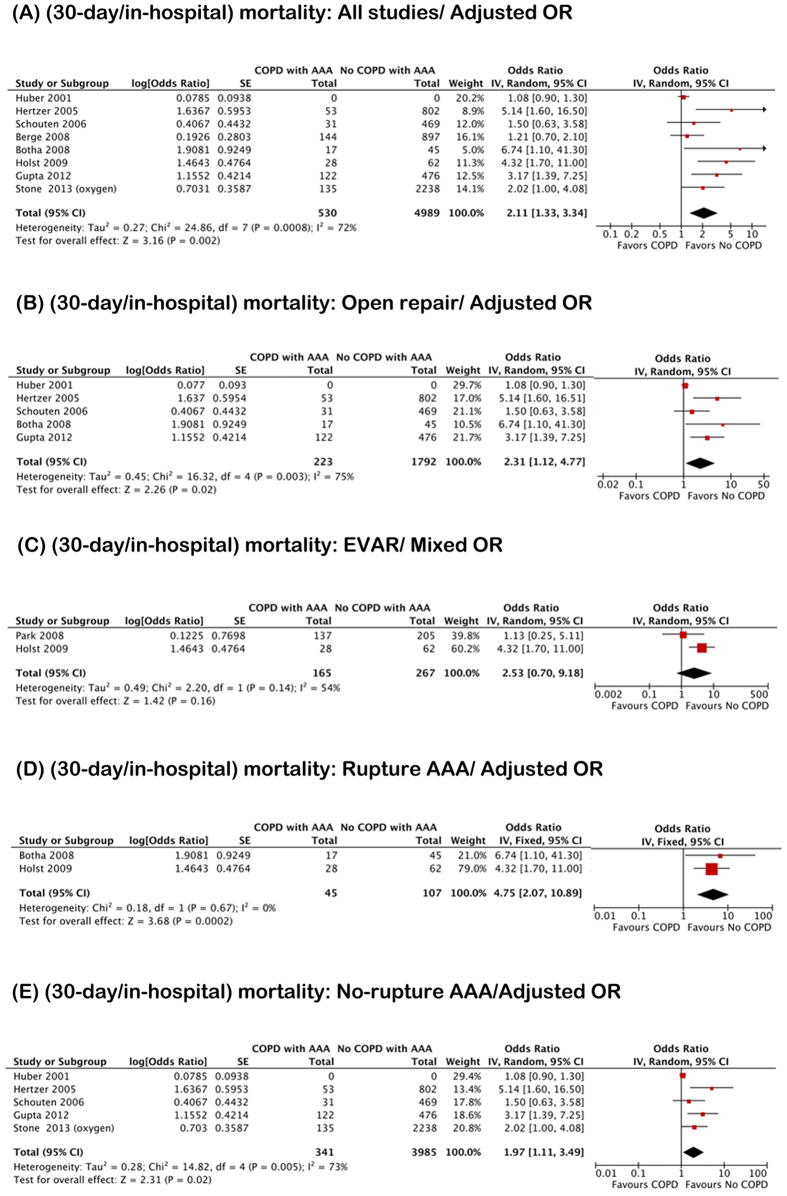
Eighteen studies (COPD/Non-COPD: 3493/14150) reported 30-day/in-hospital mortality of AAA after repair. The in-hospital mortality was found in 6 studies and 30-day mortality in 12 studies. When combining adjusted and unadjusted data, the pooled result suggested that COPD was positively associated with 30-day/in-hospital mortality of AAA after repair (OR 1.58; 95% CI 1.22–2.03; I2 60%) (Online Fig. 3A). The possibility of publication bias was high (P = 0.01). (Online Fig. 2D). If analysis was restricted to studies providing adjusted data (COPD/No-COPD: 530/4989), pooled analysis showed an increased 30-day/in-hospital mortality in COPD patients compared with non-COPD patients (OR 2.11; 95% CI 1.33–3.34; I2 72%) (Fig. 4A).
Figure 4. Cumulative operative mortality in abdominal aortic aneurysm (AAA) patients with chronic obstructive pulmonary disease: Pooled adjusted/mixed odd ratio.
(A) All studies. (B) Studies with AAA accepted open repair. (C) Studies with AAA accepted endovascular aneurysm repair. (D) Studies with rupture AAA. (E) Studies with no rupture AAA.
To evaluate the treatment effect on AAA clinic outcome, the studies were divided into two subgroups according to type of treatment (open repair vs. EVAR). The data for estimating 30-day/in-hospital mortality of AAA after open repair was identified from 13 studies (COPD/Non-COPD: 2955/10801). The pooled result (including both adjusted and unadjusted data) suggested that COPD was strongly and positively associated with the 30-day/in-hospital mortality of AAA after open repair (OR 1.43; 95% CI 1.08–1.90; I2 62%) (Online Fig. 3B). The possibility of publication bias was low (P = 0.057) (Online Fig. 2E). If analysis was restricted to the 5 studies with adjusted data (COPD/Non-COPD: 223/1792), pooled analysis showed an increased 30-day/in-hospital mortality rate in COPD patients compared with non-COPD patients (OR 2.31; 95% CI 1.12–4.77; I2 75%) (Fig. 4B). Two studies (COPD/Non-COPD: 165/267) provided data for estimating the 30-day/in-hospital mortality of AAA after EVAR. The pooled result revealed no significant association between COPD and the 30-day/in-hospital mortality of AAA after EVAR (OR 2.53; 95% CI 0.70–9.18; I2 54%) (Fig. 4C). The above results suggested that COPD increased postoperative mortality of AAA patients with the receipt of open repair but not EVAR.
To evaluate the effect of AAA types on the clinical outcomes, the studies were divided into two subgroups (rupture AAA vs. non-rupture AAA). The data for estimating the postoperative 30-day/in-hospital mortality for patients with rupture AAA was identified from 6 studies (COPD/No-COPD: 152/342). The mixed pooled result suggested that COPD was positively associated with postoperative 30-day/in-hospital mortality of rupture AAA patients (OR 2.72; 95% CI 1.14–6.48; I2 69%) (Online Fig.3C). If analysis was restricted to studies providing adjusted data (COPD/Non-COPD: 45/107), the pooled analysis showed that the postoperative 30-day/in-hospital mortality of rupture AAA patients was strongly positive associated with COPD status (OR 4.75; 95% CI 2.07–10.89; I2 0%) (Fig. 4D). Ten studies (COPD/Non-COPD: 1405/6518) provided data for estimating 30-day/in-hospital mortality for non-rupture AAA patients after receiving elective repair. The mixed pooled result suggested that COPD was slightly and positively associated with the 30-day/in-hospital mortality of non-rupture AAA after elective repair (OR 1.23; 95% CI 1.05–1.44; I2 50%) (Online Fig. 3D). The possibility of publication bias was low (P = 0.162) (Online Fig. 2F). When the unadjusted data were removed from the analysis, the pooled adjusted data (COPD/No-COPD: 341/3985) suggested the postoperative 30-day/in-hospital mortality of non-rupture AAA patients was slightly positive associated with COPD status (OR 1.97; 95% CI 1.11–3.49; I2 73%) (Fig. 4E).
30-day/in-hospital morbidity
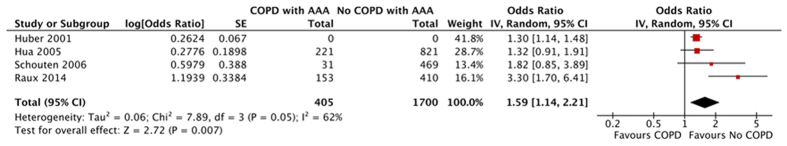
Eight studies (COPD/Non-COPD: 712/2499) were included for meta-analysis regarding 30-day/in hospital morbidity among AAA patients. The mixed pooled result suggested that COPD was positively associated with 30-day/in-hospital morbidity of AAA receiving repair surgery (OR1.37; 95% CI 1.23–1.54; I2 33%) (Online Fig. 4). Pooled estimates based on adjusted data (COPD/No-COPD: 405/1700) showed similar odds of getting complications among COPD patients and non-COPD patients (OR 1.59; 95% CI 1.14–2.21; I2 62%) (Fig. 5).
Figure 5. Cumulative operative morbidity in abdominal aortic aneurysm patients with chronic obstructive pulmonary disease: Pooled adjusted odd ratio.
Long-term mortality
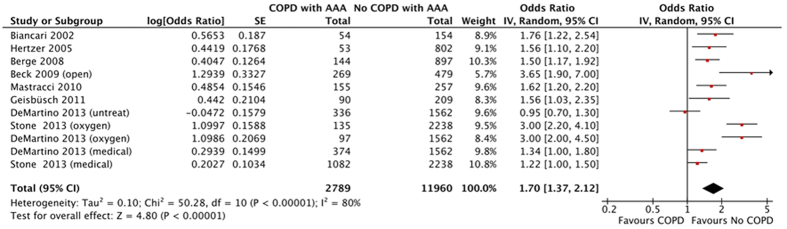
Thirteen studies (COPD/Non-COPD: 2917/12243) were included for meta-analysis regarding AAA long-term mortality. The definition of long-term varied from 1 year to 20 years. The mixed pooled estimate suggested that COPD was positively associated with long-term postoperative mortality of AAA patients (OR1.59; 95% CI 1.26–2.01; I2 84%) (Online Fig. 5). The possibility of publication bias was low (P = 0.47). (Online Fig. 2G). After removing studies with unadjusted data, the pooled adjusted estimate (COPD/No-COPD: 2789/11960) indicated higher AAA long-term mortality in COPD patients compared with non-COPD patients (OR 1.70; 95% CI 1.37–2.12; I2 80%) (Fig. 6). The possibility of publication bias was low (P=0.061) (Online Fig. 2H).
Figure 6. Cumulative long-term mortality in abdominal aortic aneurysm patients with chronic obstructive pulmonary disease: Pooled adjusted odd ratio.
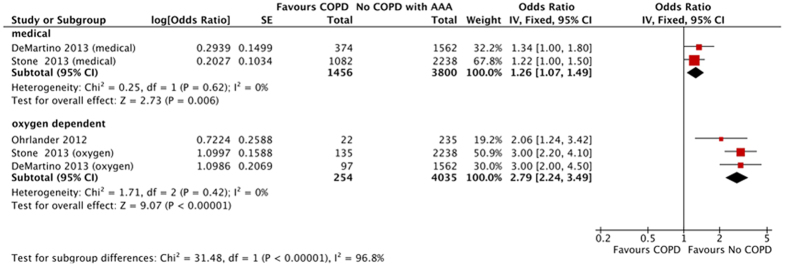
Five studies were included for meta-analysis focusing on the influence of COPD grade (medical and oxygen dependent) on AAA long-term mortality. The pooled results showed that COPD is associated with increased AAA long-term mortality regardless of COPD grade (medical COPD: OR 1.26; 95% CI 1.07–1.49; I2 0%; oxygen dependent COPD: OR 2.79; 95% CI 2.24–3.49; I2 0%) (Fig. 7).
Figure 7. Pooled result of long-term mortality in abdominal aortic aneurysm patients with chronic obstructive pulmonary disease: Medical treatment vs. Oxygen dependent treatment.
Long-term morbidity
Two studies reported long-term postoperative morbidity among AAA patients. In a prospective, multicenter clinical trail of EVAR (923 patients), COPD was not found to be associated with the probability of AAA rupture (P = 0.98) and the probability of conversion events (P = 0.18) during a 5-year follow up period21. However, EUROSTAR collaborators registry published the result of conversion from 1871 enrolled patients who underwent EVAR, and supported that COPD was significantly and positively associated with the increased risk of conversion in a 1.5-year follow up period22.
Discussion
According to our pooled mixed data and pooled adjusted data, we found that patients with COPD had 1.22 (pooled adjusted OR) to 1.78 (pooled mixed OR)-fold increased risk of AAA presence than those without COPD. When categorized by type of study, the pooled result showed that COPD was significantly and positively associated with AAA in PBS and PS studies, but CCS indicated no significant association. After excluding 6 studies with poor control group (5 studies with arteriosclerosis and 1 study with TAA) in CCS, the pooled result was found to be consistent with that were found in PBS and PS studies. As a result, in general, COPD was found to be positively associated with AAA regardless of study types, however, confounding effect resulted from recruitment of poor control group may bias the estimation. Previous studies indicated a possible association between smoking status and AAA prevalence in COPD patients. For example, Pitoulias et al. suggested that the patients’ smoking status might affect the statistical outcome of risk estimation23. Axelrod et al. believed that smoking but not COPD determined the positive association between COPD and AAA, which was supported by two viewpoints: smoking is the primary risk factor of COPD and most patients with COPD have smoking history24. To confirm this assumption we made pooled estimation based on data adjusted for smoking status, and the result suggested that the positive association between AAA and COPD was independent of smoking status. Our estimation is consistent with Meijer et al.’s study which suggested that COPD was more prevalent in aneurysm patients than in controls (OR 3.0; 95% CI 1.6–5.5), and the association was independent of smoking stauts25. The above-mentioned findings suggested that COPD might be a risk factor for AAA regardless of patients’ smoking status.
The pooled adjusted data for short-term mortality showed that COPD was associated with increased risk of postoperative mortality. When categorized by type of treatment, our result suggested a significant association between COPD and short-term mortality in open surgery subgroup, but not in EVAR group. This difference might be attributed to the larger trauma of open repair. When categorized by type of AAA, our result suggested that the positive association between COPD and short-term mortality was stronger in rupture AAA subgroup than that in non-rupture AAA group. One of the possible explanation is the unstable hemodynamic status in patients with rupture AAA, and the COPD might aggravate the risk trend of short-term mortality.
Our result also showed that COPD is associated with increased short-term morbidity among AAA patients. These results were also supported by clinical based studies. For instance, several clinical studies confirmed that a significant proportion (9–40%) of pulmonary complications were developed in severe COPD patients undergoing abdominal surgery26. In addition, based on a retrospectively study involving 181 patients, COPD was found to be a high-risk factor and onset of postoperative respiratory failure in abdominal aortic surgery27. Similar to this study, Prinssen and colleagues found a significantly higher rate of pulmonary complications in the open repair group28. Moreover, Huber et al. analyzed the outcome of 16450 intact AAA underwent open repair by performing multivariate regression, and the result showed that COPD was positive associated with negative outcomes (adjusted OR 1.3; 95% CI 1.1–1.4). Unfortunately, these studies did not comprehensively analyze morbidities and evaluate the morbidity regarding different complications. Moreover, in evaluation of the long-term mortality, our study found that the patients with oxygen dependent COPD presented a 2-folder increased risk of AAA than those with medical COPD. This is also consistant with Van Laarhoven et al.’s finding which indicate that COPD appears to have a linear dose response to AAA, which meant that patients with more severe COPD (forced expiratory volume in the first second [FEV1]/vital capacity ratio <53% predicted) were more likely to have AAA than patients with less severe disease (19.3% vs 7.6%)10. However, in our review, different studies had different period of follow up, spanning from 1 year to 20 years, which might increase the discrepancy of outcome.
This meta-analysis had some inherent limitations. First, we did not control for confounding effect due to the development and changes in treatment, postoperative treatment protocols and patient selection criteria over time. When comparing outcomes of open versus endovascular repair in AAA patients with COPD from 1982 to 2015, there is a huge improvement during the last 10–15 years (compared to 25–30 years before). This improvement can be partially attributed to dramatic changes in preoperative evaluation, selections of treatment approach and postoperative treatment algorithms for AAA patients with COPD, especially considering the use of bronchodilators, epidural analgesia and changes in ICU protocols. Also, some AAA patients with severe comorbidities possibly had not been offered with any treatment (open repair or EVAR) due to the publication of EVAR trial 129,30,31 and EVAR trial 232 report. As a result, postoperative mortality and morbidity may have been decreased due to different patient selection criteria; therefore, mortality of either open repair or EVAR in COPD may have been differed over time. However, due to lack of such detailed information, we were unable to control for those potential confounding. Second, due to lack of well-conducted randomized trial, the validity of our study findings were compromised by limited confounder control. About 50% of the included studies were PBS, among which the heterogeneity can be resulted from differences in populations, definition of AAA and variable measurements, adjusted factors, and duration of follow-up. Additionally, the small number of studies limited our ability to compare the outcomes across subgroups. For example, EVAR subgroup and medical COPD subgroup only included 2 studies and oxygen dependent COPD subgroup included 3 studies, which might weaken the credibility of the results of sensitivity analysis. Finally, although the Egger test confirmed that most of pooled results had low publication bias, it is possible that we have missed some studies published in other languages rather than in English language.
Conclusions
As there is an increasing prevalence of AAA in COPD patients, and the AAA postoperative mortality and morbidity rate is also higher among AAA patients with COPD compared with those without COPD, our study shows the epidemiological and clinical value to pay more attention for AAA prevention in COPD patients and make low-risk treatment for AAA patients with COPD. To better understand the association between COPD and AAA, as well as the rationale behind it, more well-conducted, large sample sized multicenter RCTs will be required to draw more valid conclusion.
Additional Information
How to cite this article: Xiong, J. et al. Chronic obstructive pulmonary disease effect on the prevalence and postoperative outcome of abdominal aortic aneurysms: A meta-analysis. Sci. Rep. 6, 25003; doi: 10.1038/srep25003 (2016).
Supplementary Material
Footnotes
Author Contributions Conception and design: J.X. and W.G.; Analysis and interpretation: J.X., Z.W., C.C. and W.G.; Data collection: J.X. and Z.W.; Writing the article: J.X. and C.C.; Critical revision of the article: J.X., C.C. and W.G.; Final approval of the article: J.X., C.C. and W.G.; Statistical analysis: J.X. and C.C.; Overall responsibility: J.X. and W.G.
References
- Imperato J. & Sanchez L. D. Pulmonary emergencies in the elderly. Emergency medicine clinics of North America. 24, 317–338, vi (2006). [DOI] [PubMed] [Google Scholar]
- Vos T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380, 2163–2196 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder R. M., Yun J. H., Bhunia A. & Fijalkowska I. Hypoxia and chronic lung disease. Journal of molecular medicine (Berlin, Germany). 85, 1317–1324 (2007). [DOI] [PubMed] [Google Scholar]
- Simoni G. et al. Screening for abdominal aortic aneurysms and associated risk factors in a general population. Eur J Vasc Endovasc Surg. 10, 207–210 (1995). [DOI] [PubMed] [Google Scholar]
- Pleumeekers H. J. et al. Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am J Epidemiol. 142, 1291–1299 (1995). [DOI] [PubMed] [Google Scholar]
- Smith F. C., Grimshaw G. M., Paterson I. S., Shearman C. P. & Hamer J. D. Ultrasonographic screening for abdominal aortic aneurysm in an urban community. Br J Surg. 80, 1406–1409 (1993). [DOI] [PubMed] [Google Scholar]
- Lucarotti M., Shaw E., Poskitt K. & Heather B. The Gloucestershire Aneurysm Screening Programme: the first 2 years’ experience. Eur J Vasc Surg. 7, 397–401 (1993). [DOI] [PubMed] [Google Scholar]
- Jones H. J., Ibrahim A. E., Hoskins C. & Derodra J. K. Aortic aneurysm screening in general practice. Lancet. 348, 1320 (1996). [DOI] [PubMed] [Google Scholar]
- Bengtsson H., Bergqvist D., Ekberg O. & Janzon L. A population based screening of abdominal aortic aneurysms (AAA). Eur J Vasc Surg. 5, 53–7 (1991). [DOI] [PubMed] [Google Scholar]
- van Laarhoven C. J. et al. Chronic obstructive pulmonary disease and abdominal aortic aneurysms. Eur J Vasc Surg. 7, 386–390 (1993). [DOI] [PubMed] [Google Scholar]
- Lindholt J. S., Heickendorff L., Antonsen S., Fasting H. & Henneberg E. W. Natural history of abdominal aortic aneurysm with and without coexisting chronic obstructive pulmonary disease. J Vasc Surg. 28, 226–233 (1998). [DOI] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. The American review of respiratory disease. 132, 417–433 (1985). [DOI] [PubMed] [Google Scholar]
- Janoff A. Investigations into the biochemical mechanisms of pulmonary emphysema: effects of cigarette smoke on enzymes and anti-enzymes in the lung. Respiration. 50, Suppl 1, 13–25 (1986). [DOI] [PubMed] [Google Scholar]
- Fujita J. et al. Evaluation of elastase and antielastase balance in patients with chronic bronchitis and pulmonary emphysema. The American review of respiratory disease. 142, 57–62 (1990). [DOI] [PubMed] [Google Scholar]
- Schriver E. E., Davidson J. M., Sutcliffe M. C., Swindell B. B. & Bernard G. R. Comparison of elastin peptide concentrations in body fluids from healthy volunteers, smokers, and patients with chronic obstructive pulmonary disease. The American review of respiratory disease. 145, 762–766 (1992). [DOI] [PubMed] [Google Scholar]
- Spencer C., Jamrozik K., Kelly S., Bremner P. & Norman P. Is there an association between chronic lung disease and abdominal aortic aneurysm expansion? ANZ J Surg. 73, 787–789 (2003). [DOI] [PubMed] [Google Scholar]
- Lederle F. A., Walker J. M. & Reinke D. B. Selective screening for abdominal aortic aneurysms with physical examination and ultrasound. Arch Intern Med. 148, 1753–1756 (1988). [PubMed] [Google Scholar]
- Lederle F. A. et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 126, 441–449 (1997). [DOI] [PubMed] [Google Scholar]
- Chang J. B., Stein T. A., Liu J. P. & Dunn M. E. Risk factors associated with rapid growth of small abdominal aortic aneurysms. Surgery. 121, 117–122 (1997). [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 25, 603–605 (2010). [DOI] [PubMed] [Google Scholar]
- Zarins C. K. et al. Endovascular aneurysm repair at 5 years: Does aneurysm diameter predict outcome? J Vasc Surg. 44, 920–931 (2006). [DOI] [PubMed] [Google Scholar]
- Cuypers P. W., Laheij R. J. & Buth J. Which factors increase the risk of conversion to open surgery following endovascular abdominal aortic aneurysm repair? The EUROSTAR collaborators. Eur J Vasc Endovasc Surg. 20, 183–189 (2000). [DOI] [PubMed] [Google Scholar]
- Pitoulias G. A., Donas K. P., Chatzimavroudis G., Torsello G. & Papadimitriou D. K. The role of simple renal cysts, abdominal wall hernia, and chronic obstructive pulmonary disease as predictive factors for aortoiliac aneurysmatic disease. World J Surg. 36, 1953–1957 (2012). [DOI] [PubMed] [Google Scholar]
- Axelrod D. A. et al. Impact of chronic obstructive pulmonary disease on elective and emergency abdominal aortic aneurysm repair. J Vasc Surg. 33, 72–76 (2001). [DOI] [PubMed] [Google Scholar]
- Meijer C. A. et al. An association between chronic obstructive pulmonary disease and abdominal aortic aneurysm beyond smoking: results from a case-control study. Eur J Vasc Endovasc Surg. 44, 153–157 (2012). [DOI] [PubMed] [Google Scholar]
- Berardi G. et al. Combined spinal and epidural anesthesia for open abdominal aortic aneurysm surgery in vigil patients with severe chronic obstructive pulmonary disease ineligible for endovascular aneurysm repair. Analysis of results and description of the technique. Int Angiol. 29, 278–283 (2010). [PubMed] [Google Scholar]
- Calligaro K. D. et al. Pulmonary risk factors of elective abdominal aortic surgery. J Vasc Surg. 18, 914–921 (1993). [PubMed] [Google Scholar]
- Prinssen M. et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 351, 1607–1618 (2004). [DOI] [PubMed] [Google Scholar]
- Greenhalgh R. M., Brown L. C., Kwong G. P., Powell J. T. & Thompson S. G. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 364, 843–848 (2004). [DOI] [PubMed] [Google Scholar]
- EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 365, 2179–2186 (2005). [DOI] [PubMed] [Google Scholar]
- Greenhalgh R. M. et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 362, 1863–1871 (2010). [DOI] [PubMed] [Google Scholar]
- EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 365, 2187–2192 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.