Abstract
Traditionally, Type II heterogeneous photo-oxidations produce singlet oxygen via external irradiation of a sensitizer and external supply of ground-state oxygen. A potential improvement is reported here. A hollow-core fiber-optic device was developed with an “internal” supply of light and flowing oxygen, and a porous photosensitizer-end capped configuration. Singlet oxygen was delivered through the fiber tip. The singlet oxygen steady-state concentration in the immediate vicinity of the probe tip was ca 20 fM by N-benzoyl-DL-methionine trapping. The device is portable and the singlet oxygen-generating tip is maneuverable, which opened the door to simple disinfectant studies. Complete Escherichia coli inactivation was observed in 2 h when the singlet oxygen sensitizing probe tip was immersed in 0.1 mL aqueous samples of 0.1–4.4 × 107 cells. Photobleaching of the probe tip occurred after ca 12 h of use, requiring baking and sensitizer reloading steps for reuse.
INTRODUCTION
Only recently was a fiber-optic singlet oxygen (FOSG) device developed for the localized “delivery” of singlet oxygen (Scheme 1) (1). The device consisted of a porous Vycor glass (PVG) cap coated with meso-tetra(N-methyl-4-pyridyl)porphine (1) fixed to the end of a hollow fiber-optic (1). The hollow fiber flowed O2 gas and guided 532 nm light from a continuous-wave or pulsed laser. The lifetime of photoexcited triplet-state adsorbed 1 was 57 μs and O2 quenching at the water–PVG interface resulted in the formation of 1O2 in aqueous solution (2).
Scheme 1.
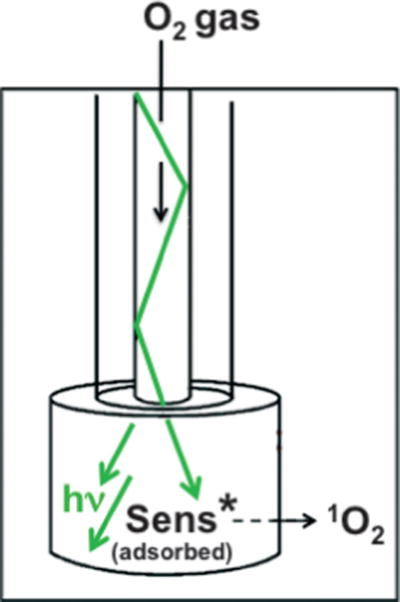
Distal end of fiber-optic device where singlet oxygen exits.
To date, there are few devices specifically designed to deliver singlet oxygen through space (3–5). Perhaps the best documented example is that of Eisenberg et al. (5) who 24 years ago reported a Pyrex tube-bound rose bengal photosensitizer flowing singlet oxygen upon irradiation (Scheme 2). Singlet oxygen exited the distal end of the Pyrex tube and was transported through space ~1.5 mm due to a 54 ms lifetime. Despite the novelty of the Eisenberg Pyrex tube method (5) and other gas–solid methods to generate external 1O2, they are not compatible in aqueous solution because of high oxygen gas flow rates, which would lead to water cooling and evaporation. Thus, interesting questions remain about devices whose distal ends generate singlet oxygen in aqueous solution.
Scheme 2.
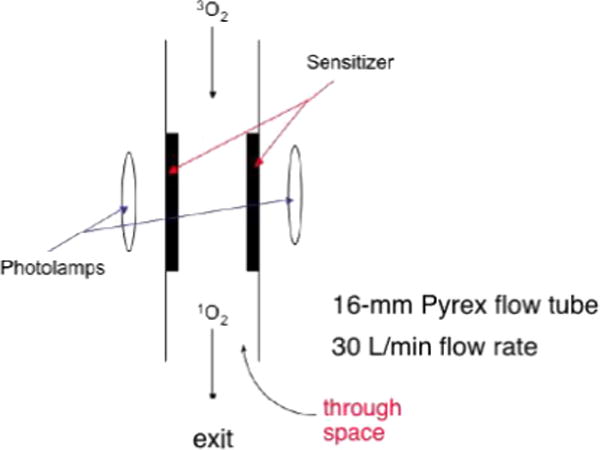
Device of Eisenberg et al. where singlet oxygen exited the distal end of the flow tube (5).
We wondered whether the FOSG device could be exploited to inactivate Escherichia coli or whether the short diffusion distance of 1O2 in water (~160 nm) (6–8) would prohibit it. Our interest in a disinfection application was due to interest in the fundamental factors of singlet oxygen generation water–solid interfaces, and because of the great potential that singlet oxygen photocatalysts possess for E. coli killing under visible light conditions (9). Fiber optics have been coupled to heterogeneous photosensitizers for the generation of 1O2 (10–14), and used in the sensing of 3O2 or 1O2 in biological media (15,16), but no such device has been developed for E. coli inactivation.
MATERIALS AND METHODS
Device construction
A diagram of the fiber-optic apparatus is shown in Fig. 1. A similar fiber-optic geometry was chosen because it gave good results in our previous paper on singlet oxygen delivery (1). In the present study, a different fiber and different illumination source was used than in Ref. (1). The hollow core photonic bandgap fiber was Crystal Fibre’s HC-440 having a 84 μm diameter, coated with a 136 μm layer of acrylate (attenuation of <2 dB m−1), which guided 473 nm light from a blue CW laser (20 mW, 2.0 mm beam diameter; Dragon Laser), which was within the wavelength range covered by the photonic bandgap in the cladding (415–485 nm). Unwanted melting of the acrylate coating by the incident 20 mW laser beam required the removal of a 0.5-cm segment of the acrylate, which was done by dipping the proximal end (beginning) of the fiber into dichloromethane while flowing oxygen (40 psi). This acrylate stripping method reduced the amount of light that could be transported through the fiber. The intensity of the illumination was 2.2 mW, incident into the photosensitizing cap hole during the experiment. The immobilization of 1 on PVG pieces was conducted as described previously for this heterogeneous reaction (1). PVG has pore sizes around 40 Å and increases in weight by 30% from its dry weight after soaking in water. Typically, 0.015 g PVG caps were loaded with 4 × 10−9 mol 1 producing ~0.5% sensitizer coverage. Caps were cut and polished into cylindrical shapes, ~2 mm o.d. × 3–4 mm. The sensitizer-impregnated PVG cap was then fixed to the distal end of the hollow fiber and glued into place at the hole entrance with ethyl-2-cyanoacrylate. The sensitizer-coated PVG cap absorbs 473 nm light at the tail of the Soret band (λmax = 422 nm). Concentrations of oxygen delivered to 0.1 mL water samples via the FOSG generator system were determined with a pO2 micro-oxygen electrode.
Figure 1.
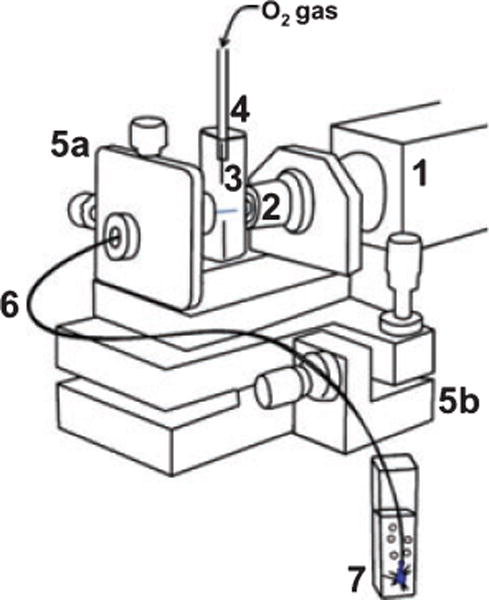
Schematic of the fiber-optic-based singlet oxygen generator (FOSG): (1) continuous-wave laser; (2) microscope objective; (3) cuvette; (4) cuvette top with tube coupled to compressed oxygen tank; (5) laser-to-fiber coupler with micrometer x–y (a) and y–z (b) translation resolution; (6) hollow core fiber-optic transporting oxygen gas and 473 nm light; (7) aqueous reaction solution containing the distal end of the fiber capped with the singlet oxygen sensitizing porous Vycor glass tip.
E. coli viability
Singlet oxygen toxicity was judged by E. coli K-12 cultures grown in tryptic soy broth to an early logarithmic phase (A590 = 0.2) and following the reduction in the number of colonies in treated versus control samples. Standard protocols were used in the growth and maintenance of the E. coli cultures and the experiments were carried out at ambient temperature (25°C). Two milliliters of the E. coli solutions was centrifuged, washed once with distilled water, re-suspended and diluted to 1.1 × 107 to 4.4 × 108 cells mL−1 in 100 μL reaction volumes. In a dark room, E. coli cells were exposed to singlet oxygen via the fiber-optic-based singlet oxygen generator with illumination with the 473 nm laser and introduction of the fiber tip into the 100 μL water solution. There was no headspace above the cell culture medium. The E. coli viability was evaluated at hourly intervals, in which 10 μL aliquots were removed and serial dilutions made ranging from 10−4 to 10−6. Each 100 μL serial dilution was then added to 3 mL molten tryptic soy top agar (TSA), briefly vortexed and overlaid onto the TSA plates. Dilutions were made in duplicate. When the overlay solidified, the plates were inverted and incubated for 48 h. Temperatures were kept at 25°C throughout the course of the experiments. The number of colony-forming units was determined by direct count and the final concentration of E. coli is reported as the number of viable cells mL−1.
Photo-oxidation
Reports of N-benzoyl-DL-methionine anion (2), 9,10-anthracenedipropionic acid (4) and trans-2-methyl-2-pentenoate anion (6) as chemical probes for the evaluation of singlet oxygen in aqueous solution are available in the literature (1,17,18). The experiments in this manuscript were conducted at room temperature with the FOSG device with 40 psi oxygen gas and blue CW laser light passing through a hollow core bandgap fiber. Steady-state concentrations of singlet oxygen were determined as described previously (19, 20), but with the use of methionine anion 2 rather than furfuryl alcohol due to adsorption of the latter onto the PVG surface.
Chemicals
Deionized H2O was obtained from a U.S. Filter Corporation deionization system. PVG (Corning 7930) was purchased from Advanced Glass and Ceramics. N-benzoyl-DL-methionine sodium salt (Aldrich), 9,10-anthracenedipropionic acid (Aldrich), sodium azide (Aldrich), meso-tetra(N-methyl-4-pyridyl)porphine tetratosylate (Frontier Scientific) and adipic acid (Monsanto Chemical Co.) were used as received. Deuterium oxide-d2 (Aldrich), chloroform-d1 (Aldrich) and acetonitrile-d3 (Isotec, Inc.) were of spectrophotometric grade.
RESULTS AND DISCUSSION
A mechanism that involves the generation of singlet oxygen as outlined in Scheme 3 is consistent with our results. As can be seen, the FOSG generator is essentially a three-phase system: the gas phase is the hollow core of the fiber, the solid phase is the fiber cap and the aqueous phase is the bulk solution. Blue light and ground-state triplet O2 were delivered through a hollow fiber to the porous cap (Eq. [1]) and sensitized to 1O2 at the water–solid interface (Eq. [2]). The heterogeneous sensitizer was the probe tip coated with porphyrin 1 (2.5 × 10−7 mol 1 g−1 PVG). Subsequent physical and chemical quenching reactions of singlet oxygen can take place by processes involving solvent, substrates or biological materials (Eqs. [3–5]).
Scheme 3.
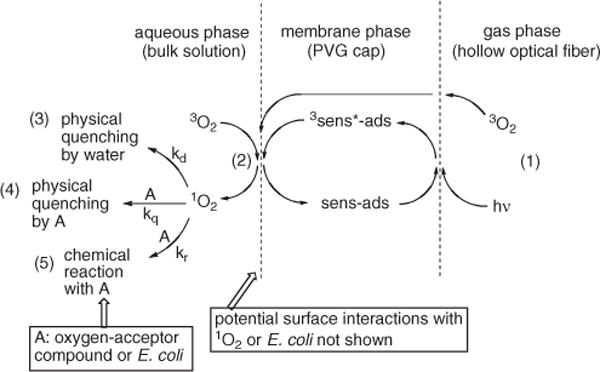
Three-phase system.
Flowing oxygen through the probe tip
Gas diffusion through hollow core fibers has been studied before (21), but not with the aim of generating singlet oxygen. The FOSG generator was operated at 40 psi O2 pressure as the pieces glued at the cuvette/fiber and fiber/cap junctions otherwise come apart. The transmission rate of O2 through the porous tip was ~1.0 μL min−1 (~0.1 ppm h−1). After 4 h at 40 psi, an increase in oxygen concentration of 4–12% (~2 × 10−5 M) can be obtained beyond the starting air-saturated concentration (2.6 × 10−4 M; 4.68 ppm). The variation in the percent of oxygen transported across the PVG tip can be explained by the differences in the cap shape, its length and outer diameter, and the hole inner diameter (Fig. 2). When illuminated, oxygen rapidly quenches the triplet PVG adsorbed 1; its lifetime in N2-purged water (τ0 = 57 ± 1 μs) was reduced by oxygen in air-saturated solution (τ = 7 ± 1 μs) (2).
Figure 2.
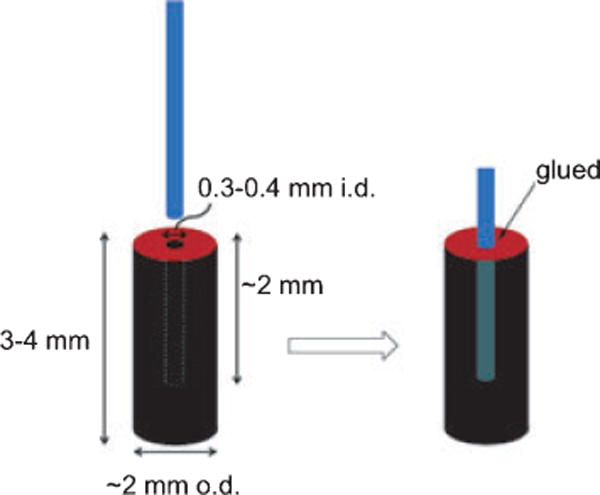
Schematic of the singlet oxygen sensitizing porous Vycor glass tip.
Photo-oxidation and kinetics
Ene and [4+2]-cycloaddition reactions are often diagnostic and verify the presence of singlet oxygen (22–24) in solution. Here, we found the formation of 9,10-anthracene-9,10-endoperoxide dipropionate dianion (5) took place via a [4+2] cycloaddition of singlet oxygen with 9,10-anthracene dipropionate dianion (0.1–0.2 M, pH = 10, 4) (Scheme 4). Control reactions demonstrated that >99% of 4 was photo-oxidized by the device tip, and was not self-sensitized under the reaction conditions. N-benzoyl-DL-methionine 2 was also photo-oxidized by the FOSG giving predominantly N-benzoyl-DL-methionine S-oxide (3). Products 3 and 5 were stable in aqueous solution at room temperature and identified by 1H NMR and by LCMS. We next wanted to learn if the FOSG production could be quantified with the chemical trap 2.
Scheme 4.
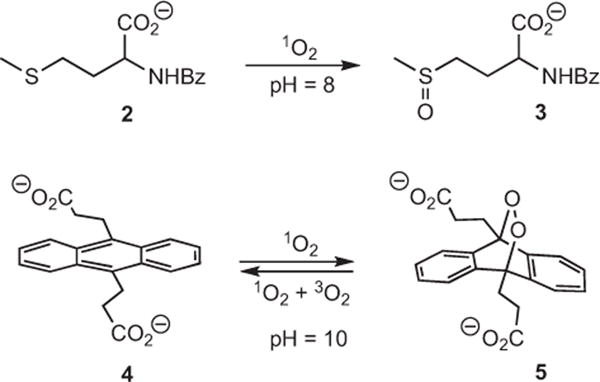
Photo-oxidation of N-benzoyl-DL-methionine anion and 9,10-anthracene dipropionate dianion.
Control experiments showed that anions, such as methionine anion 2, do not penetrate through nor associate with PVG (Scheme 5), suggesting 2 could be used as a trapping agent for the FOSG and its concentration followed in the surrounding aqueous solution. The chemical quenching (kr), and solvent (kd) and substrate (kq)-induced physical quenching reactions of singlet oxygen are shown in Eqs. (3–5) (Scheme 3). The quantum yield of singlet oxygen production is Φ, and the rate of absorption of 473 nm light by the PVG-sensitizer 1 is Ia (Eq. [6]). As a 2 mW output of the CW laser through the fiber was constant to ±0.1 mW over the course of the experiments, we applied a steady-state approximation for singlet oxygen (Eqs. [7] and [8]) in a similar fashion as Haag and Hoigné (19). Protic solvents suppress the physical quenching (kq) of singlet oxygen by organic sulfides (typically 0% physical quenching) (25) thus permitting the rate of formation of singlet oxygen in the solution around the probe tip to be estimated by the reduction in the concentration of 2. The rate constant of the reaction between 1O2 and DL-methionine (kT) has been examined previously (~3 × 107 M−1 s−1 in water at pH 6–11) (26) and we assumed a similar or identical rate constant between 1O2 and N-benzoyl-DL-methionine 2. The initial concentration of 2 was 0.1 mM so that kd was seven times greater than kT[2] and the loss of 2 was first-order in 2. The kd of singlet oxygen in water is 2.5 × 105 s−1 (27). Under constant 2-mW light intensity, pseudo-first order plots with the FOSG generator were obtained by Eq. (8), which suggested a [1O2]ss of ~1–4 × 10−14 M in the immediate vicinity of the probe tip.
| (6) |
| (7) |
(ss = steady state)
| (8) |
Scheme 5.
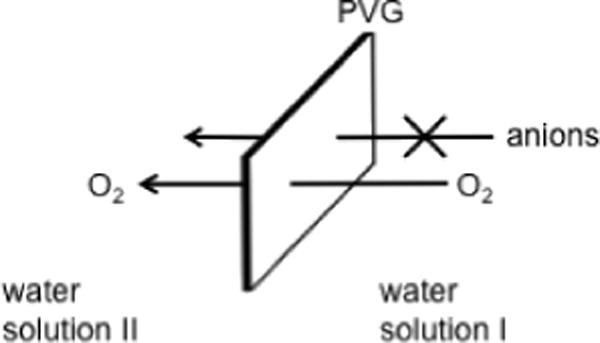
Inability of anions to penetrate through or adsorb onto PVG.
Inactivation of E. coli
Figure 3 shows the results of E. coli inactivation through singlet oxygen sensitized damage together with control data. The reduced number of cells was compared against blanks containing from 1.1 × 107 to 4.4 × 108 cells mL−1 (entry a). A decrease in the number of viable cells was observed over a 1 h period with the FOSG (cf. control [2.1 × 108 cells mL−1] with photo-oxidation sample [9.7 × 107 cells mL−1]) (entry b). Escherichia coli was completely killed after 2 h (entry c). Additional data with higher oxygen concentrations (O2-saturated solution [1.4 mM] versus air-saturated solution [0.26 mM]) revealed enhanced E. coli killing by a factor of ~2. Higher oxygen concentrations are known to enhance photodynamic bacterial inactivation (28). After a 4 h irradiation period the E. coli samples turned yellow, which we attribute to photochemical oxygen uptake. Escherichia coli was completely killed after 1 h when a 0.02 g piece of heterogeneous PVG sensitizer was irradiated externally with 473 nm light (entry d). A homogeneous solution of 5.0 × 10−8 M 1 in the presence of 473 nm light was also lethal concentration to E. coli growth after 2 h (entry e), which was expected due to previous reports of the phototoxicity of 1 toward E. coli in homogeneous solution (29). Singlet oxygen inactivation of E. coli with a number of homogeneous photosensitizers has been established (30,31). The viable number of E. coli significantly increased in the presence of blue light and/or oxygen without sensitizer. Twenty percent E. coli inactivation was observed in the presence of sparging oxygen in the dark (entry f), and in the presence of 473 nm light and sparging oxygen without sensitizer (entry g). The sensitizer 1-coated PVG showed negligible dark toxicity toward E. coli (entry h), which was similar to the low dark toxicity previously reported for homogeneous 1 (32).
Figure 3.
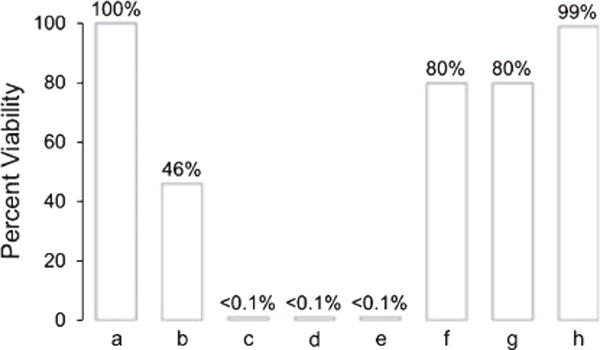
Percent survival of Escherichia coli cells (initial concentration = 1.1 × 107 to 4.4 × 108 cells mL−1, 0.1 mL, pH = 7) in aqueous media. Each bar represents an average of two to three runs. (a) The values are shown as the increase in cell numbers relative to blanks containing the same initial E. coli cell concentration without introduction of the singlet oxygen generator tip. Escherichia coli inactivation in the presence of (b) the FOSG delivering 473 nm light and 40 psi O2 over a 1 h period; (c) the FOSG delivering 473 nm light and 40 psi O2 over a 2 h period; (d) the heterogeneous PVG sensitizer irradiated externally with 473 nm light over a 1 h period; (e) the homogeneous sensitizer 1 in the presence of oxygen and 473 nm light over a 2 h period; (f) sparging 20 μL min−1 oxygen in the dark over a 2 h period; (g) sparging 20 μL min−1 oxygen with 473 nm laser light over a 2 h period; and (h) the heterogeneous PVG sensitizer in the dark over a 2 h period.
Sensitizer leaching and E. coli adhesion onto the PVG surface
Control experiments suggested that E. coli inactivation was not due to desorption of the sensitizer off the PVG and functioning as a homogeneous photosensitizer catalyst. Absorption spectra of E. coli cell solutions were identical in the 300–1100 nm range prior to and after removal of the PVG/adsorbed sensitizer 1. Sensitizer 1 desorption was not detected when PVG (1.1 × 10−8 mol 1 adsorbed onto 0.04 g PVG) was placed in 2 mL of H2O solution at pH = 7, and stirred for 8 h in the presence of E. coli in the dark. Figure 4 shows that even 1.6% desorption of 1 would be readily detected in the presence of E. coli M (Soret band, λmax = 422 nm). Previous results also showed that porphyrin 1 remained immobilized on PVG when sensitizer-coated PVG was placed in H2O solution at pH = 3, 7 and 10 (1), and that cations and metals tend to adsorb strongly onto PVG (2,33). The possible adhesion of E. coli to the PVG surface was then examined. A 0.3 g PVG sample coated with 8.0 × 10−8 mol 1 was submerged in 1.0 mL E. coli suspension (2.1 × 10−8 cells mL−1) for 2 h. The PVG sample was washed with sterilized water and then plated onto TSA. An aliquot of the sterilized water from the last rinse was also plated onto TSA. The two plated samples showed no difference in colony growth, suggesting that E. coli adhesion did not take place onto the PVG photocatalyst surface. Electrostatic repulsion may be expected between E. coli and the negatively charged PVG surface, E. coli adhesiveness is known to correlate inversely with surface electro-negativity (34). Escherichia coli adhesion is generally favored on positively rather than negatively charged surfaces (35).
Figure 4.
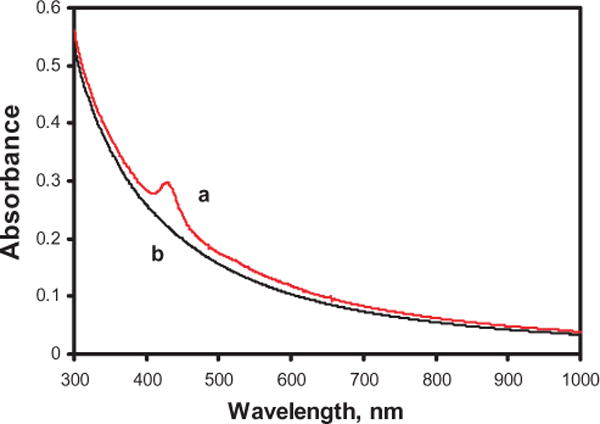
(a) 1.0 × 108 Escherichia coli cells mL−1 in aqueous solution with 6.6 × 10−7 M sensitizer 1. (b) 1.0 × 108 E. coli cells mL−1 in aqueous solution.
Photocatalyst bleaching and reuse
An important matter concerned the photostability of the probe tip. After 12 h of use, the probe tip began to photobleach and the rate of E. coli killing declined. We analyzed the photo-bleaching process with blue-light irradiation of a PVG sample (2.2 × 10−8 mol 1 adsorbed onto 0.084 g PVG) in D2O. A dichloromethane solvent extract of the D2O solution after removal of the PVG catalyst revealed organics presumably from fragmented/oxidized sensitizer molecules by GCMS in the m/z range 206.0–246.1. Baking the PVG catalyst at 500°C removed the residual bleached and unbleached material from the surface, and an active photocatalyst tip was regenerated by baking of the PVG and following the procedure of Ref. (1) to reload the tip with sensitizer 1. Such baking and reloading steps could be done more than 10 times.
CONCLUSION
Heterogeneous 1O2-photosensitizers typically require externally supplied oxygen and light, and in some cases cannot be recovered for reuse. One way of improving heterogeneous photosensitizer performance would be if a hollow-core fiber-optic system flowing oxygen were coupled to a porous Type II heterogeneous photosensitizer tip, which is the subject of this paper. In this paper, it was shown that singlet oxygen can be delivered through the fiber tip into aqueous solution. Escherichia coli inactivation was analyzed to help establish conditions that can generate singlet oxygen, which extends our previous study (1) and provides a potential application in water disinfection. The porous fiber tip can be reused with baking and sensitizer reloading steps to enhance the photodynamic E. coli killing.
Acknowledgments
This work was supported by the NIH (GM076168-01) and the PSC-CUNY Grants Program.
References
- 1.Zamadar M, Aebisher D, Greer A. Singlet oxygen delivery through the porous cap of a hollow-core fiber-optic device. J Phys Chem B. 2009;113(48):15803–15806. doi: 10.1021/jp907945c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giaimuccio J, Zamadar M, Aebisher D, Meyer GJ, Greer A. Singlet oxygen chemistry in water. 2. Photoexcited sensitizer quenching by O2 at the water–porous glass interface. J Phys Chem B. 2008;112(49):15646–15650. doi: 10.1021/jp807556x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Midden WR, Wang SY. Singlet oxygen generation for solution kinetics: Clean and simple. J Am Chem Soc. 1983;105(13):4129–4135. [Google Scholar]
- 4.Naito K, Tachikawa T, Cui S-C, Sugimoto A, Fujitsuka M, Majima T. Single-molecule detection of airborne singlet oxygen. J Am Chem Soc. 2006;128(51):16430–16431. doi: 10.1021/ja066739b. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg WC, Taylor K, Murray RW. Gas-phase kinetics of the reaction of singlet oxygen with olefins at atmospheric pressure. J Phys Chem. 1986;90(9):1945–1948. [Google Scholar]
- 6.Moan J. On the diffusion length of singlet oxygen in cells and tissues. J Photochem Photobiol B, Biol. 1990;6(3):343–344. [Google Scholar]
- 7.Kanofsky JR. Quenching of singlet oxygen by human plasma. Photochem Photobiol. 1990;51(3):299–303. doi: 10.1111/j.1751-1097.1990.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 8.Skovsen E, Snyder JW, Lambert JDC, Ogilby PR. Lifetime and diffusion of singlet oxygen in a cell. J Phys Chem. 2005;109(18):8570–8573. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- 9.Manjón F, Villén L, García-Fresnadillo D, Orellana G. On the factors influencing the performance of solar reactors for water disinfection with photosensitized singlet oxygen. Environ Sci Technol. 2008;42(1):301–307. doi: 10.1021/es071762y. [DOI] [PubMed] [Google Scholar]
- 10.Ulatowska-Jarza A, Bindig U, Podbielska H, Holowacz I, Strek W, Muller G, Eichler HJ. Spectroscopic properties of a chlorophyll-based photosensitive dye entrapped in sol-gel fiber-optic applicators. Mater Sci Poland. 2005;23(1):111–122. [Google Scholar]
- 11.Podbielska H, Bindig U, Ulatowska-Jarza A, Holowacz I, Mueller G, Scheller EE. Optical properties of sol-gel fiber optic applicators for laser interstitial therapy. Laser Phys. 2006;16(5):816–826. [Google Scholar]
- 12.Pradhan AR, Uppili S, Shailaja J, Sivaguru J, Ramamurthy V. Zeolite-coated quartz fibers as media for photochemical and photophysical studies. Chem Commun. 2002;6:596–597. doi: 10.1039/b110959f. [DOI] [PubMed] [Google Scholar]
- 13.Leshem B, Sarfati G, Novoa A, Breslav I, Marks RS. Photochemical attachment of biomolecules onto fibre-optics for construction of a chemiluminescent immunosensor. Luminescence. 2004;19(2):69–77. doi: 10.1002/bio.760. [DOI] [PubMed] [Google Scholar]
- 14.Konry T, Novoa A, Shemer-Avni Y, Hanuka N, Cosnier S, Lepellec A, Marks RS. Optical fiber immunosensor based on a poly(pyrrole-benzophenone) film for the detection of antibodies to viral antigen. Anal Chem. 2005;77(6):1771–1779. doi: 10.1021/ac048569w. [DOI] [PubMed] [Google Scholar]
- 15.Wolfbeis OS. Fiber-optic chemical sensors and biosensors. Anal Chem. 2004;76(12):3269–3284. doi: 10.1021/ac040049d. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Vu DH, Hinds MF, Davis SJ, Liang A, Hasan T. Pulsed diode laser-based singlet oxygen monitor for photodynamic therapy: In vivo studies of tumor-laden rats. J Biomed Opt. 2008;13(6):064035. doi: 10.1117/1.3042265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindig BA, Rodgers MAJ, Schaap AP. Determination of the lifetime of singlet oxygen in water-d2 using 9,10-anthracenedipropionic acid, a water-soluble probe. J Am Chem Soc. 1980;102(17):5590–5593. [Google Scholar]
- 18.Aebisher D, Azar NS, Zamadar M, Gafney HD, Gandra N, Gao R, Greer A. Singlet oxygen chemistry in water. A porous Vycor glass-supported photosensitizer. J Phys Chem B. 2008;112(7):1913–1917. doi: 10.1021/jp709829z. [DOI] [PubMed] [Google Scholar]
- 19.Haag WR, Hoigné J. Singlet oxygen in surface waters. 3. Photochemical formation and steady-state concentrations in various types of waters. Environ Sci Technol. 1986;20(4):341–348. doi: 10.1021/es00146a005. [DOI] [PubMed] [Google Scholar]
- 20.Latch DE, Stender BL, Packer JL, Arnold WA, McNeill K. Photochemical fate of pharmaceuticals in the environment: Cimetidine and ranitidine. Environ Sci Technol. 2003;37(15):3342–3350. doi: 10.1021/es0340782. [DOI] [PubMed] [Google Scholar]
- 21.Hoo YL, Ho JHL, Ju J, Wang DN. Gas diffusion measurement using hollow-core photonic bandgap fiber. Sensors Actuators B Chem. 2005;105(2):183–186. [Google Scholar]
- 22.Martinez GR, Medeiros MHG, Ravanat J, Cadet J, Di Mascio P. Naphthalene endoperoxide as a source of [18O]-labeled singlet oxygen for oxidative DNA damage studies. Trends Photochem Photobiol. 2002;9:25–39. [Google Scholar]
- 23.Stratakis M, Orfanopoulos M. Regioselectivity in the ene reaction of singlet oxygen with alkenes. Tetrahedron. 2000;56(12):1595–1615. [Google Scholar]
- 24.Zamadar M, Greer A. Singlet oxygen as a reagent in organic synthesis. In: Albini A, Fagnoni M, editors. Handbook of Synthetic Photochemistry. Wiley-VCH; Weinheim: 2010. pp. 353–386. [Google Scholar]
- 25.Liang JJ, Gu CL, Kacher ML, Foote CS. Chemistry of singlet oxygen. 45. Mechanism of the photooxidation of sulfides. J Am Chem Soc. 1983;105(14):4717–4721. [Google Scholar]
- 26.Wilkinson F, Helman WP, Ross AB. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J Phys Chem Ref Data. 1995;24(2):663–1021. [Google Scholar]
- 27.Schmidt R. Influence of heavy atoms on the deactivation of singlet oxygen (1.DELTA.g) in solution. J Am Chem Soc. 1989;111(18):6983–6987. [Google Scholar]
- 28.Maisch T, Baier J, Franz B, Maier M, Landthaler M, Szeimies R-M, Bäumler W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc Natl Acad Sci USA. 2007;104(17):7223–7228. doi: 10.1073/pnas.0611328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddi E, Ceccon M, Valduga G, Jori G, Bommer JC, Elisei F, Latterini L, Mazzucato U. Photophysical properties and antibacterial activity of meso-substituted cationic porphyrins. Photochem Photobiol. 2002;75(5):462–470. doi: 10.1562/0031-8655(2002)075<0462:ppaaao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Agnez-Lima LF, Di Mascio P, Napolitano RL, Fuchs RP, Menck CFM. Mutation spectrum induced by singlet oxygen in Escherichia coli deficient in exonuclease III. Photochem Photobiol. 1999;70(4):505–511. [PubMed] [Google Scholar]
- 31.Dahl TA, Midden WR, Neckers DC. Comparison of photodynamic action by Rose Bengal in gram-positive and gram-negative bacteria. Photochem Photobiol. 1988;48(5):607–612. doi: 10.1111/j.1751-1097.1988.tb02870.x. [DOI] [PubMed] [Google Scholar]
- 32.Valduga G, Breda B, Giacometti GM, Jori G, Reddi E. Photosensitization of wild and mutant strains of Escherichia coli by meso-tetra(N-methyl-4-pyridyl)porphine. Biochem Biophys Res Commun. 1999;256(1):84–88. doi: 10.1006/bbrc.1999.0190. [DOI] [PubMed] [Google Scholar]
- 33.Gafney HD. Photochemistry of metal carbonyls physisorbed on porous Vycor glass. In: Anpo M, Matsuura T, editors. Photochemistry on Solid Surfaces. Elsevier; New York: 1989. pp. 272–287. [Google Scholar]
- 34.Gilbert P, Evans DJ, Evans E, Duguid IG, Brown MRW. Surface characteristics and adhesion of Escherichia coli and Staphylococcus epidermidis. J Appl Bacteriol. 1991;71(1):72–77. [PubMed] [Google Scholar]
- 35.Li B, Logan BE. Bacterial adhesion to glass and metal-oxide surfaces. Colloids Surf B Biointerfaces. 2004;36(2):81–90. doi: 10.1016/j.colsurfb.2004.05.006. [DOI] [PubMed] [Google Scholar]


