Abstract
Widely targeted metabolomic assays are useful because they provide quantitative data on large groups of related compounds. We report a high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method that utilizes benzoyl chloride labeling for 70 neurologically relevant compounds, including catecholamines, indoleamines, amino acids, polyamines, trace amines, antioxidants, energy compounds, and their metabolites. The method includes neurotransmitters and metabolites found in both vertebrates and insects. This method was applied to analyze microdialysate from rats, human cerebrospinal fluid, human serum, fly tissue homogenate, and fly hemolymph, demonstrating its broad versatility for multiple physiological contexts and model systems. Limits of detection for most assayed compounds were below 10 nM, relative standard deviations were below 10%, and carryover was less than 5% for 70 compounds separated in 20 min, with a total analysis time of 33 min. This broadly applicable method provides robust monitoring of multiple analytes, utilizes small sample sizes, and can be applied to diverse matrices. The assay will be of value for evaluating normal physiological changes in metabolism in neurochemical systems. The results demonstrate the utility of benzoyl chloride labeling with HPLC-MS/MS for widely targeted metabolomics assays.
1. Introduction
Metabolomics is a valuable approach for studying physiological mechanisms and identifying biomarkers. Both untargeted and targeted assays, also called metabolite profiling, are used in such studies. Targeted assays measuring a limited number of metabolites allow focus on important known compounds or pathways and offer better quantification, but they provide lower metabolome coverage compared to untargeted methods. Targeted assays that measure relatively large numbers of compounds (i.e., over 50) help mitigate the disadvantage of limited metabolome coverage. Gas chromatography-mass spectrometry (MS) and high performance liquid chromatography (HPLC)-MS are well-suited platforms for developing such widely targeted assays. Several methods for measuring over 100 known metabolites in a single assay using these techniques have been reported [1–6]. These widely targeted assays are powerful, but they rarely use more than a few internal standards, and for HPLC often make use of ion-pairing reagents [3, 6] or multiple LC pumps [4, 5] to account for the wide polarity range of the metabolites. Here we report a targeted method for 70 neurochemicals that uses HPLC-MS/MS with benzoyl chloride (BzCl) as a derivatizing agent and avoids these limitations.
HPLC- tandem mass spectrometry (MS/MS) using a triple quadrupole mass spectrometer is well established as a sensitive, quantitative, and selective technique for metabolite profiling [7, 8]. Although compounds can be detected by MS/MS without labeling, the use of BzCl provides numerous advantages with only minor drawbacks. In particular, addition of a phenyl group to the polar analytes increases retention on reversed phase columns, which aids resolution and decreases ion suppression. Many compounds are detected with greater sensitivity after labeling, e.g. 1,000-fold increases in sensitivity have been reported for BzCl labeling [9]. The labeling step allows rapid creation of stable-isotope labeled internal standards by using 13C-BzCl for labeling standards, thereby improving quantification for every analyte. BzCl is widely applicable because it derivatizes primary and secondary amines, phenols, thiols, and some alcohols (e.g., ribose hydroxyls and glucose). Indeed, it has previously been used with MS or ultraviolet absorption detection for monitoring neurochemicals in dialysate [9, 10], plasma [10], and human cerebrospinal fluid (CSF) [11]. It has also been used for other amine [12, 13] and alcohol [14, 15] containing compounds. These previous assays targeted a relatively narrow group of compounds.
Although we focus on BzCl, other reagents such as dansyl chloride may provide similar utility for metabolomics [16–19]. We favor BzCl because it reacts faster (seconds at room temperature compared to 20 min at elevated temperature), has a wider pH range for reaction, is less prone to photodegradation, and is commercially available in 13C-labeled form. Additionally benzoylated products are stable for a week at room temperature [9], and standards and internal standards are stable for six months at −80°C (data not shown).
The 70 compound assay described here targets neurochemicals. Neurons specialize in storing and transmitting information using neurotransmitters and neuromodulators. Low molecular weight polar compounds represent an important class of neurotransmitters including acetylcholine, adenosine, catecholamines, indoleamines, amino acids, trace amines, and dipeptides. A variety of other compounds, such as energy metabolites, antioxidants, and polyamines that affect neuronal function or have been linked to neurological disease are also included in the assay. (A complete list of the compounds included in the assay and their functions is in Supplemental A.) This assay focuses on these compounds and their precursors and degradation products, as their measurement can provide insights into neuronal function to better understand the neurochemical changes in brain diseases. Although this is not a comprehensive assay for all neurochemicals, it demonstrates the wide applicability of BzCl derivatization. The method is an improvement over previous neurochemical assays which were limited to even smaller subsets of neurochemicals [10, 20–26], including our previously described 17 compound method based on similar technology [9].
This report demonstrates the utility of BzCl with HPLC-MS for targeted metabolomics on several sample types including tissue, serum, CSF, and microdialysate. Tissue samples are used to characterize concentrations at fixed time points and are best used for determining the overall production and metabolism of neurochemicals. We demonstrate the assay for Drosophila melanogaster tissue and hemolymph, an important neurochemical model system. Serum and CSF assays are useful for biomarker studies and assessment of overall physiological state. Microdialysis samples the brain extracellular space and enables the measurement of released neurochemicals over time, making it valuable for correlating neurochemical dynamics to behavior, monitoring drug effects, and assessing the effect of disease states on neurochemical concentrations. However, the low samples volumes of microdialysate in our studies (typically 1 µL) makes analysis challenging.
Although BzCl labeling with HPLC-MS/MS has been used for neurochemicals before, the current work increases the number of analyzed compounds by 4-fold, streamlines reagent addition, and improves labeling conditions to give better sensitivity and reproducibility for some compounds. Finally, the assay is shown to be useful for a wider range of sample types.
2. Experimental
2.1 Chemicals and reagents
All chemicals were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted. Water, methanol, and acetonitrile for mobile phases are Burdick & Jackson HPLC grade purchased from VWR (Radnor, PA). Stock solutions of 500 mM Glc; 10 mM DOMA, DOPAC (Acros Organics, Geel, Belgium), MOPEG, GABA, 5HIAA, 5HTP, Agm, Ala, Ans, Arg, Asn, Asp, βAla, Carn, Cit, CA, Cys, DA, E, ETA, Glu, Gln, GSH, Gly, Hist, His, HCA, HCY, HSer, HVA (Tocris, Bristol, UK), HTau, Kyo (MP Biomedicals, Santa Ana, CA), Leu, Lys, Met, NAP, NAS, NE, NM, OA, Orn (Acros Organics, Geel, Belgium), PhEt (MP Biomedicals, Santa Ana, CA), Pro, Put, Ser, 5HT, Spd, Spm, Syn, Tau, Thr, Val, and VMA; 5 mM ACh, 5HTOL (Cayman Chemical, Ann Arbor, MI), Ado, Kyn, LDOPA, Phe, and Trp; 2.5 mM 3HK; 2 mM Tyr; 1 mM DOPEG, 3HAA, 3MT, KA, and TyrA; 0.25 mM TrpA; and 20 mM isotopically labeled d4-ACh and d4-Ch (C/D/N isotopes, Pointe-Claire, Canada); were made in HPLC water and kept at −80 °C. A standard mixture was diluted from stocks with artificial cerebrospinal fluid (aCSF) consisting of 145 mM NaCl, 2.68 mM KCl, 1.4 mM CaCl2, 1.0 mM MgSO4, 1.55 mM Na2HPO4, and 0.45 mM NaH2PO4 adjusted pH to 7.4 with NaOH. Preparation of calibration standards and internal standards is described in Supplemental B. Calibration standard and internal standard stocks were frozen at −80 °C in aliquots to prevent multiple freeze/thaw cycles. A single internal standard stock aliquot was thawed the day of use, diluted 100-fold in 20% (v/v) acetonitrile containing 1% (v/v) sulfuric acid, and spiked with deuterated acetylcholine and choline (C/D/N isotopes, Pointe-Claire, Canada) to a final concentration of 20 nM. A fresh benzoyl chloride solution was made daily.
2.2 Microdialysis in Anesthetized Rat
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250–275 g were used for microdialysis collection. Rats were housed with access to food and water ad libitum in a temperature and humidity controlled room with 12 h light/dark cycles. All animals were treated as approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan, the National Institute of Health (NIH) Guidelines for the Care and Use of Laboratory Animals. All precautions were taken to prevent animal discomfort through the course of the experiments. In addition, all animal experiments were conducted within the guidelines of Animal Research Reporting in vivo Experiments (ARRIVE).
A custom-made concentric microdialysis probe (4 mm dialyzing membrane), made using regenerated cellulose (Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA), was implanted into the striatum. Rats were anesthetized with 1–4% isoflurane and placed into a stereotaxic frame (David Kopf, Tujunga, CA). A burr hole was placed above the striatum using the anterior-posterior +1.0 mm and lateral ±3.0 mm coordinates from bregma. The microdialysis probe was flushed with aCSF at a flow rate of 2 µL/min using a Fusion 400 syringe pump (Chemyx, Stafford, TX) as it was lowered −6.15 mm from top of skull. Once the probe was positioned, the probe was flushed at 2 µL/min for 30 min followed by 30 min at 1.0 µL/min prior to collection. 10 µL dialysate was derivatized using the modified method reported: 5 µL of 100 mM sodium carbonate, 5 µL BzCl (2% (v/v) in acetonitrile), and 5 µL internal standard mixture were added sequentially, with vortex mixing after each addition. At the completion of the experiment, animals were euthanized, brains were extracted and stored at 4 °C in 4% paraformaldehyde. Probe placement was confirmed with histology.
2.3 Human CSF
Pooled human CSF from healthy patients was obtained from the Batemen lab at Washington University School of Medicine, St. Louis, MO [27]. Samples were diluted 100-fold in water, and a 10 µL aliquot was derivatized using 5 µL of 100 mM sodium carbonate, 5 µL BzCl (2% (v/v) in acetonitrile), and 5 µL internal standard mixture before LC-MS analysis.
2.4 Human Serum
Pooled human serum from the American Red Cross Detroit National Testing Lab was provided by the Michigan Regional Comprehensive Metabolomics Resource Core (MRC2). To remove proteins, 20 µL of serum were diluted with 80 µL of ice-cold acetonitrile. The samples were vortexed briefly, then centrifuged for 10 min at 12,100×g. 20 µL of the supernatant was derivatized by sequential addition of 10 µL of 100 mM sodium carbonate, 10 µL of BzCl (2% (v/v) in acetonitrile), and 10 µL of the internal standard mixture. 50 µL of water were added to reduce the organic content of the samples. Calibration standards were prepared in aCSF, which is similar in composition to serum without proteins [28]. Five µL aliquots of the standards were diluted with 20 µL acetonitrile to match the sample composition, and then derivatized in the same manner as the serum supernatant.
2.5 Fly Tissue Homogenate
Homogenized Drosophila samples were provided by the Pletcher lab at the University of Michigan, Ann Arbor. Female flies were treated with 250 µM 5HTP for four days prior to harvesting. The flies were snap frozen in liquid nitrogen and vortexed to remove heads. The heads were homogenized in 4 µL of ice cold acetonitrile per head, and 20 µL ice-cold acetonitrile per body, using a pestle grinder. The homogenate was centrifuged at 18,000×g for 5 min and the supernatant was removed and stored at −80 °C until derivatization. 20 µL of the supernatant was derivatized by sequential addition of 10 µL of 100 mM sodium carbonate, 10 µL of BzCl (2% (v/v) in acetonitrile), and 10 µL of the internal standard mixture. Finally, 50 µL of water were added to reduce the organic content of the samples. Calibration standards were prepared in aCSF. Five µL aliquots of the standards were diluted with 20 µL acetonitrile to match the sample composition, and then derivatized in the same manner as the tissue homogenate supernatant.
2.6 Fly Hemolymph
Hemolymph from Drosophila was provided by the Dus lab at the University of Michigan. Flies were reared in standard cornmeal-glucose medium at 25 °C in a 12:12 light/dark cycle. After eclosion groups of 100 w1118CS (w1118 backcrossed to CS for 10 generations) males were placed in bottles and aged for 7–10 days until the time of hemolymph collection. Fresh food was provided every 2 days.
Hemolymph collection was performed as previously described, with modifications to the sated condition [29]. w118CS males in groups of 100 were moved into bottles containing agar and fasted for 24 h. For the starved condition, males were collected directly from the starvation bottles; for the sated condition, males were moved to bottles containing 5% sucrose agar and red food dye for 1 h, and then gathered for hemolymph collection. To generate a sufficient sample volume, multiple collections of hemolymph each from 100 males were pooled together. Hemolymph was stored at −80 °C until derivatization.
20 µL of hemolymph were diluted with 80 µL of ice-cold acetonitrile. The samples were vortexed, then centrifuged for 10 min at 12,100×g. 20 µL of the supernatant was derivatized by sequential addition of 10 µL of 100 mM sodium carbonate, 10 µL of BzCl (2% (v/v) in acetonitrile), and 10 µL of the internal standard mixture. 50 µL of water were added. Calibration standards were prepared in aCSF. Five µL aliquots of the standards were diluted with 20 µL acetonitrile to match the sample composition, and then derivatized in the same manner as the hemolymph supernatant.
2.7 Protein precipitation method validation
To validate the method and test recovery of the solvent precipitation, we spiked a mixture of isotopically labeled metabolites (500 nM 13C5-glutamate, 50 nM d6-GABA, 50 nM d4-serotonin, and 50 nM 13C6-dopamine) into 50 µL of pooled human serum. The spiked serum was diluted with 200 µL ice cold acetonitrile, followed by centrifugation for 10 minutes at 12,100×g. 20 µL of supernatant was derivatized by sequential addition of 10 µL of 100 mM sodium carbonate, 10 µL of BzCl (2% (v/v) in acetonitrile), and 10 µL of the internal standard mixture. Three spiked serum samples were extracted and derivatized in parallel for triplicate analysis. Calibration standards were prepared in aCSF, and 5 µL aliquots of the standards were diluted with 20 µL acetonitrile to match the sample composition, and then derivatized in the same manner as the serum supernatant.
2.8 Small molecule neurochemical analysis using QQQ MS/MS
Derivatized samples were analyzed by LC-MS (as further described in the results section) using a Waters nanoAcquity UPLC (Milford, MA) coupled to an Agilent 6410 (Santa Clara, CA) triple quadrupole mass spectrometer operating in dynamic multiple reaction monitoring (dMRM) mode. Five µL were injected onto an Acquity HSS T3 C18 column (1 mm×100 mm, 1.8 µm, 100 Å pore size) in partial loop injection mode. Samples were analyzed in triplicate. Mobile phase A was 10 mM ammonium formate with 0.15% formic acid, and mobile phase B was acetonitrile. The flow rate was 100 µL/min and the elution gradient was as follows: initial, 0% B; 0.01 min, 15% B; 0.5 min, 17% B; 14 min, 55% B; 14.5 min, 70% B; 18 min, 100% B; 19 min, 100% B; 19.1 min, 0% B; and 20 min, 0% B. An additional 10 min of column equilibration at 0% B were required to achieve reproducible chromatography. The required pressure over the gradient was from 2,500 – 8,000 psi. The autosampler was kept at ambient temperature and the column was kept at 27 °C. Electrospray ionization was used in positive mode at 4 kV. The gas temperature was 350 °C, gas flow was 11 L/min, and the nebulizer was at 15 psi. Automated peak integration was performed using Agilent MassHunter Workstation Quantitative Analysis for QQQ, version B.05.00; all peaks were visually inspected to ensure proper integration.
2.9 Statistical analyses
All statistical analyses were performed in Prism 7 (GraphPad, La Jolla, CA). For statistical analysis unpaired Student’s t test were applied. Differences were deemed significant if P < 0.05.
3. Results and discussion
BzCl labeling has been previously reported for the analysis of small molecule neurotransmitters, polyamines and steroids with HPLC-MS or ultraviolet-absorption detection [9, 10, 30, 31]. Here we identify new reaction conditions that improve sensitivity for LC-MS/MS for many of the neurochemicals tested and demonstrate the wide applicability of BzCl derivatization for low molecular weight metabolites in a variety of complex sample matrices.
3.1 Effect of buffer and solvents on reaction conditions
The initial report of using BzCl with HPLC-MS/MS for neurochemicals utilized four reagent addition steps [9]: 1) sodium tetraborate buffer (100 mM) to the sample to achieve basic pH conditions required for BzCl labeling; 2) 2% (v/v) BzCl in acetonitrile; 3) internal standards diluted in dimethyl sulfoxide (DMSO) with 1% (v/v) formic acid, 4) d4-ACh in water to provide an internal standard for this neurotransmitter that does not react with BzCl. Tetraborate buffer was originally selected because it forms a reversible complex with catechol groups [9, 32] to prevent oxidation under high pH conditions. Our present work first focused on modifying reaction conditions to improve sensitivity and reduce the number of steps. These initial studies used 17 neurochemicals as test analytes (Figure 1, Table 1).
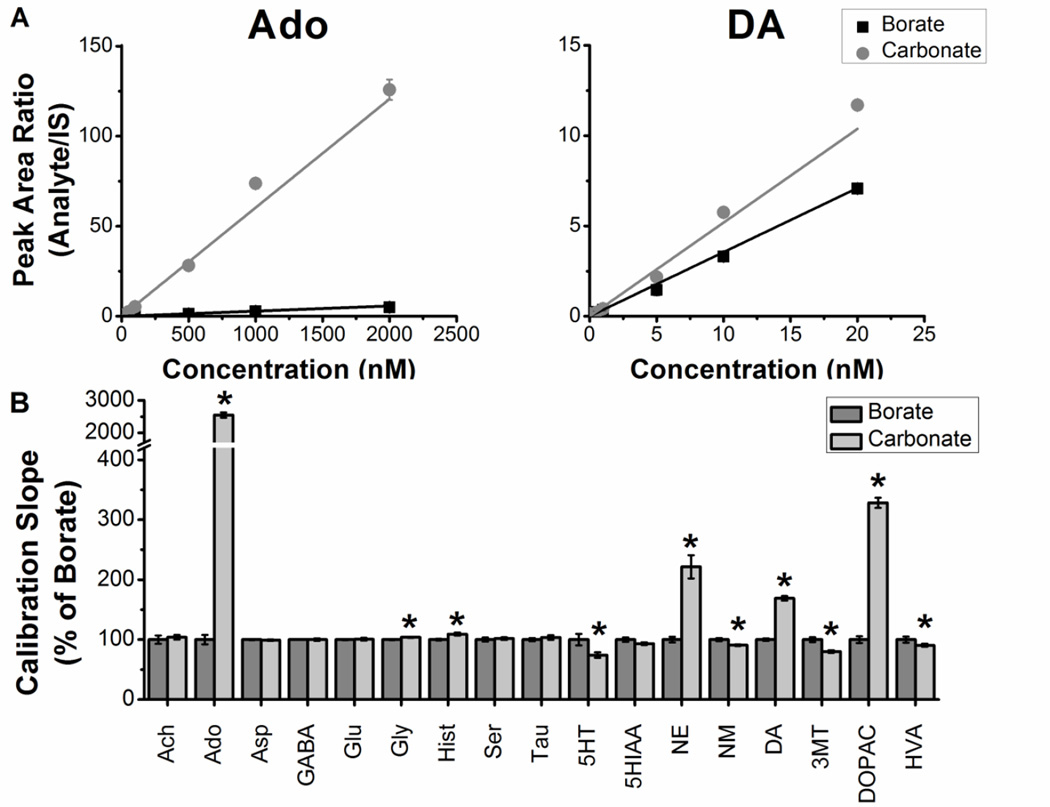
Figure 1.
Normalized effect of sodium borate versus sodium carbonate buffer on calibration slope for select analytes. Standards made using sodium borate buffer and sodium carbonate buffer were analyzed with LC-MS in triplicate. A 6-point calibration curve for all analytes of interest was made to determine the average calibration slope for each analyte (n = 3 for each concentration tested). For the calibrations, the high concentrations were 20 nM for ACh, 5HT, NE, NM, DA and 3MT; 200 nM for Hist, GABA, 5HIAA, HVA, and DOPAC; and 2 µM for Tau, Ser, Asp, Ado, Gly, and Glu; followed by serial dilution. Analyte to internal standard ratios were plotted against known concentrations and a linear trend line was applied to determine slope (A). Sodium carbonate slopes were normalized to sodium borate slopes. Significant improvements to Ado, Gly, Hist, NE, DA, and DOPAC occurred when using 100 mM sodium carbonate as the buffer. Slopes were decreased for 5HT, NM, 3MT, and HVA. Unpaired two-tailed Students t test statistics were performed (B). Data expressed as percent borate ± SD. *p < 0.05, n = 3.
Table 1.
Improvements in sensitivity using sulfuric acid compared to formic acid additive to reagent mixture. Standards were derivatized with sodium carbonate (100 mM), BzCl (2% (v/v) in acetonitrile), and an internal mixture that contained 20% (v/v) acetonitrile with 1% (v/v) formic acid or sulfuric acid.
| Analyte | Retention Time (min) |
Concentration (nM) |
Formic Acid Peak Area |
Sulfuric Acid Peak Area |
Increase with Sulfuric (%) |
|---|---|---|---|---|---|
| ACh | 1.2 | 50 | 9409 | 8219 | 87 |
| Tau | 2.3 | 2000 | 17373 | 17723 | 102 |
| Hist | 2.3 | 200 | 78624 | 81494 | 104 |
| Ser | 2.6 | 5000 | 19108 | 20770 | 109 |
| Asp | 2.8 | 200 | 1118 | 1324 | 118 |
| Gly | 2.9 | 5000 | 4163 | 4695 | 113 |
| Glu | 3.1 | 2000 | 19442 | 23809 | 122 |
| GABA | 3.7 | 200 | 23076 | 30285 | 131 |
| Ado | 4.6 | 200 | 8894 | 12443 | 140 |
| 5HIAA | 5.2 | 500 | 12758 | 16814 | 132 |
| HVA | 5.3 | 500 | 54869 | 69455 | 127 |
| NM | 5.5 | 20 | 13240 | 31391 | 237 |
| DOPAC | 5.9 | 500 | 158474 | 261578 | 165 |
| 5HT | 5.9 | 20 | 2221 | 10703 | 482 |
| NE | 6.0 | 20 | 5974 | 28457 | 476 |
| 3MT | 6.0 | 20 | 16229 | 71462 | 440 |
| DA | 6.4 | 20 | 21510 | 100588 | 468 |
We found that sodium carbonate instead of borate buffer significantly improved sensitivity (i.e. slope of the calibration curve; Figure 1) for compounds containing a 1,2 diol group, such as dopamine, norepinephrine, and DOPAC (Figure 2a). Use of 100 mM carbonate buffer instead of 100 mM borate increased the slope of norepinephrine 221% (t(4) = 19.6, p < 0.0001), dopamine 170% (t(4) = 27.7, p < 0.0001), and DOPAC 330% (t(4) = 39.5, p < 0.0001). The slope was increased 2550% for adenosine (t(4) = 52.0, p < 0.0001), which is also a diol. The slope increased slightly for two compounds without diols: glycine 103% (t(4) = 5.6, p < 0.01) and histamine 110% (t(4) = 5.4, p < 0.01). Although these compounds improved, the calibration slope was reduced 25% for serotonin (t(4) = 4.2, p < 0.05), 10% for normetanephrine (t(4) = 5.3, p < 0.01), 20% for 3MT (t(4) = 7.7, p < 0.01), and 10% for HVA (t(4) = 2.9, p < 0.05). These small decreases in slope are a reasonable trade-off for the large gains for the diols.
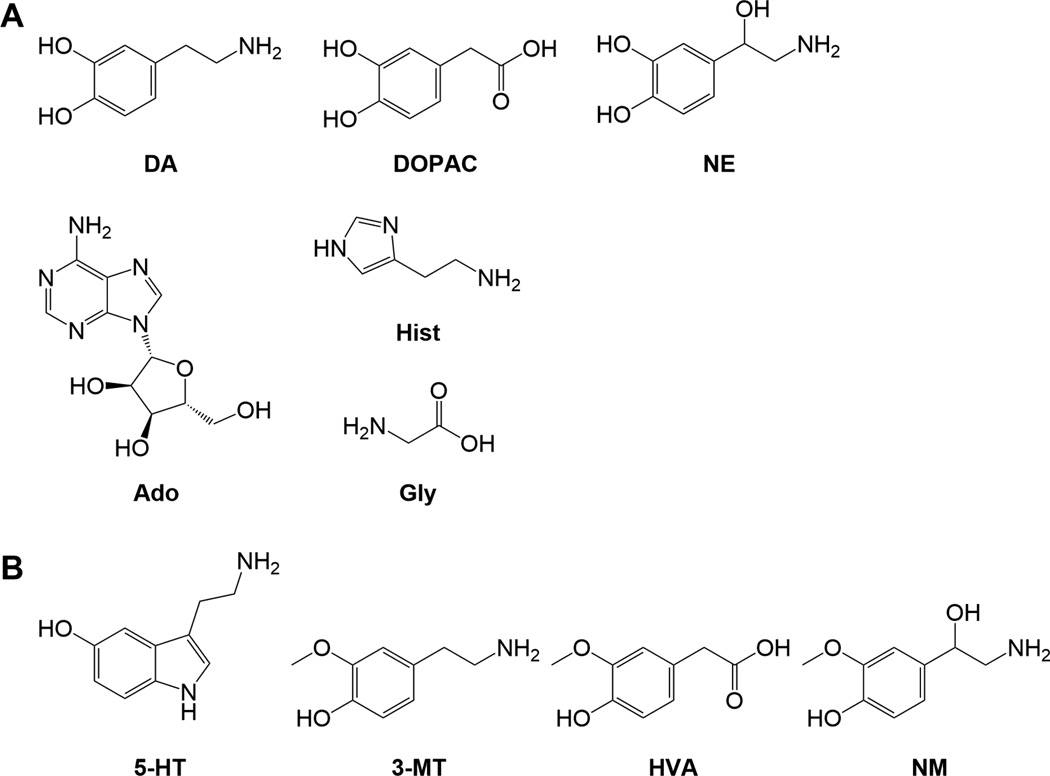
Figure 2.
Chemical structures of neurochemicals enhanced by sodium carbonate buffer (A). Structures of neurochemical reduced with carbonate buffer (B).
Improved catechol detection sensitivity may relate to how the buffers interact with 1,2 diol groups. Both borate and carbonate can be used as protecting groups for 1,2 diols [33]; however, cyclic borates are deprotected using dilute acid [33, 34], while cyclic carbonates hydrolyze in water [33, 35]. The protection of the carbonate group is more readily reversed than borate due to the high aqueous content of the sample, allowing for greater access of the diols for BzCl. The reason for the decreases in sensitivity of some compounds is unclear, but several of the compounds with decreased slopes have an ortho configuration of an alcohol and methoxy group (Figure 2b).
A potential problem with the BzCl assay is that organic solvent in the injected sample could cause poor peak shape for the most polar analytes, particularly ACh (Supplemental C). However, some organic solvent is needed to maintain solubility of the hydrophobic internal standards that are added to the sample. Replacing DMSO in the internal standard mixture with 20% (v/v) acetonitrile improved peak shape and signal intensity for acetylcholine, while retaining sufficient organic content to maintain solubility of hydrophobic compounds. The peak area for acetylcholine standards treated with internal standards in 20% (v/v) acetonitrile increased 5-fold relative to samples treated with internal standards in DMSO. This change in solvent reduces the final organic content of the samples, so band broadening is reduced for polar metabolites such as acetylcholine, as the sample composition is more closely matched in elution strength to the initial gradient conditions.
Substituting 1% (v/v) sulfuric acid for 1% (v/v) formic acid in the internal standard mixture improved signals of late eluting compounds by 237% for normetanephrine, 165% for DOPAC, 481% for serotonin, 476% for norepinephrine, 440% for 3MT, and 468% for dopamine (Table 1). While the explanation for this increased signal is unclear, we hypothesize that it is due to the decreased formation of formate adducts late in the gradient. The production of undetectable formate adducts limits the production of detectable proton adducts. Formic acid is used in our mobile phase A, so early eluting compounds may still form formate adducts; whereas later eluting compounds have less likelihood of formate adducts due to the lack of formic acid in sample and mobile phase B.
To reduce the number of reagent addition steps and the dilution associated with derivatization, we added the d4-acetylcholine internal standard to the 13C-labeled internal standards and introduced all internal standards in one step. This modification had no effect on d4-acetylcholine or the 13C-labeled compounds.
3.2 Addition of new compounds
To illustrate the potential for more comprehensive measurement of neurochemical pathways with this assay, 53 compounds were added to the original 17 compound assay (Supplemental A). The selected 70 compounds include 19 proteinogenic amino acids and intermediates in the metabolism of phenylalanine, tyrosine, tryptophan, and arginine. Phenylalanine and tyrosine are precursors to the catecholamines and several trace amines, so many metabolites in this pathway were added. These include several norepinephrine metabolites (e.g. VMA and MOPEG), as well as tyrosine derivatives such as tyramine and octopamine. Trace amines (tyramine, octopamine, tryptamine, and phenethylamine) play prominent roles in many invertebrate species [36–40], and are present as metabolic by-products in the mammalian central nervous system, where they may neuromodulate biogenic amine signaling [40, 41]. Serotonin is derived from tryptophan, so several intermediates in tryptophan metabolism were included (Supplemental D). These include 5HTP, the direct precursor to serotonin, as well as serotonin metabolite N-acetylserotonin, which is particularly relevant in flies [42, 43]. Monoamine oxidase (MAO) activity is limited in flies, so metabolites produced via MAO (e.g., 5HIAA) are not typically observed. Instead, monoamines are preferentially metabolized by N-acetylation, producing compounds such as N-acetylserotonin [42–44]. Tryptophan is also the precursor to kynurenine and its metabolites, which may have both neuroprotective and neurotoxic properties [45]. Several intermediates in arginine metabolism were also included in the method. Arginine is involved in the urea cycle and nitric oxide production. Ornithine, another member of the urea cycle, serves as a precursor for many polyamines, ubiquitous small molecules with a broad array of functions [46], whose dysfunction are associated with neurodegenerative disease [47, 48]. Thiol-containing dipeptide glutathione, and histidine-containing dipeptides, carnosine and metabolite anserine, have antioxidative effects in the brain [49–51], and decreased glutathione activity is associated with oxidative stress. Postmortem prefrontal-cortex tissue from human patients with psychiatric conditions such as bipolar, depression, and schizophrenia, show decreased levels of glutathione [51]. Carnosine may be neuroprotective by inhibiting the formation of β-amyloid polymerization and α-synuclein oligomerization [50], toxic species in Alzheimer’s and Parkinson’s diseases. Glucose indicates neuronal energy expenditure, and alterations of normal glucose metabolism can lead to synaptic dysfunction, including glucose hypometabolism in Alzheimer’s disease and Parkinsonian patients with dementia [52–55].
All 70 analytes of interest and their internal standards are benzoylated, except acetylcholine and choline, and detected by MS/MS (Supplemental Eexcept acetylcholine and choline, and detected by MS/MS (Supplemental E and F). Analytes were labeled 1–4 times with BzCl depending on the functional groups. In all cases, only the fully labeled compounds were observed, indicating quantitative (i.e., complete labeling) reactions. As an example, dopamine is triply labeled; singly and doubly labeled dopamine were not detectable. Protonated benzoylation products (MW + 1) were observed for most compounds with ESI in positive mode. A protonated water loss was observed for octopamine, normetanephrine, and synephrine (MW − 18 + 1), and the ammonium adduct (MW + 18) was detected for VMA, MOPEG, 5HIAA, HVA. DOMA, DOPEG, and DOPAC. A sodium adduct was observed for glucose (MW + 23). Other hexoses (e.g. fructose, mannose, and galactose) were resolved chromatographically or by MRM. For acetylcholine and choline, the unlabeled molecular ions were used for detection.
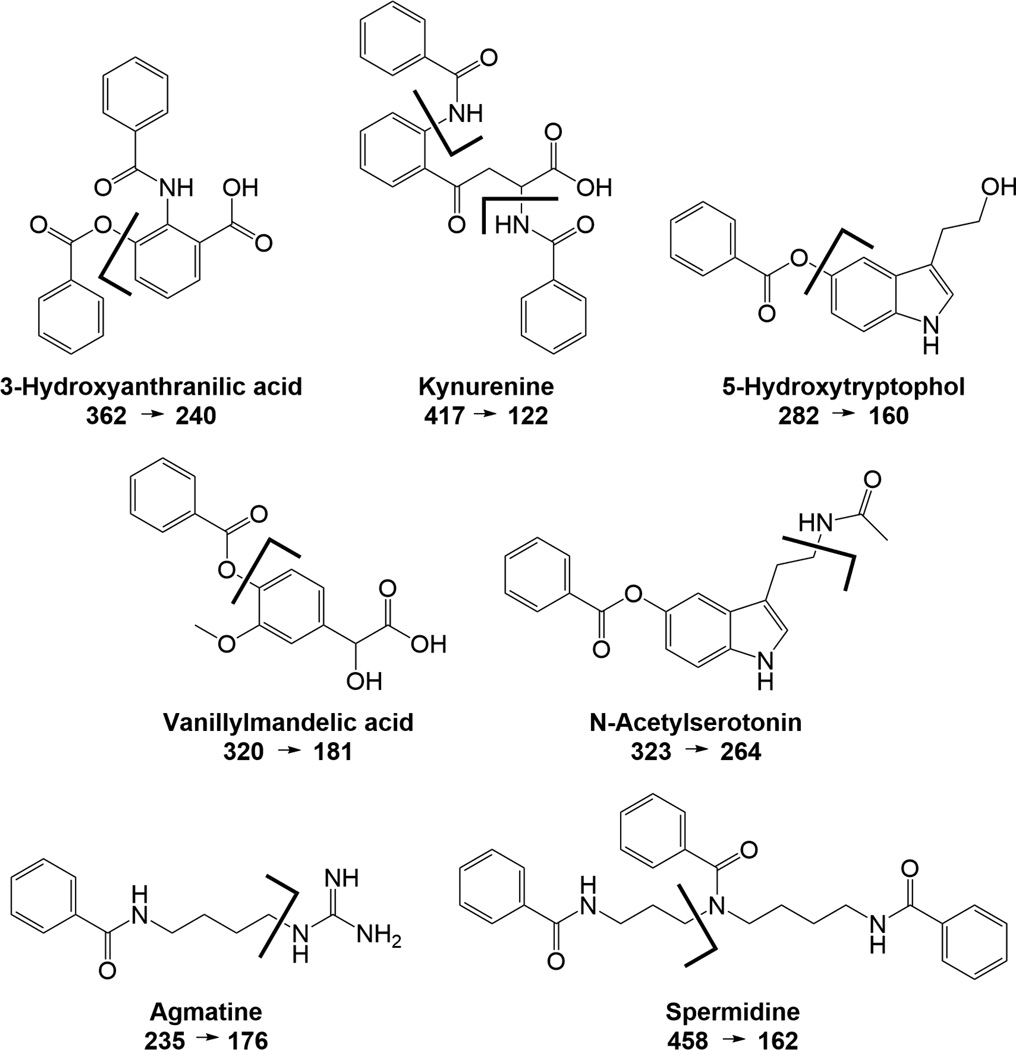
Analytes were detected by MS/MS under collision activated dissociation (CAD) conditions. The fragmentation of each analyte was examined to determine the best product ion to use for quantification (Supplemental E and F). For benzoylated analytes, the benzoyl fragment of 105 m/z was usually the most abundant product ion, and used for dMRM. Unique fragments for acetylcholine, choline, histidine, carnosine, phenylalanine, kynurenine, adenosine, tryptamine, 5HIAA, tryptophan, 5HTOL, spermidine, ornithine, kyotorphin, agmatine, N-acetylputrescine, VMA, glucose, lysine, 3HAA, 3HK, N-acetylserotonin, serotonin, 5HTP, and spermine were identified. These correspond to immonium ions for histidine, phenylalanine, and tryptophan, y ions for kyotorphin and carnosine, and the adenine moiety for adenosine. Several fragmentation patterns are shown in Figure 3. When possible, unique fragments were chosen for quantification to increase the selectivity of the assay for these compounds, reducing the likelihood of interferences from unknowns with similar precursor masses. The unique fragments have comparable or increased sensitivity relative to the 105 fragments for those compounds.
Figure 3.
Fragmentation patterns for select benzoyl labeled compounds. Analytes were detected by MS/MS under collision activated dissociation (CAD) conditions. While the benzoyl fragment of 105 m/z was the most abundant product ion for most analytes detected, unique fragments were chosen for detection to increase the selectivity of the assay for these compounds.
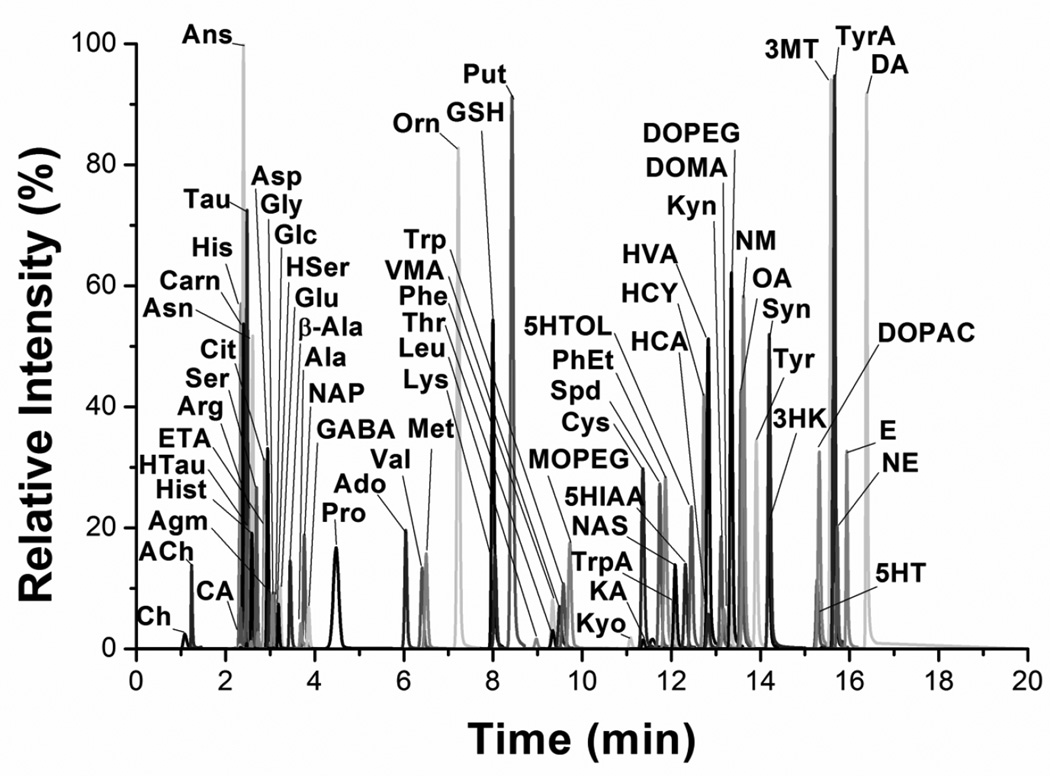
After determining the MS/MS transitions, a gradient was developed to separate the analytes (Figure 4). The gradient was not designed to fully resolve all analytes but to spread the analytes out over the 20 min separation time and minimize the number of dMRMs at any given time. The gradient results show that even very polar compounds like dopamine can be well retained after benzoylation. Total analysis time for each sample is around 33 min including injection time, elution and column re-equilibration on the Waters nanoAcquity system. Higher throughput may be possible. In preliminary tests, the method was transferred to a higher flow rate HPLC with higher pressure limits and the separation time was reduced to 12 min, with a total analysis time around 14 min. Further reductions in the analysis time per sample can be achieved as LC pressure limits and MS scan rates continue to increase.
Figure 4.
Reconstructed ion chromatogram of 70 compounds detected in 20 min. Extracted ion chromatograms for each compound at the highest concentration calibration standard run, were normalized to highest intensity and overlaid.
The method yields good detection limits, linearity, reproducibility and low carryover for all detected compounds (Table 2). All detection limits were better than 10 nM except for glutathione, alanine, citrulline, glycine, serine, and glucose [56]. While limits of detection for these select compounds were higher than other reported compounds in the assay, these levels were below the observed concentrations in dialysate and CSF.
Table 2.
Summary of limits of detection (LOD), carryover, relative standard deviation (n = 3), and R2 value of a six-point calibration for aqueous standards.
| Analyte | LoD (nM) |
Carryover (%) |
RSD (%) |
Fit (R2) |
Analyte | LoD (nM) |
Carryover (%) |
RSD (%) |
Fit (R2) |
|---|---|---|---|---|---|---|---|---|---|
| Ach | 1 | 1 | 0.7 | 0.9996 | Thr | 5 | 0.3 | 1 | 0.9995 |
| Ch | 3 | 0.2 | 1 | 0.9997 | VMA | 1 | 0.1 | 1 | 0.9999 |
| CA | 4 | 0.02 | 2 | 1.0000 | Trp | 1 | 0.1 | 0.9 | 0.9997 |
| His | 2 | 0.1 | 2 | 0.9996 | MOPEG | 0.7 | 0.08 | 0.6 | 0.9999 |
| Ans | 0.4 | 0.03 | 2 | 0.9999 | Kyo | 0.2 | 0.2 | 2 | 0.9998 |
| Carn | 0.8 | 0.04 | 2 | 0.9999 | Cys | 1 | 0.1 | 5 | 0.9971 |
| HTau | 7 | 0.05 | 4 | 0.9977 | KA | 1 | 0.1 | 0.9 | 0.9997 |
| Tau | 3 | 0.02 | 1 | 0.9998 | Spd | 0.09 | 0.1 | 0.5 | 0.9994 |
| Arg | 1 | 0.5 | 3 | 0.9576 | PhEt | 0.08 | 0.09 | 2 | 0.9989 |
| Hist | 0.09 | 0.04 | 3 | 0.9996 | TrpA | 0.1 | 0.09 | 3 | 0.9993 |
| Asn | 2 | 0.1 | 2 | 0.9999 | NAS | 0.09 | 0.06 | 0.8 | 0.9997 |
| Ser | 70 | 0.6 | 1 | 0.9976 | 5HIAA | 0.7 | 0.08 | 0.8 | 0.9999 |
| Gln | 4 | 0.2 | 3 | 0.9998 | 5HTOL | 0.9 | 0.07 | 1 | 0.9997 |
| HSer | 11 | 0.5 | 3 | 0.9994 | HCY | 0.9 | 0.08 | 1 | 0.9968 |
| Cit | 20 | 2 | 2 | 0.9965 | 3HAA | 1 | 0.1 | 0.9 | 0.9996 |
| ETA | 6 | 0.4 | 2 | 0.9997 | HCA | 1 | 0.09 | 2 | 0.9998 |
| Asp | 8 | 0.05 | 1 | 0.9999 | HVA | 0.6 | 0.07 | 0.4 | 1.0000 |
| Agm | 1 | 0.03 | 4 | 0.9997 | DOMA | 0.3 | 0.2 | 3 | 0.9998 |
| Glc | 160 | 0.06 | 6 | 0.9997 | Kyn | 1 | 0.1 | 3 | 0.9994 |
| Gly | 30 | 0.09 | 7 | 0.9997 | Spm | 0.1 | 0.1 | 2 | 0.9984 |
| Glu | 0.3 | 0.2 | 1 | 1.0000 | DOPEG | 0.1 | 0.1 | 1 | 0.9992 |
| BAla | 5 | 0.09 | 1 | 1.0000 | 5HTP | 2 | 0.2 | 3 | 0.9996 |
| Ala | 20 | 2 | 2 | 0.9994 | OA | 0.2 | 0.2 | 1 | 0.9983 |
| NAP | 0.5 | 0.05 | 1 | 0.9999 | NM | 0.08 | 0.09 | 2 | 0.9988 |
| GABA | 0.5 | 0.4 | 2 | 0.9997 | Tyr | 4 | 2 | 3 | 0.9950 |
| Pro | 5 | 0.5 | 0.4 | 0.9996 | 3HK | 8 | 0.06 | 2 | 0.9941 |
| Ado | 1 | 0.1 | 0.2 | 0.9956 | Syn | 0.2 | 0.2 | 0.1 | 0.9982 |
| Val | 7 | 0.6 | 3 | 0.9998 | 5HT | 0.4 | 0.4 | 1 | 0.9970 |
| Met | 0.7 | 0.09 | 1 | 0.9998 | DOPAC | 0.2 | 0.2 | 2 | 0.9999 |
| Orn | 7 | 0.4 | 0.2 | 0.9963 | 3MT | 0.2 | 0.2 | 2 | 0.9987 |
| GSH | 10 | 0.1 | 2 | 0.9999 | LDOPA | 1 | 0.9 | 2 | 0.9999 |
| Lys | 4 | 2 | 0.7 | 0.9867 | TyrA | 0.2 | 0.3 | 1 | 0.9964 |
| Put | 0.1 | 0.1 | 1 | 0.9999 | NE | 0.3 | 0.2 | 2 | 0.9970 |
| Leu | 5 | 4 | 2 | 0.9894 | E | 0.3 | 0.2 | 1 | 0.9964 |
| Phe | 3 | 0.2 | 0.8 | 0.9999 | DA | 0.3 | 0.3 | 3 | 0.9965 |
3.3 Application of 70 compound assay in various matrices
To test the versatility of the assay, we analyzed several types of biological samples, including rat striatal dialysate, human CSF, human serum, and Drosophila tissue homogenate (Tables 3–5). 57 compounds were detected in dialysate samples, whereas 35 and 50 compounds were above the limits of detection in human CSF and serum samples, respectively. Drosophila heads and bodies were isolated and analyzed separately, with detection of 44 compounds in head and 42 compounds in bodies. 54 compounds were detected in hemolymph from Drosophila.
Table 3.
Application of 70 compound method to analyze rat dialysate and human CSF. The average of 3 repeated injections with standard deviation is reported below. Values for analytes were reported only if they were above the LoD.
| Analyte | Concentration (nM) | Analyte | Concentration (nM) | ||
|---|---|---|---|---|---|
| Rat Dialysate | Human CSF | Rat Dialysate | Human CSF | ||
| ACh | 12.2 ± 0.1 | 1.19 ± 0.04 | Thr | 691 ± 7 | 65 ± 2 |
| Ch | 1212 ± 5 | 14 ± 2 | VMA | ||
| CA | 2170 ± 90 | 300 ± 30 | Trp | 141 ± 4 | 20.9 ± 0.2 |
| His | 930 ± 10 | 91.4 ± 0.8 | MOPEG | 4.3 ± 0.3 | |
| Ans | Kyo | ||||
| Carn | 14.0 ± 0.4 | Cys | 503 ± 9 | ||
| HTau | KA | ||||
| Tau | 1820 ± 70 | 33 ± 2 | Spd | 2.38 ± 0.08 | 0.19 ± 0.02 |
| Arg | 1380 ± 70 | 211 ± 6 | PhEt | 0.53 ± 0.02 | |
| Hist | 0.76 ± 0.08 | 0.20 ± 0.03 | TrpA | ||
| Asn | 40 ± 1 | 6.6 ± 0.3 | NAS | 0.23 ± 0.02 | |
| Ser | 4100 ± 250 | 570 ± 40 | 5HIAA | 390 ± 6 | 2.4 ± 0.2 |
| Gln | 37300 ± 1300 | 4080 ± 60 | 5HTOL | 1.9 ± 0.1 | |
| HSer | 2920 ± 20 | 266 ± 7 | HCY | 4.91 ± 0.03 | |
| Cit | 390 ± 10 | 3HAA | |||
| ETA | 6980 ± 310 | 124 ± 4 | HCA | 1050 ± 30 | 4.7 ± 0.3 |
| Asp | 108 ± 7 | 15 ± 2 | HVA | 1130 ± 40 | 4.4 ± 0.4 |
| Agm | DOMA | 0.47 ± 0.02 | |||
| Glc | 633000 ± 85000 | 55400 ± 840 | Kyn | 7.1 ± 0.3 | |
| Gly | 690 ± 10 | 52 ± 5 | Spm | 2.9 ± 0.1 | |
| Glu | 21 ± 1 | 4.9 ± 0.1 | DOPEG | 1.26 ± 0.03 | |
| BAla | 5.8 ± 0.9 | 5HTP | |||
| Ala | 5260 ± 160 | 306 ± 8 | OA | ||
| NAP | 1.0 ± 0.1 | 0.74 ± 0.02 | NM | 0.30 ± 0.01 | |
| GABA | 40.5 ± 0.6 | 3.3 ± 0.2 | Tyr | 350 ± 20 | 76.6 ± 0.3 |
| Pro | 1326 ± 8 | 13.8 ± 0.2 | 3HK | ||
| Ado | 112.8 ± 0.6 | Syn | |||
| Val | 1760 ± 20 | 104 ± 2 | 5HT | 0.89 ± 0.02 | |
| Met | 855 ± 6 | 26.2 ± 0.5 | DOPAC | 598 ± 5 | 0.6 ± 0.1 |
| Orn | 196 ± 3 | 54.9 ± 0.8 | 3MT | 8.5 ± 0.2 | |
| GSH | 74 ± 2 | LDOPA | 4.00 ± 0.04 | ||
| Lys | 4030 ± 170 | 249 ± 7 | TyrA | 0.21 ± 0.02 | |
| Put | 0.83 ± 0.03 | 0.41 ± 0.03 | NE | 1.00 ± 0.08 | |
| Leu | 2200 ± 140 | 102 ± 4 | E | ||
| Phe | 710 ± 5 | 66.9 ± 0.8 | DA | 29.4 ± 0.8 | |
Table 5.
Application of 70 compound method with a protein precipitation step for fly bodies and heads. The average of 3 repeated injections with standard deviation is reported below. Values for analytes were reported only if they were above the LoD.
| Analyte | Amount (pmol/fly) | Analyte | Amount (pmol/fly) | ||
|---|---|---|---|---|---|
| Fly Bodies | Fly Heads | Fly Bodies | Fly Heads | ||
| ACh | 2.64 ± 0.08 | 0.212 ± 0.011 | Thr | 18.4 ± 1.8 | 0.98 ± 0.04 |
| Ch | 312 ± 8.8 | 66.36 ± 1.14 | VMA | ||
| CA | Trp | 17.1 ± 0.9 | 0.44 ± 0.02 | ||
| His | 2.7 ± 0.1 | 0.27 ± 0.04 | MOPEG | ||
| Ans | Kyo | ||||
| Carn | Cys | ||||
| HTau | 21 ± 6 | KA | 1.9 ± 0.1 | 0.4 ± 0.04 | |
| Tau | 278.5 ± 4.1 | 88.81 ± 3.19 | Spd | 0.03 ± 0.002 | 0.01 ± 0.0003 |
| Arg | 0.45 ± 0.01 | 0.06 ± 0.004 | PhEt | 0.336 ± 0.015 | 0.005 ± 0.0004 |
| Hist | 1.92 ± 0.13 | 0.772 ± 0.011 | TrpA | 1.85 ± 0.09 | 0.024 ± 0.002 |
| Asn | 6.5 ± 0.7 | 0.74 ± 0.04 | NAS | 29.61 ± 0.7 | 4.24 ± 0.242 |
| Ser | 0.8 ± 0.4 | 5HIAA | 0.47 ± 0.07 | 0.025 ± 0.001 | |
| Gln | 54.99 ± 0.59 | 5.61 ± 0.14 | 5HTOL | ||
| HSer | 4.5 ± 1.1 | 0.4 ± 0.01 | HCY | ||
| Cit | 3HAA | ||||
| ETA | 44.03 ± 0.92 | 5.56 ± 0.27 | HCA | 0.07 ± 0.01 | |
| Asp | HVA | ||||
| Agm | DOMA | ||||
| Glc | 3170 ± 295 | 1111.5 ± 0.2 | Kyn | 8 ± 0.7 | |
| Gly | 8 ± 1 | 0.8 ± 0.004 | Spm | ||
| Glu | 1.1 ± 0.2 | 0.21 ± 0.03 | DOPEG | ||
| BAla | 83.5 ± 0.7 | 3.04 ± 0.13 | 5HTP | 184.1 ± 3.6 | 1.83 ± 0.04 |
| Ala | 90.4 ± 5.4 | 22.84 ± 2.69 | OA | 0.003 ± 0.0002 | |
| NAP | 0.16 ± 0.01 | 0.009 ± 0.0001 | NM | ||
| GABA | 6.25 ± 0.18 | 1.661 ± 0.055 | Tyr | 8.4 ± 0.2 | 0.35 ± 0.007 |
| Pro | 614.3 ± 2.8 | 100.46 ± 2.2 | 3HK | 36 ± 3 | 1.7 ± 0.1 |
| Ado | 31.1 ± 0.12 | 2.599 ± 0.038 | Syn | ||
| Val | 22.3 ± 0.3 | 2.17 ± 0.1 | 5HT | 81.66 ± 10.34 | 5.98 ± 0.666 |
| Met | 16.17 ± 0.58 | 1.186 ± 0.025 | DOPAC | 0.09 ± 0.003 | 0.013 ± 0.001 |
| Orn | 3MT | ||||
| GSH | LDOPA | 1.5 ± 0.13 | 0.541 ± 0.026 | ||
| Lys | 0.2 ± 0.02 | TyrA | 11.89 ± 0.64 | 0.062 ± 0.001 | |
| Put | 0.08 ± 0.001 | 0.0074 ± 0.0003 | NE | ||
| Leu | 53.6 ± 1.9 | 3.84 ± 0.04 | E | ||
| Phe | 18.4 ± 0.3 | 1.02 ± 0.03 | DA | 0.22 ± 0.01 | 0.005 ± 0.0001 |
All commonly studied neurotransmitters (i.e. GABA, glutamate, and monoamines) were within expected ranges in rat striatal dialysate (Table 3). Several detectable compounds were not previously reported in rat dialysate or tissue homogenate studies, and include homoserine, a precursor to amino acids threonine and methionine; N-acetylputrescine, a metabolite of polyamine putrescine; and DOMA, a norepinephrine metabolite. Polyamines putrescine, spermidine, and spermine were also detected in the dialysate sample. The norepinephrine and normetanephrine metabolites MOPEG, DOPEG, and DOMA (but not epinephrine or VMA) were detected in rat dialysate, demonstrating the potential for analysis of metabolic pathways.
Analysis of human serum, derivatized after protein precipitation, revealed kyotorphin at 31 nM concentration. Kyotorphin is an endogenous analgesic dipeptide with potential neuroprotective properties. It has previously been found in rat brain tissue and human CSF samples [57, 58]. This is the first report of quantitative detection of kyotorphin in human serum. Kyotorphin is proposed to have indirect opioid-like actions by modulating enkephalin release [59]. Kyotorphin does not cross the blood brain barrier, and is a candidate biomarker for neurodegenerative diseases such as Alzheimer’s disease [57]. While previous studies detected kyotorphin in CSF samples obtained from lumbar puncture, less invasive blood sample collection would be beneficial for patients, with subsequent detection as reported here. Interestingly, kyotorphin was not detected in our analysis of pooled human CSF from healthy patients, which did not undergo a protein precipitation step prior to analysis.
Fly tissue homogenate contained detectable levels of tyramine and octopamine, which was expected as they are the fly analogs of epinephrine and norepinephrine, respectively. 5HTP pretreatment of the flies resulted in high levels of 5HTP in both bodies and heads. 5HTP metabolites serotonin and N-acetylserotonin were also elevated, though the effect was more pronounced in the bodies. Interestingly, 5HIAA was observed in both bodies and heads, despite the expected lack of MAO activity in flies, likely due to the excess of 5HTP [44].
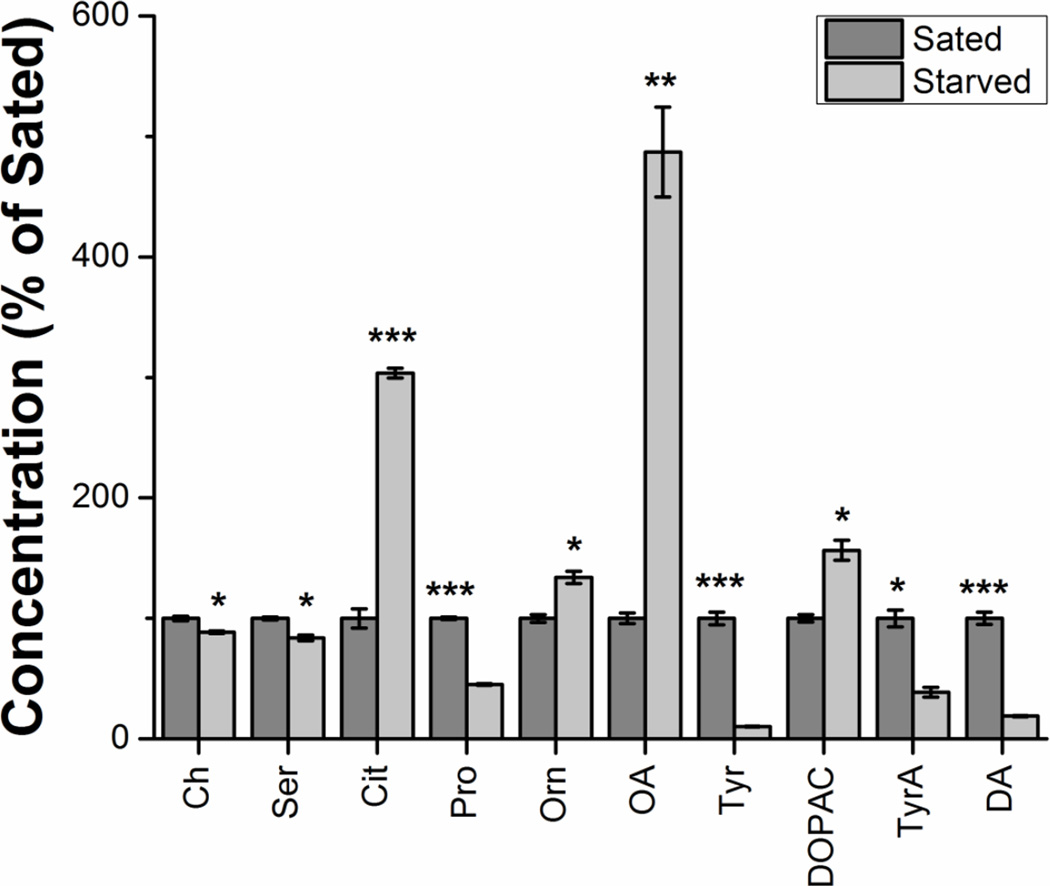
Of the 54 compounds detected in fly hemolymph, 10 showed significant (p < 0.05) changes between starved and sated states (Figure 5). These compounds were Ch (t(4) = 9.7, p < 0.0001); Ser (t(4) = 10.5, p < 0.0001), Cit (t(4) = 39.3, p < 0.0001), Pro (t(4) = 80.2, p < 0.0001), Orn (t(4) = 9.8, p < 0.0001), OA (t(4) = 17.9, p < 0.0001), Tyr (t(4) = 29.5, p < 0.0001), DOPAC (t(4) = 11.0, p < 0.0001), TyrA (t(4) = 13.1, p < 0.0001), DA (t(4) = 27.2, p < 0.0001). Of particular note was a nearly 5 fold increase of octopamine in starved flies relative to sated flies. Increased octopamine activity has been reported in flies upon starvation, and has been linked to foraging-like behaviors as the flies presumably try to locate food [60, 61]. The roles of other implicated metabolites are currently undergoing further investigation.
Figure 5.
Metabolites showing significant differences between sated and starved states in fly hemolymph. Metabolite concentrations were normalized to total protein content, and then normalized to the sated sample. Each sample was run in triplicate. Unpaired two-tailed Students t tests were performed, and the Holm-Bonferroni correction was used. Data expressed as average ± SD. *p < 0.05; **p < 0.01; ***p < 0.001.
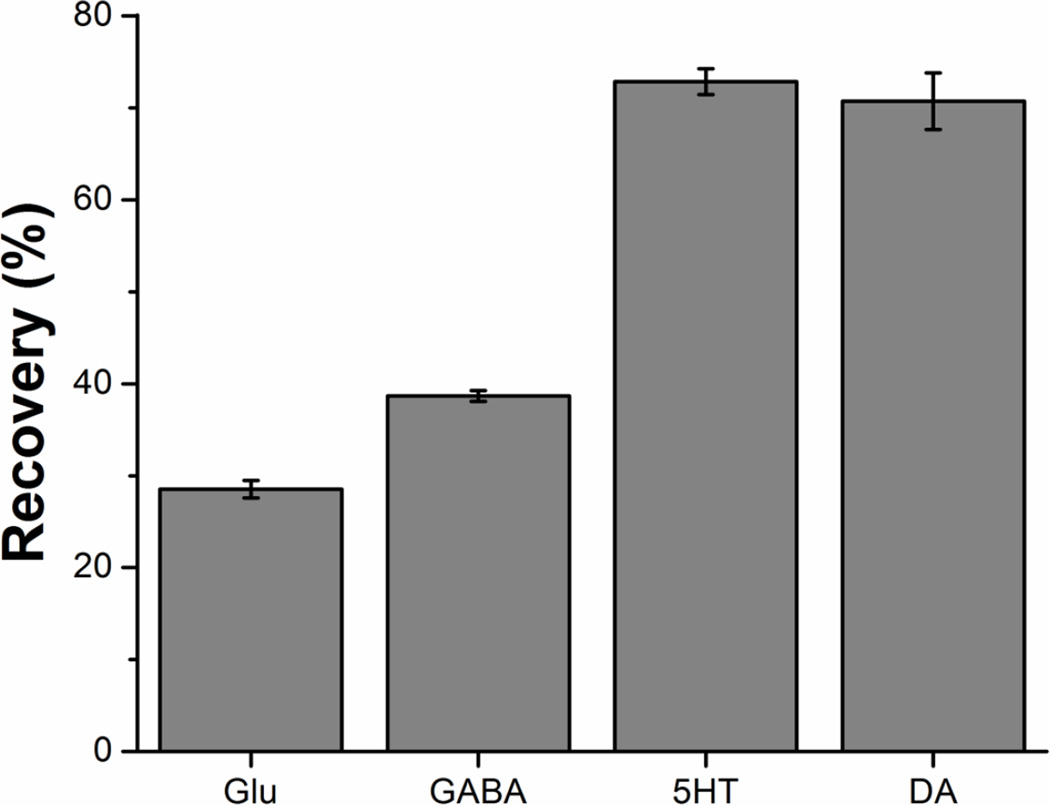
Protein removal prior to analysis of bodily fluids and tissue homogenate prevents column contamination and exposure of the HPLC-MS to high protein concentrations. Many extraction techniques are used in metabolomics [62–64]. These methods vary in effectiveness based on the sample type and target metabolites, and require optimization for each assay. Solvent precipitation with cold acetonitrile was selected for its simplicity and reproducibility. To evaluate the effect of protein precipitation on recovery and reproducibility we spiked known amounts of isotopically labeled glutamate, GABA, serotonin, and dopamine into serum prior to solvent precipitation. We then measured concentrations of the isotopically labeled compounds after solvent precipitation and derivatization, and compared these measured concentrations to the known amount spiked into serum to determine the relative recovery (Figure 6). The recovery varied for each tested metabolite, but was reproducible (RSD < 8%). As such, we concluded that fair comparison could be made between samples analyzed using this method, though comparisons to other methods would require correction for recovery.
Figure 6.
Recovery of four isotopically labeled metabolites spiked into plasma prior to solvent precipitation and derivatization. Percent recovery calculated as measured concentration after precipitation, relative to concentration spiked into serum. The average of three extraction replicates is shown. Error bars represent the standard error of the mean.
4. Conclusions
These results demonstrate the utility of BzCl derivatization with HPLC-MS/MS for targeted neurochemical metabolomics. Improvements to the benzoylation of small neurochemicals resulted in a comprehensive, robust, and quantitative method to monitor 70 neurochemicals. This modified method improves sensitivity for compounds containing 1,2-diols and early eluting peaks such as acetylcholine, and was expanded to 4-fold more neurochemicals compared to prior studies. The method is suitable for multiple samples types, including CSF, serum, and tissue homogenate.
The results also indicate considerable potential for even wider use of BzCl as a MS labeling reagent. For example, the MRC2 maintains a library of over 1,000 metabolites. Based on the reactivity of BzCl towards amines, phenols, thiols, and some alcohols, we estimate it could be used to label approximately 25% of these compounds. BzCl labeling is fast and simple to implement. Benzoylation improves sensitivity, retention, and quantification (via easily generated internal standards) with few drawbacks compared to direct detection of analytes.
Supplementary Material
Table 4.
Application of 70 compound method after protein precipitation for human serum. The average of 3 repeated injections with standard deviation is reported below. Values for analytes were reported only if they were above the LoD.
| Analyte | LoD (nM) |
Concentration (nM) Human Serum |
Analyte | LoD (nM) |
Concentration (nM) Human Serum |
|---|---|---|---|---|---|
| Ach | 4 | 380 ± 10 | Thr | 30 | 62000 ± 5000 |
| Ch | 50 | 15200 ± 200 | VMA | 20 | |
| CA | 50 | Trp | 30 | 51000 ± 3000 | |
| His | 30 | 37000 ± 1000 | MOPEG | 5 | 20 ± 5 |
| Ans | 3 | 160 ± 20 | Kyo | 5 | 31 ± 3 |
| Carn | 6 | 14 ± 3 | Cys | 30 | 1600 ± 100 |
| HTau | 500 | 160000 ± 10000 | KA | 40 | 220 ± 30 |
| Tau | 60 | 115000 ± 2000 | Spd | 2 | 41 ± 0.6 |
| Arg | 10 | 7100 ± 300 | PhEt | 1 | |
| Hist | 2 | 41 ± 2 | TrpA | 2 | |
| Asn | 70 | 20900 ± 800 | NAS | 2 | |
| Ser | 700 | 45000 ± 4000 | 5HIAA | 5 | 67 ± 4 |
| Gln | 10 | 180000 ± 10000 | 5HTOL | 10 | 16 ± 2 |
| HSer | 100 | 65000 ± 2000 | HCY | 30 | |
| Cit | 20 | 9400 ± 200 | 3HAA | 20 | 76 ± 6 |
| ETA | 10 | 6300 ± 100 | HCA | 10 | 56 ± 7 |
| Asp | 200 | 2300 ± 200 | HVA | 20 | 57 ± 1 |
| Agm | 20 | DOMA | 6 | ||
| Glc | 500 | 1050000 ± 90000 | Kyn | 40 | 2380 ± 50 |
| Gly | 200 | 102000 ± 4000 | Spm | 2 | 39 ± 3 |
| Glu | 30 | 21800 ± 700 | DOPEG | 2 | |
| BAla | 20 | 2300 ± 400 | 5HTP | 30 | |
| Ala | 70 | 254000 ± 8000 | OA | 2 | |
| NAP | 7 | 24 ± 2 | NM | 2 | |
| GABA | 4 | 115 ± 4 | Tyr | 40 | 56000 ± 1000 |
| Pro | 30 | 24300 ± 3000 | 3HK | 200 | |
| Ado | 3 | Syn | 4 | ||
| Val | 40 | 160000 ± 10000 | 5HT | 3 | 300 ± 40 |
| Met | 8 | 21800 ± 300 | DOPAC | 5 | |
| Orn | 200 | 27400 ± 300 | 3MT | 4 | |
| GSH | 90 | LDOPA | 2 | 390 ± 20 | |
| Lys | 40 | 37000 ± 1000 | TyrA | 8 | |
| Put | 0.4 | 13 ± 1 | NE | 3 | 3.2 ± 0.7 |
| Leu | 40 | 139000 ± 3000 | E | 4 | |
| Phe | 30 | 63000 ± 3000 | DA | 2 |
Highlights.
Improved reaction conditions for benzoyl chloride labeling for HPLC-MS/MS analysis
Novel assay of 70 neurologically relevant compounds using benzoyl chloride labeling
Analysis of rat dialysate, fly tissue homogenate and hemolymph, human CSF and serum
Stable-isotope labeled internal standard for all analytes for quantification
Acknowledgments
The authors thank Prof. Charles Burant for providing human CSF samples and University of Michigan’s MRC2 (P30DK020572) for human serum and access to compounds used to make standards. O.S.M was funded by the Michael J. Fox Foundation Dyskinesia Challenge 2013. Fly tissue homogenate sample collection was supported by NIH F31 AG047696 (to J.R.) and NIH R01 AG023166. Fly hemolymph was provided by M.D. lab, with assistance from Jalal Taleb and Jeanna Yu; this work was supported by 4R00ND097141 to M.D. This work was supported by NIH R37 EB003320 and NIH R37 DK046960 to R.T.K.
Compound Abbreviations
- DOMA
3,4-Dihydroxymandelic acid
- DOPAC
3,4-Dihydroxyphenylacetic acid
- LDOPA
3,4-Dihydroxyphenylalanine
- DOPEG
3,4-Dihydroxyphenylglycol
- 3HAA
3-Hydroxyanthranilic acid
- 3HK
3-Hydroxykynurenine
- MOPEG
3-Methoxy-4-hydroxyphenylglycol
- 3MT
3-Methoxytyramine
- GABA
4-Aminobutyric acid
- 5HIAA
5-Hydroxyindoleacetic acid
- 5HTP
5-Hydroxytryptophan
- 5HTOL
5-Hydroxytryptophol
- Ach
Acetylcholine
- Ado
Adenosine
- Agm
Agmatine
- Ala
Alanine
- Ans
Anserine
- Arg
Arginine
- Asn
Asparagine
- Asp
Aspartate
- BAla
β-Alanine
- Carn
Carnosine
- Ch
Choline
- Cit
Citrulline
- CA
Cysteic acid
- Cys
Cysteine
- DA
Dopamine
- E
Epinephrine
- ETA
Ethanolamine
- Glc
Glucose
- Glu
Glutamate
- Gln
Glutamine
- GSH
Glutathione
- Gly
Glycine
- Hist
Histamine
- His
Histidine
- HCA
Homocysteic acid
- HCY
Homocysteine
- HSer
Homoserine
- HVA
Homovanillic acid
- HTau
Hypotaurine
- KA
Kynurenic acid
- Kyn
Kynurenine
- Kyo
Kyotorphin
- Leu
Leucine
- Lys
Lysine
- Met
Methionine
- NAP
N-Acetylputrescine
- NAS
N-Acetylserotonin
- NE
Norepinephrine
- NM
Normetanephrine
- OA
Octopamine
- Orn
Ornithine
- Phe
Phenylalanine
- PhEt
Phenylethylamine
- Pro
Proline
- Put
Putrescine
- Ser
Serine
- 5HT
Serotonin
- Spd
Spermidine
- Spm
Spermine
- Syn
Synephrine
- Tau
Taurine
- Thr
Threonine
- TrpA
Tryptamine
- Trp
Tryptophan
- TyrA
Tyramine
- Tyr
Tyrosine
- Val
Valine
- VMA
Vanillylmandelic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knee JM, Rzezniczak TZ, Barsch A, Guo KZ, Merritt TJS. A novel ion pairing LC/MS metabolomics protocol for study of a variety of biologically relevant polar metabolites. Journal of Chromatography B. 2013;936:63–73. doi: 10.1016/j.jchromb.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Michopoulos F, Whalley N, Theodoridis G, Wilson ID, Dunkley TPJ, Critchlow SE. Targeted profiling of polar intracellular metabolites using ion-pair-high performance liquid chromatography and -ultra high performance liquid chromatography coupled to tandem mass spectrometry: Applications to serum, urine and tissue extracts. Journal of Chromatography A. 2014;1349:60–68. doi: 10.1016/j.chroma.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Virgiliou C, Sampsonidis I, Gika HG, Raikos N, Theodoridis GA. Development and validation of a HILIC-MS/MS multitargeted method for metabolomics applications. Electrophoresis. 2015;36:2215–2225. doi: 10.1002/elps.201500208. [DOI] [PubMed] [Google Scholar]
- 4.Wei R, Li G, Seymour AB. High-throughput and multiplexed LC/MS/MRM method for targeted metabolomics. Analytical chemistry. 2010;82:5527–5533. doi: 10.1021/ac100331b. [DOI] [PubMed] [Google Scholar]
- 5.Yan Z, Yan R. Increase the accessibility and scale of targeted metabolomics: Construction of a human urinary metabolome-wide multiple reaction monitoring library using directly-coupled reversed-phase and hydrophilic interaction chromatography. Analytica Chimica Acta. 2015;894:65–75. doi: 10.1016/j.aca.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 6.Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nature protocols. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass spectrometry reviews. 2007;26:51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gummer J, Banazis M, Maker G, Solomon P, Oliver R, Trengove R. Use of mass spectrometry for metabolite profiling and metabolomics. Australian Biochemist. 2009;40:5–8. [Google Scholar]
- 9.Song P, Mabrouk OS, Hershey ND, Kennedy RT. In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Analytical chemistry. 2012;84:412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Kang A, Dai C, Liang Y, Xie T, Xie L, Peng Y, Wang G, Hao H. Quantitative analysis of neurochemical panel in rat brain and plasma by liquid chromatography–tandem mass spectrometry. Analytical chemistry. 2012;84:10044–10051. doi: 10.1021/ac3025202. [DOI] [PubMed] [Google Scholar]
- 11.Cox JM, Butler JP, Lutzke BS, Jones BA, Buckholz JE, Biondolillo R, Talbot JA, Chernet E, Svensson KA, Ackermann BL. A validated LC-MS/MS method for neurotransmitter metabolite analysis in human cerebrospinal fluid using benzoyl chloride derivatization. Bioanalysis. 2015;7:2461–2475. doi: 10.4155/bio.15.170. [DOI] [PubMed] [Google Scholar]
- 12.Aflaki F, Ghoulipour V, Saemian N, Salahinejad M. A simple method for benzoyl chloride derivatization of biogenic amines for high performance liquid chromatography. Analytical Methods. 2014;6:1482–1487. [Google Scholar]
- 13.Özdestan Ö, Üren A. A method for benzoyl chloride derivatization of biogenic amines for high performance liquid chromatography. Talanta. 2009;78:1321–1326. doi: 10.1016/j.talanta.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Asan A, Isildak I. Determination of major phenolic compounds in water by reversed-phase liquid chromatography after pre-column derivatization with benzoyl chloride. Journal of Chromatography A. 2003;988:145–149. doi: 10.1016/s0021-9673(02)02056-3. [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Wilson DM, Edinboro LE, McGuire GM, Williams SG, Thomas Karnes H. Improvement of sensitivity for the determination of propylene glycol in rat plasma and lung tissue using HPLC/tandem MS and derivatization with benzoyl chloride. Journal of liquid chromatography & related technologies. 2003;26:3413–3431. [Google Scholar]
- 16.Cai HL, Zhu RH, Li HD. Determination of dansylated monoamine and amino acid neurotransmitters and their metabolites in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. Analytical biochemistry. 2010;396:103–111. doi: 10.1016/j.ab.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Dai W, Huang Q, Yin P, Li J, Zhou J, Kong H, Zhao C, Lu X, Xu G. Comprehensive and highly sensitive urinary steroid hormone profiling method based on stable isotope-labeling liquid chromatography-mass spectrometry. Analytical chemistry. 2012;84:10245–10251. doi: 10.1021/ac301984t. [DOI] [PubMed] [Google Scholar]
- 18.Guo K, Li L. Differential 12C-/13C–isotope dansylation labeling and fast liquid chromatography/mass spectrometry for absolute and relative quantification of the metabolome. Analytical chemistry. 2009;81:3919–3932. doi: 10.1021/ac900166a. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Fang C, Smagin G. Derivatization for the simultaneous LC/MS quantification of multiple neurotransmitters in extracellular fluid from rat brain microdialysis. Journal of pharmaceutical and biomedical analysis. 2014;100:357–364. doi: 10.1016/j.jpba.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Bhandare P, Madhavan P, Rao B, Someswar rao N. Determination of amino acid without derivatization by using HPLC - HILIC column. J. Chem. Pharm. Res. 2010;2:372–380. [Google Scholar]
- 21.Buck K, Voehringer P, Ferger B. Rapid analysis of GABA and glutamate in microdialysis samples using high performance liquid chromatography and tandem mass spectrometry. J Neurosci Methods. 2009;182:78–84. doi: 10.1016/j.jneumeth.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Chirita-Tampu R-I, Fougere L, West C. Advantages of HILIC mobile phases for LC– ESI–MS–MS analysis of neurotransmitters. LCGC Europe. 2013;26 [Google Scholar]
- 23.González RR, Fernández RF, Vidal JLM, Frenich AG, Pérez MLG. Development and validation of an ultra-high performance liquid chromatography-tandem mass-spectrometry (UHPLC-MS/MS) method for the simultaneous determination of neurotransmitters in rat brain samples. J Neurosci Methods. 2011;198:187–194. doi: 10.1016/j.jneumeth.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Tufi S, Lamoree M, de Boer J, Leonards P. Simultaneous analysis of multiple neurotransmitters by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry. Journal of Chromatography A. 2015;1395:79–87. doi: 10.1016/j.chroma.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L-H, Cai H-L, Jiang P, Li H-D, Cao L-J, Dang R-L, Zhu W-Y, Deng Y. Simultaneous determination of multiple neurotransmitters and their metabolites in rat brain homogenates and microdialysates by LC-MS/MS. Analytical Methods. 2015;7:3929–3938. [Google Scholar]
- 26.Zhang X, Rauch A, Lee H, Xiao H, Rainer G, Logothetis NK. Capillary hydrophilic interaction chromatography/mass spectrometry for simultaneous determination of multiple neurotransmitters in primate cerebral cortex. Rapid Communications in Mass Spectrometry. 2007;21:3621–3628. doi: 10.1002/rcm.3251. [DOI] [PubMed] [Google Scholar]
- 27.Wardlaw SL, Burant CF, Klein S, Meece K, White A, Kasten T, Lucey BP, Bateman RJ. Continuous 24-hour leptin, proopiomelanocortin, and amino acid measurements in human cerebrospinal fluid: correlations with plasma leptin, soluble leptin receptor, and amino acid levels. The Journal of clinical endocrinology and metabolism. 2014;99:2540–2548. doi: 10.1210/jc.2013-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang L, He L, Fountoulakis M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. Journal of Chromatography A. 2004;1023:317–320. doi: 10.1016/j.chroma.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Alfa RW, Topper SM, Kim GES, Kockel L, Kim SK. A genetic strategy to measure circulating drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genet. 2014;10:e1004555. doi: 10.1371/journal.pgen.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novotny M, Alasandro M, Konishi M. Microcolumn liquid chromatography of benzoyl derivatives of steroid metabolites. Analytical chemistry. 1983;55:2375–2377. doi: 10.1021/ac00264a038. [DOI] [PubMed] [Google Scholar]
- 31.Redmond JW, Tseng A. High-pressure liquid chromatographic determination of putrescine, cadaverine, spermidine and spermine. Journal of Chromatography A. 1979;170:479–481. [Google Scholar]
- 32.Higa S, Suzuki T, Hayashi A, Tsuge I, Yamamura Y. Isolation of catecholamines in biological fluids by boric acid gel. Analytical biochemistry. 1977;77:18–24. doi: 10.1016/0003-2697(77)90285-8. [DOI] [PubMed] [Google Scholar]
- 33.Wuts PG, Greene TW. Greene's protective groups in organic synthesis. John Wiley & Sons; 2006. [Google Scholar]
- 34.Scheline RR. A rapid synthesis of 3-o-methylgallic acid. Acta Chemica Scandinavica. 1966;20 [Google Scholar]
- 35.Hillemann H, Beiträge zur kenntnis des phenazins, mitteil I, Über die einwirkung von dimethylsulfat auf phenazin 1-methoxy-phenazin und 1-oxy-phenazin. Berichte der deutschen chemischen Gesellschaft (A and B Series) 1938;71:34–41. [Google Scholar]
- 36.El-Kholy S, Stephano F, Li Y, Bhandari A, Fink C, Roeder T. Expression analysis of octopamine and tyramine receptors in Drosophila. Cell Tissue Res. 2015;361:669–684. doi: 10.1007/s00441-015-2137-4. [DOI] [PubMed] [Google Scholar]
- 37.Evans P, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- 38.Livingstone MS, Tempel BL. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983;303:67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- 39.Monastirioti M, Linn CE, Jr, White K. Characterization of drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. British Journal of Pharmacology. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berry MD. Mammalian central nervous system trace amines Pharmacologic amphetamines, physiologic neuromodulators. Journal of Neurochemistry. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- 42.Evans PH, Michael Fox P. Enzymatic N-acetylation of indolealkylamines by brain homogenates of the honeybee, Apis mellifera. Journal of Insect Physiology. 1975;21:343–353. [Google Scholar]
- 43.Finocchiaro L, Callebert J, Launay JM, Jallon JM. Melatonin biosynthesis in Drosophila: Its nature and Its effects. Journal of Neurochemistry. 1988;50:382–387. doi: 10.1111/j.1471-4159.1988.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 44.Paxon TL, Powell PR, Lee H-G, Han K-A, Ewing AG. Microcolumn separation of amine metabolites in the fruit fly. Analytical chemistry. 2005;77:5349–5355. doi: 10.1021/ac050474m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PSF, Bodurka J, Teague TK, Dantzer R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology. 2015;40:463–471. doi: 10.1038/npp.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pegg AE, Casero RA., Jr Current status of the polyamine research field. Methods in molecular biology (Clifton, N.J.) 2011;720:3–35. doi: 10.1007/978-1-61779-034-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewandowski NM, Ju S, Verbitsky M, Ross B, Geddie ML, Rockenstein E, Adame A, Muhammad A, Vonsattel JP, Ringe D, Cote L, Lindquist S, Masliah E, Petsko GA, Marder K, Clark LN, Small SA. Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16970–16975. doi: 10.1073/pnas.1011751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison LD, Kish SJ. Brain polyamine levels are altered in Alzheimer's disease. Neurosci Lett. 1995;197:5–8. doi: 10.1016/0304-3940(95)11881-v. [DOI] [PubMed] [Google Scholar]
- 49.Aldini G, Orioli M, Carini M, Maffei Facino R. Profiling histidine-containing dipeptides in rat tissues by liquid chromatography/electrospray ionization tandem mass spectrometry. Journal of mass spectrometry. 2004;39:1417–1428. doi: 10.1002/jms.696. [DOI] [PubMed] [Google Scholar]
- 50.Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiological reviews. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 51.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 52.Edison P, Ahmed I, Fan Z, Hinz R, Gelosa G, Ray Chaudhuri K, Walker Z, Turkheimer FE, Brooks DJ. Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacology. 2013;38:938–949. doi: 10.1038/npp.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Annals of the New York Academy of Sciences. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peppard RF, Martin WR, Clark CM, Carr GD, McGeer PL, Calne DB. Cortical glucose metabolism in Parkinson's and Alzheimer's disease. Journal of neuroscience research. 1990;27:561–568. doi: 10.1002/jnr.490270417. [DOI] [PubMed] [Google Scholar]
- 55.Vander Borght T, Minoshima S, Giordani B, Foster NL, Frey KA, Berent S, Albin RL, Koeppe RA, Kuhl DE. Cerebral metabolic differences in Parkinson's and Alzheimer's diseases matched for dementia severity, Journal of nuclear medicine : official publication. Society of Nuclear Medicine. 1997;38:797–802. [PubMed] [Google Scholar]
- 56.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. The Clinical Biochemist Reviews. 2008;29:S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 57.Santos SM, Garcia-Nimo L, Sa Santos S, Tavares I, Cocho JA, Castanho MA. Neuropeptide kyotorphin (tyrosyl-arginine) has decreased levels in the cerebro-spinal fluid of Alzheimer's disease patients: potential diagnostic and pharmacological implications. Frontiers in aging neuroscience. 2013;5:68. doi: 10.3389/fnagi.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueda H, Shiomi H, Takagi H. Regional distribution of a novel analgesic dipeptide kyotorphin (Tyr-Arg) in the rat brain and spinal cord. Brain research. 1980;198:460–464. doi: 10.1016/0006-8993(80)90761-1. [DOI] [PubMed] [Google Scholar]
- 59.Ribeiro MM, Pinto AR, Domingues MM, Serrano I, Heras M, Bardaji ER, Tavares I, Castanho MA. Chemical conjugation of the neuropeptide kyotorphin and ibuprofen enhances brain targeting and analgesia. Molecular pharmaceutics. 2011;8:1929–1940. doi: 10.1021/mp2003016. [DOI] [PubMed] [Google Scholar]
- 60.Sigrist SJ, Andlauer TFM. Fighting the famine with an amine: synaptic strategies for smart search. Nat Neurosci. 2011;14:124–126. doi: 10.1038/nn0211-124. [DOI] [PubMed] [Google Scholar]
- 61.Yang Z, Yu Y, Zhang V, Tian Y, Qi W, Wang L. Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proceedings of the National Academy of Sciences. 2015;112:5219–5224. doi: 10.1073/pnas.1417838112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canelas AB, ten Pierick A, Ras C, Seifar RM, van Dam JC, van Gulik WM, Heijnen JJ. Quantitative evaluation of intracellular metabolite extraction techniques for yeast metabolomics. Analytical chemistry. 2009;81:7379–7389. doi: 10.1021/ac900999t. [DOI] [PubMed] [Google Scholar]
- 63.Dietmair S, Timmins NE, Gray PP, Nielsen LK, Krömer JO. Towards quantitative metabolomics of mammalian cells: development of a metabolite extraction protocol. Analytical biochemistry. 2010;404:155–164. doi: 10.1016/j.ab.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 64.Mushtaq MY, Choi YH, Verpoorte R, Wilson EG. Extraction for metabolomics: access to the metabolome. Phytochemical Analysis. 2014;25:291–306. doi: 10.1002/pca.2505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.