Abstract
Background
Ginsenoside Rd (GSRd), a main component of the root of Panax ginseng, exhibits anti-inflammation functions and decreases infarct size in many injuries and ischemia diseases such as focal cerebral ischemia. M1 Macrophages are regarded as one of the key inflammatory cells having functions for disease progression.
Methods
To investigate the effect of GSRd on renal ischemia/reperfusion injury (IRI) and macrophage functional status, and their regulatory role on mouse polarized macrophages in vitro, GSRd (10–100 mg/kg) and vehicle were applied to mice 30 min before renal IRI modeling. Renal functions were reflected by blood serum creatinine and blood urea nitrogen level and histopathological examination. M1 polarized macrophages infiltration was identified by flow cytometry analysis and immunofluorescence staining with CD11b+, iNOS+/interleukin-12/tumor necrosis factor-α labeling. For the in vitro study, GSRd (10–100 μg/mL) and vehicle were added in the culture medium of M1 macrophages to assess their regulatory function on polarization phenotype.
Results
In vivo data showed a protective role of GSRd at 50 mg/kg on Day 3. Serum level of serum creatinine and blood urea nitrogen significantly dropped compared with other groups. Reduced renal tissue damage and M1 macrophage infiltration showed on hematoxylin–eosin staining and flow cytometry and immunofluorescence staining confirmed this improvement. With GSRd administration, in vitro cultured M1 macrophages secreted less inflammatory cytokines such as interleukin-12 and tumor necrosis factor-α. Furthermore, macrophage polarization-related pancake-like morphology gradually changed along with increasing concentration of GSRd in the medium.
Conclusion
These findings demonstrate that GSRd possess a protective function against renal ischemia/reperfusion injury via downregulating M1 macrophage polarization.
Keywords: ginsenoside Rd, macrophage, renal ischemia/reperfusion injury
1. Introduction
Ginseng, the root of Panax ginseng Meyer (Araliaceae), has been used as medication in Asia for thousands of years. Recent studies have shown that ginseng saponins (ginsenosides) are the components responsible for therapeutic and pharmacologic effects. Dammar-24(25)-ene-3b,12b,20(S)-triol-(20-O-b-D-glucopyranosyl)-3-O-b-D-glucopyranosyl-(1→2)-b-D-glucopyranoside (ginsenoside Rd, GSRd, C48H82O18·3H2O) is one of the main ginsenosides. Studies have shown that GSRd have antitumor and anti-inflammation properties [1], [2]. In a rat myocardial ischemia/reperfusion injury (IRI) model, GSRd reduced myocardial infarct size, apoptotic cell death, and blood creatine kinase/lactate dehydrogenase levels [3]. GSRd has also shown neuroprotection in transient focal cerebral ischemia, which may involve early free radical scavenging pathways and late anti-inflammatory effects, and such protection effect appears to have connections with microglial cells [2].
Renal IRI is commonly seen in kidney transplantation causing delayed graft function, rejection, and renal failure. Potential molecular and cellular mechanisms have long been investigated for this pathological damage. Caspase activation, cytochrome C release, cytosolic calcium overload, and burst of free radicals would cause cell injury, apoptosis and necrosis [4], [5]. Immune cells are also involved in the process. Neutrophils attracted by chemokines lead to vascular congestion and cell damage via adhering to endothelial cells [6] and degranulation [7].
Macrophages are a group of innate immune cells that have great phagocytosis and secretion capability. Based on their functions, two subgroups of macrophage are defined as classically activated (M1) and alternatively activated (M2) macrophages [8], [9]. M1 macrophages, marked as CD11b+ iNOS+ in mouse, have strong phagocytic capability for scavenging microorganisms or cell debris. They can secrete proinflammatory cytokines such as interleukin (IL)-6, IL-12, and tumor necrosis factor (TNF)-α, and attract more inflammatory cells to promote further progress of inflammation. These functions would aggravate cell and tissue damage [10]. It has been reported that iNOS+ M1 macrophages are recruited into kidney in the first 48 hours after ischemia/reperfusion injury. However, predepletion of these macrophages could reduce renal injury, and depletion at 3–5 days after injury slows tubular cell proliferation and repair, indicating that M1 macrophages exacerbate renal injury [11]. By contrast, M2 macrophages have lower ability of phagocytosis and proinflammatory function. In some study, these alternatively activated macrophages could promote renal fibrosis by promoting formation of extracellular matrix and recruiting fibroblasts [12], [13].
Although there are studies that discuss GSRd functions on ischemia injury [3], and its potential role on M1 macrophages by in vitro study [14], the role GSRd played on renal IRI and on M1 macrophages in vivo is still unknown. In the present study, we investigated the therapeutic effect of GSRd on renal IRI and functional status of macrophages in injured tissue, and further discuss its regulatory role on in vitro mouse polarized macrophages.
2. Materials and methods
2.1. Renal IRI
This study was performed in adherence with the National Institutes of Health Guide for the care and use of laboratory animals, and was approved by the Fourth Military Medical University Committee on animal care. Male C57 BL/6 mice, age 6–8 weeks and weighing 16–18 g, were obtained from the Experimental Animal Center of Fourth Military Medical University (Xi'an, China). In brief, mice were anesthetized with ketamine (100 mg/kg intraperitoneally) and xylazine (10 mg/kg intraperitoneally). Bilateral renal pedicles were clamped for 25 min with nontraumatic microaneurysm clamps. After removing the clamp and suturing the incision, mice were kept on a heating pad to maintain constant body temperature at 37°C and received analgesia. The control sham group was only given anesthesia and laparotomy. On Days 1, 3, 5, and 7 postsurgery, kidneys were obtained in each group at the scheduled end points, and mice blood was collected as well. GSRd (purity 98%; Tai-He Biopharmaceutical Co. Ltd, Guangzhou, China) stock solutions were prepared in saline containing 10% 1, 3-propanediol (v/v). GSRd at 10 mg/kg, 20 mg/kg, 50 mg/kg, and 100 mg/kg and vehicle were applied intraperitoneally 30 min before renal IRI. These groups are referred to as IRI+10, IRI+20, IRI+50, IRI+100 and IRI+vehicle hereinafter.
2.2. Renal functions
Mice orbital blood was collected in heparinized tubes when sacrificed. Blood was centrifuged at 14,000 g for 10 min to obtain plasma. Serum creatinine (Scr) and blood urea nitrogen (BUN) were analyzed with Scr and BUN assay kit (Nanjing Jiancheng Bioengineering Institute, China) by Roche Hitachi Modular Chemistry analyzer.
2.3. Histological analysis
Kidney was perfused with cold phosphate-buffered saline and formalin before harvesting and fixed in formalin for 24 hours and embedded in paraffin follow routine protocol. The samples were sectioned as 4-μm thick slices for hematoxylin–eosin staining. Tissue sections were examined and scored by light microscopy and evaluated for peritubular leukocyte infiltration, tubular cell swelling and epithelial necrosis on scale of 0–4 according to the following: 0: no involvement; 1: <25% involvement; 2: 25–50% involvement; 3: 50–75% involvement; and 4: >75% involvement.
For immunofluorescence staining, tissues were fixed in 4% paraformaldehyde for 12 h at 4°C, followed by 25% sucrose in phosphate-buffered saline dehydration overnight. Samples were embedded in optimal cutting temperature compound (Sakura Finetek, Inc., Torrance, CA, USA), sectioned at 10-μm thickness, and then dried at room temperature for 2 h before sectioning. Macrophages were stained with Biotin CD11b antibody (ab24954; Abcam, Cambridge, MA, USA) and ExtrAvidin-Cy3 conjugate (E4142; Sigma–Aldrich, St Louis, MO, USA); M1 phenotype was marked by anti-iNOS antibody (ab15323; Abcam), anti-IL12 antibody (ab203031; Abcam), anti-TNFα (ab6671; Abcam), and donkey anti-rabbit IgG H&L (Alexa Fluor 488; ab150073; Abcam). Cell nuclei were stained with Hoechst Stain solution (H6024; Sigma–Aldrich).
2.4. Flow cytometry
Macrophages in kidney were labeled and identified by flow cytometry. Briefly, kidney was harvested and minced by scissors and strainer. Acquired tissues were then fully digested using collagenase D (0.05%; Sigma–Aldrich). After filtering impurities, cells were counted and stained with allophycocyanin (APC)-CD11b (17-0112; eBioscience, San Diego, CA, USA) at 4°C for 30 min. Then they were fixed and permeabilized using Intracellular Fixation & Permeabilization Buffer (plus Brefeldin A) kit (88-8823; eBioscience) following manufacturer's protocols. After that, cells were stained with Alexa Fluor 488-NOS2 (iNOS; 53-5920; eBioscience) at 4°C for 30 min. All staining procedures were carried out in the dark. Flow cytometry analysis was performed by using a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA, USA).
2.5. Macrophage culture and polarization
Bone marrow stem cells were acquired from mice femur and tibia, maintained in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 2mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin sulfate and stimulated by macrophage colony stimulating factor (10 ng/mL; PeproTech, Rocky Hill, NJ, USA) for 7 days. Macrophages were collected and identified by flow cytometry. M1 macrophage polarization was performed by adding lipopolysaccharide (LPS; 100 ng/mL; Sigma–Aldrich) plus interferon (IFN)-γ (50 ng/mL; PeproTech) for 24 h. GSRd concentration at 10 μg/mL, 20 μg/mL, 50 μg/mL, and 100 μg/mL and vehicle were added in the culture medium when polarized, a dose selected based on previous investigations [3], [15].
2.6. Real-time polymerase chain reaction
Macrophage total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was prepared with reverse transcription (RT) system (Takara, Dalian, China). Quantitative real-time polymerase chain reaction (PCR) was performed in triplicates using SYBR Premix EX Taq kit (Takara, Dalian, China) and the ABI PRISM 7500 Real-Time PCR System, with β-actin as an internal control. The following primers were used: β-actin (forward: CATCCGTAAAGACCTCTATGCCAAC; reverse: ATGGAGCCACCGATCCACA); IL-12β (forward: GCTCGCAGCAAAGCAAGGTAA; reverse: CCATGAGTGGAGACACCAGCA); iNOS (forward: CAAGCACATTTGGGAATGGAGA; reverse: CAGAACTGAGGGTACATGCTGGAG); TNF-α (forward: TATGGCCCAGACCCTCACA; reverse: GGAGTAGACAAGGTACAACCCATC).
2.7. Statistical analysis
Data were analyzed with GraphPad Prism software version 5.0 (GraphPad Software, San Diego, CA). Results are expressed as the mean ± SD. Differences between groups were assessed using one-way ANOVA followed by Bonferroni posthoc test. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. GSRd alleviates mouse acute renal ischemia/reperfusion injury
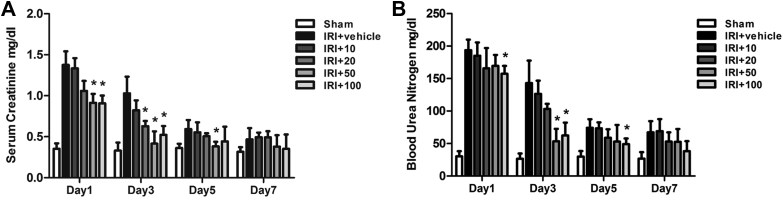
All animals in the study showed that GSRd has no effects on body temperature, blood pressure, or renal function in the absence of renal IRI. GSRd-pretreated mice received surgery and were sacrificed on Days 1, 3, 5, and 7, as were the sham and vehicle groups. Mice kidney and peripheral blood were acquired. Blood Scr and BUN were tested for all groups. The content became very high in the first day after surgery and gradually decreased as the time passed. GSRd showed protective role on Day 1 with relatively high dose (50 mg/kg, 100 mg/kg). Group IRI+50 had a significant effect on reducing blood Scr and BUN to nearly the baseline level on Day 3. However, group IRI+100 did not show further protective function than group IRI+50 as expected, which might indicate a dose-dependent relationship between GSRd concentration and therapeutic effect (Fig. 1).
Fig. 1.
Ginsenoside Rd significantly reduces renal ischemia/reperfusion injury (IRI)-induced increase in (A) serum creatinine (Scr) and (B) blood urea nitrogen (BUN). The levels of Scr and BUN in the surgery group compared to sham group were markedly high on Day 1 and Day 3. Group IRI+50, IRI+100 significantly reduced Scr and BUN level on Day 3 compared to the sham group, while group IRI+vehicle and IRI+10 did not display differences. IRI+10: administration of 10 mg/kg ginsenoside Rd combined with renal IRI surgery. Other groups ibid. (n = 8–10 mice/group, values represent mean ± standard deviation in the histograms. *p < 0.05 vs. vehicle.)
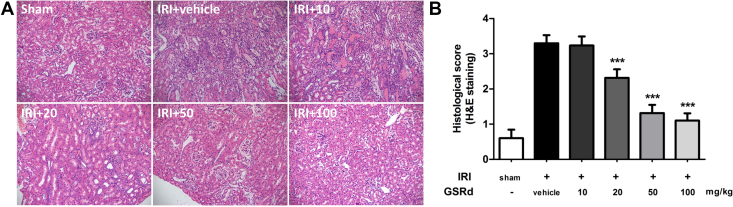
Hematoxylin–eosin staining of group IRI+50 on Day 3 also showed the protective role of GSRd on tissue damage. Tubular cell swelling and leukocyte infiltration decreased with incremental concentration (Fig. 2A). Tissue damage could be observed on group IRI+vehicle and IRI+10, whereas it began to lighten gradually in groups IRI+20, IRI+50, and IRI+100. Histological scores assessed by two senior pathologists showed therapeutic effect of GSRd on renal IRI (Fig. 2B).
Fig. 2.
Ginsenoside Rd (GSRd) alleviates renal tissue injury caused by ischemia/reperfusion. (A) Hematoxylin–eosin (H&E) staining showed that ischemia/reperfusion injury (IRI) caused severe renal tissue damage compared to the sham group. Leukocyte infiltration and tissue structure damage were mainly appeared on group IRI+vehicle and IRI+10. Tissue morphology started to improve from a GSRd concentration of 20 mg/kg. Tubular cell swelling and inflammatory cell infiltration significantly reduced on group IRI+50 and IRI+100. (B) Histology score assessed by two senior pathologists showed the improvement of tissue injury of the group receiving GSRd treatment. (Eight randomly-selected fields/slice, n = 6 mice for each group, values represent mean ± standard deviation in the histograms, ***p < 0.0001 vs. vehicle.)
3.2. GSRd reduces M1 macrophages of renal ischemia tissue in situ
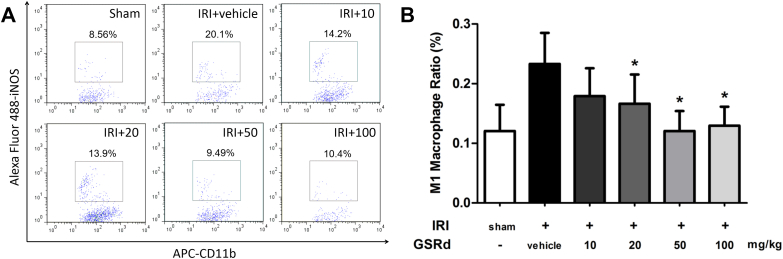
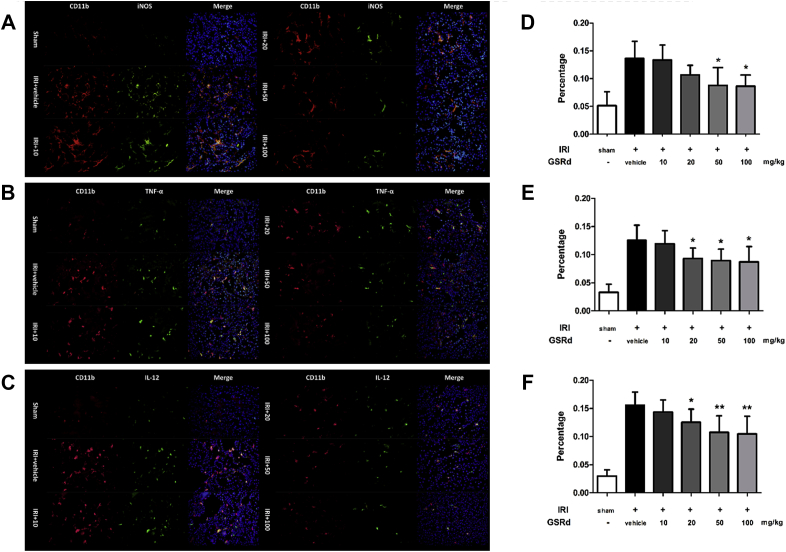
Since M1 macrophages played essential role in renal IRI induced inflammation, their phenotype and function in the case of GSRd treatment will be our concern. To investigate this, mice kidneys were immediately acquired after sacrifice for flow cytometry analysis and immunofluorescence assay. Ratio of CD11b+ iNOS+ macrophages was significantly higher in IRI group compared with sham surgery mice (Fig. 3A). These macrophages were regarded as M1 phenotype, which exacerbates inflammation and tissue damage. Administration of GSRd to the IRI mice with increased concentration resulted in reduction of iNOS+ macrophages ratio in renal tissue (Fig. 3B). Immunofluorescence staining of macrophages in kidney indicated that quantity of macrophage increased after performing renal IRI surgery. Moreover, these macrophages expressed M1 phenotype marker iNOS, IL-12, or TNF-α at the same time. As shown in Fig. 4, group IRI+20 and IRI+50 had less CD11b+ iNOS+ macrophages than group IRI+vehicle. This change had a dose-dependency in a certain density range. However, it could still be seen that some inflammatory marker negative macrophages existed within renal tissue.
Fig. 3.
Administration of ginsenoside Rd reduces M1 macrophages infiltration ratio in model rat kidney. Complete kidney cells were acquired for flow cytometry analysis. (A) The ratio of CD11b and iNOS labeled M1 macrophages increased in group IRI+vehicle and reduced significantly in group IRI+20, IRI+50 and IRI+100; (B) Histogram statistics of whole applied mice. (n = 8–10/ group, values represent mean ± standard deviation in the histograms, *p < 0.05 vs. vehicle.)
Fig. 4.
Ginsenoside Rd reduces M1 macrophage in situ infiltration in IRI tissue detected by immunofluorescence staining. (A–C)Red and green fluorescence indicates CD11b+, iNOS+/tumor necrosis factor-α+/interleukin-12+ macrophages, respectively. Blue fluorescence indicates total nuclei in renal tissue. Original magnification: 400×. (D–F) Corresponding percentage of M1 macrophage among all kidney cells. (Eight randomly selected fields/slice, n = 6 mice for each group, values represent mean ± SD in the histograms, *p < 0.05 vs vehicle.)
3.3. GSRd regulate M1 macrophages polarization status in vitro
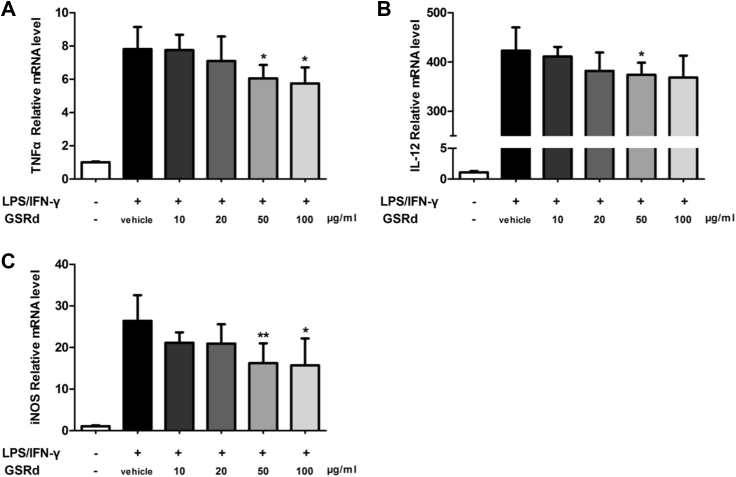
Since GSRd could suppress renal IRI accompanied with macrophages phenotype alteration, we next investigated its direct function acting on macrophage in vitro. Bone marrow stem cells were harvested from mouse bone marrow and differentiated and polarized to M1 macrophages as previous described. Concentration gradient GSRd were added in the culture medium at the time of polarization. After that, M1 macrophages related inflammatory cytokine or enzyme TNFα, IL-12, and iNOS were detected by real-time PCR. After adding LPS/IFN-γ, which were used to induce the M1 phenotype [16], expression of typical cytokines was significantly enhanced. However, this enhancement was reduced with administration of GSRd. As shown in Fig. 5, this reduction started slightly at a GSRd concentration of 10 μg/mL. The 50–100 μg/mL group showed distinct suppressive function on inflammatory cytokine expression.
Fig. 5.
Ginsenoside Rd suppresses M1 macrophage secreted inflammatory cytokines in vitro. Twenty-four hours after adding lipopolysaccharide (LPS)/interferon (IFN)-γ, expression of (A) tumor necrosis factor (TNF)α, (B) interleukin (IL)-12, and (C) iNOS were detected by real-time polymerase chain reaction. (n = 8/group; values represent mean ± standard deviation in the histograms, *p < 0.05 vs. vehicle, **p < 0.01 vs. vehicle.)
3.4. GSRd modulate M1 macrophages morphology in vitro
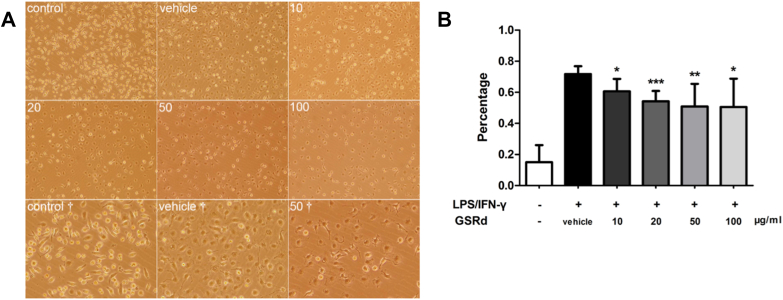
Nonpolarized macrophages had various types of shape including round, threadlike, and pancake-like. In our study, differentiated macrophages showed multiple morphology without regular pattern. After 24 h LPS stimulation, M1 macrophages began to show typical pancake-like shape. However, a proportion of these macrophages became decreased when GSRd were added in the medium. More round and threadlike cell appeared in groups IRI+20, IRI+50, and IRI+100 (Fig. 6).
Fig. 6.
Ginsenoside Rd modulates M1 macrophages morphology in vitro. (A) Nonpolarized macrophages have various types of shape (Control, Control†). After lipopolysaccharide (LPS) and interferon (IFN)-γ stimulation (all rest images), macrophages presented pancake-like shape (Vehicle, Vehicle†). 10: 10 μg/mL GSRd, Other groups ibid. Original magnification: 100×, †: 200×; (B) Percentage of pancake-like macrophages in all cultured cells. (n = 8/group, values represent mean ± standard deviation in the histograms, *p < 0.05 vs. vehicle, **p < 0.01 vs. vehicle, ***p < 0.001 vs. vehicle.)
4. Discussion
In this study, we planned to find out the role that GSRd played on renal IRI. In general, we have demonstrated protective effects of GSRd and its regulatory function on polarized macrophages. Administration of GSRd alleviated renal tissue damage, reduced iNOS+ M1 macrophages infiltration within the injury area and improved renal function. In vitro study indicated an obvious role of GSRd on macrophage polarization status. GSRd at certain concentrations suppressed inflammatory cytokine secretion of M1 macrophages and mediated cell morphology as well.
Many studies have marked the essential role of macrophages in renal ischemia/reperfusion injury [11], [18]. Macrophages depletion studies have suggested their key function during initial inflammation and subsequent remodeling/fibrosis [19], [20]. Exploration of therapeutic methods based on macrophage intervention has attracted much attention. For instance, chemokine and chemokine receptors CCR2 and CX3CR1 were studied for infiltration of inflamed monocyte/macrophage in renal IRI [21]. Toll-like receptors-2 and -4, which are mainly expressed on macrophages, were closely related to CCR2 expression and macrophage infiltration [22], [23]. Other cytokines and growth factors related to macrophage, such as TNF-α, transforming growth factor-β, granulocyte macrophage colony-stimulating factor were also investigated for relieving tissue injury [24], [25], [26].
Ginseng is one of the most famous medical herbs around the world. It has been used as medication for a long time. In previous studies, GSRd exhibited remarkable pharmacological function on multiple diseases. It has been found that GSRd relieves inflammatory injury by suppression of inducible nitric oxide synthase and cyclooxygenase-2 expression in rat transient focal cerebral ischemia [2]. In this study, GSRd attenuated oxidative DNA and protein damage, lipid peroxidation, and consumption of the endogenous antioxidant system activities, showing a neuroprotective activity. GSRD also attenuated myocardial IRI in rat by improving cardiac function, reducing infarct size and myocardial apoptosis. It could reduce cardiomyocytes intracellular reactive oxygen species generation, and activate Akt/GSK-3β signaling pathway for antiapoptotic effect [3]. However, what exact role GSRd plays on renal IRI is still unclear. Since macrophages are essential for initiating first step inflammation and following step remodeling/fibrosis, we planned to test if GSRd could regulate macrophage functions and whether it is associated with renal IRI development tendency afterwards.
This study is the first to demonstrate the renal protective role of GSRd for IRI in kidney. In the first a few days after IRI, blood Scr and BUN level dropped along with administration of GSRd in groups IRI+20, IRI+50, and IRI+100. Histology confirmed the improvement directly by observing less inflammatory cell infiltration and better tissue morphology. We next applied immunofluorescence staining together with flow cytometry to analyze M1 macrophage population changes. The results indicated GSRd might either decreased M1 macrophage infiltration or reduced incidence of cell polarization. In order to clarify the direct function of GSRd on macrophages, we carried an in vitro study to add GSRd in M1 macrophage culture medium. Real-time PCR data indicated that GSRd could reduce inflammatory cytokine secretion of polarized macrophage. These cytokines are among the primary causes of the first wave of injury. Furthermore, it is worth mentioning that numbers of M1 macrophages, which had the typical pancake-like morphology, decreased along with GSRd administration. This phenomenon was first observed among GSRd research. Together with the functional study towards shape of polarized macrophage [17], our medication showed a confirmed intervention of reducing M1 macrophage numbers.
Although our study manifested protective role of GSRd on renal IRI via regulation of macrophages, it is also necessary to discuss mechanisms of this regulating process. M1 macrophage can be activated by several signaling pathways. In IFN-γ induced signaling, Janus kinase 1 and 2 adaptors could activate signal transducers and activators of transcription (STAT) 1 and interferon regulatory factors (IRF), such as IRF-1 and IRF-8 [27]. Suppressors of cytokine signaling 1 and 3 could act as feedback inhibitor of the Janus kinase/STAT 1 signaling [28]. In LPS induced signaling, Toll-like receptor 4 activation induces MyD88 and Toll-interleukin 1 receptor domain containing adaptor protein (MaL/Tirap) dependent pathways. This pathway is controlled by nuclear factor κ light chain enhancer of activated B cells, activator protein 1, IRFs, STAT1, and early growth response family members [27], [29]. A recent in vitro study indicated an anti-inflammatory effect of GSRd by inhibition of iNOS and COX-2 via suppression of nuclear factor κ light chain enhancer of activated B cells signaling in RAW264.7 macrophage cell line [14]. However, a deeper and further study of the mechanism in vivo is still needed.
Taken together, our results provide evidence for the first time that GSRd performs a protective role in renal IRI by modulating M1 macrophage polarization. It reduced macrophages infiltration and decreased their inflammatory cytokine secretion. These results indicate that GSRd, a traditional herbal medicine, display the therapeutic potential for alleviating acute renal ischemia/reperfusion injury.
Conflicts of interest
We declare that no financial conflict of interest exists in relation to the publication of this work.
Acknowledgments
We thank Dr Gang Zhao for his kind help with providing GSRd stock solutions.
References
- 1.Kim Y.J., Yamabe N., Choi P., Lee J.W., Ham J., Kang K.S. Efficient thermal deglycosylation of ginsenoside Rd and its contribution to the improved anticancer activity of ginseng. J Agric Food Chem. 2013;61:9185–9191. doi: 10.1021/jf402774d. [DOI] [PubMed] [Google Scholar]
- 2.Ye R., Yang Q., Kong X., Han J., Zhang X., Zhang Y., Li P., Liu J., Shi M., Xiong L. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58:391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Li X., Wang X., Lau W., Wang Y., Xing Y., Zhang X., Ma X., Gao F. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS One. 2013;8:e70956. doi: 10.1371/journal.pone.0070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanden Hoek T.L., Qin Y., Wojcik K., Li C.Q., Shao Z.H., Anderson T., Becker L.B., Hamann K.J. Reperfusion, not simulated ischemia, initiates intrinsic apoptosis injury in chick cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;284:H141–H150. doi: 10.1152/ajpheart.00132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.A., Allen D.G. Changes in intracellular free calcium concentration during long exposures to simulated ischemia in isolated mammalian ventricular muscle. Circ Res. 1992;71:58–69. doi: 10.1161/01.res.71.1.58. [DOI] [PubMed] [Google Scholar]
- 6.Singbartl K., Green S.A., Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 2000;14:48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Jang H.R., Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards J.P., Zhang X., Frauwirth K.A., Mosser D.M. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez F.O., Gordon S., Locati M., Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 11.Lee S., Huen S., Nishio H., Nishio S., Lee H.K., Choi B.S., Ruhrberg C., Cantley L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 13.Nikolic-Paterson D.J., Wang S., Lan H. Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int Suppl. 2014;4:34–38. doi: 10.1038/kisup.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim D.H., Chung J.H., Yoon J.S., Ha Y.M., Bae S., Lee E.K., Jung K.J., Kim M.S., Kim Y.J., Kim M.K. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-κB in LPS-stimulated RAW264.7 cells and mouse liver. J Ginseng Res. 2013;37:54–63. doi: 10.5142/jgr.2013.37.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye R., Han J., Kong X., Zhao L., Cao R., Rao Z., Zhao G. Protective effects of ginsenoside Rd on PC12 cells against hydrogen peroxide. Biol Pharm Bull. 2008;31:1923–1927. doi: 10.1248/bpb.31.1923. [DOI] [PubMed] [Google Scholar]
- 16.Jha A.K., Huang S.C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E., Chmielewski K., Stewart K.M., Ashall J., Everts B. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 17.McWhorter F.Y., Wang T., Nguyen P., Chung T., Liu W.F. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huen S.C., Cantley L.G. Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol. 2015;30:199–209. doi: 10.1007/s00467-013-2726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L., Faubel S., He Z., Andres Hernando A., Jani A., Kedl R., Edelstein C.L. Depletion of macrophages and dendritic cells in ischemic acute kidney injury. Am J Nephrol. 2012;35:181–190. doi: 10.1159/000335582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferenbach D.A., Sheldrake T.A., Dhaliwal K., Kipari T.M., Marson L.P., Kluth D.C., Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 21.Li L., Huang L., Sung S.S., Vergis A.L., Rosin D.L., Rose C.E., Jr., Lobo P.I., Okusa M.D. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–1537. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leemans J.C., Stokman G., Claessen N., Rouschop K.M., Teske G.J., Kirschning C.J., Akira S., van der Poll T., Weening J.J., Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H., Chen G., Wyburn K.R., Yin J., Bertolino P., Eris J.M., Alexander S.I., Sharland A.F., Chadban S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnahoo K.K., Shames B.D., Harken A.H., Meldrum D.R. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- 25.Huen S.C., Moeckel G.W., Cantley L.G. Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am J Physiol Renal Physiol. 2013;305:F477–F484. doi: 10.1152/ajprenal.00624.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huen S.C., Huynh L., Marlier A., Lee Y., Moeckel G.W., Cantley L.G. GM-CSF Promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol. 2014;26:1334–1345. doi: 10.1681/ASN.2014060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin H., Holdbrooks A.T., Liu Y., Reynolds S.L., Yanagisawa L.L., Benveniste E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189:3439–3448. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saccani A., Schioppa T., Porta C., Biswas S.K., Nebuloni M., Vago L., Bottazzi B., Colombo M.P., Mantovani A., Sica A. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]