Abstract
Background
Chemotherapy-induced alopecia (CIA) is one of the most distressing side effects for patients undergoing chemotherapy. This study evaluated the protective effect of Korean Red Ginseng (KRG) on CIA in a well-established in vitro human hair follicle organ culture model as it occurs in vivo.
Methods
We examined whether KRG can prevent premature hair follicle dystrophy in a human hair follicle organ culture model during treatment with a key cyclophosphamide metabolite, 4-hydroperoxycyclophosphamide (4-HC).
Results
4-HC inhibited human hair growth, induced premature catagen development, and inhibited proliferation and stimulated apoptosis of hair matrix keratinocytes. In addition, 4-HC increased p53 and Bax protein expression and decreased Bcl2 protein expression. Pretreatment with KRG protected against 4-HC-induced hair growth inhibition and premature catagen development. KRG also suppressed 4-HC-induced inhibition of matrix keratinocyte proliferation and stimulation of matrix keratinocyte apoptosis. Moreover, KRG restored 4-HC-induced p53 and Bax/Bcl2 expression.
Conclusion
Overall, our results indicate that KRG may protect against 4-HC-induced premature catagen development through modulation of p53 and Bax/Bcl2 expression.
Keywords: alopecia, chemotherapy, hair growth, Korean Red Ginseng
1. Introduction
Hair provides protective, sensory, and sexual attractiveness attributes and is also often used to indicate personal beliefs or social position. During postnatal life, hair cyclically undergoes the following three alternating phases of rapid growth: anagen (2–6 yr), apoptosis-mediated regression (catagen, 2–3 wk), and relative quiescence (telogen, 2–3 mo) [1]. Hair matrix keratinocytes at the anagen phase are some of the fastest dividing cells in the body, with 60% of them remaining in the S phase [1]. Because chemotherapeutic drugs target rapidly proliferating cell populations, they attack not only neoplastic cancer cells but also rapidly growing hair matrix keratinocytes in anagen, which leads to hair loss (alopecia) [2].
Chemotherapy-induced alopecia (CIA) is one of the most distressing side effects for patients undergoing chemotherapy [3], [4], [5]. Although the CIA is almost always reversible, CIA can lead to negative psychological perceptions for patients, even leading to refusal of treatment [2], [6]. The incidence of CIA is ∼65% among patients receiving chemotherapy [5], [7]. As much as 47–58% of female patients consider hair loss to be the most traumatic aspect of chemotherapy and 8% would decline chemotherapy due to fears of hair loss [8], [9]. Therefore, the pursuit of more efficient management strategies for CIA remains a major research challenge in clinical oncology [10].
Korean Red Ginseng (KRG; the steamed root of Panax ginseng Meyer) has been an established traditional herbal medicine for > 2,000 y [11]. KRG has been cultivated and aged for ≥ 4–6 yr, and goes through extensive cleaning, steaming, and drying processes to enhance its pharmacological activity and stability [12]. Recent studies have reported antitumor, antiviral, antidiabetic, antioxidative, and immune-modulatory activities of KRG [13], [14], [15], [16]. Furthermore, a number of studies have also illustrated the role of KRG as a potent regulator of hair growth. KRG prevents apoptosis of hair follicles in irradiated mice, promotes hair growth in C57BL/6 mice, promotes human and murine vibrissae hair growth in organ culture, and improves hair regrowth in androgenetic alopecia and alopecia areata patients [17], [18], [19], [20], [21].
In a recently developed in vitro human hair follicle organ culture model for CIA, the cyclophosphamide (chemotherapeutic drug) metabolite 4-hydroperoxycyclophosphamide (4-HC) induces apoptosis followed by dystrophy in isolated human anagen hair follicles, like CIA in vivo [22]. This study assessed the ability of KRG to protect against CIA in a well-established in vitro human hair follicle organ culture model [22].
2. Materials and methods
2.1. Materials
The KRG extract was provided by the Korea Ginseng Corporation (Daejeon, Korea) through a standardized and reproducible process. The extract was manufactured by the Korea Ginseng Corporation (Seoul, Korea) from the roots of a 6-y-old red ginseng (P. ginseng Meyer), which was harvested in the Korea. KRG was prepared by steaming fresh ginseng at 90–100°C for 3 h and then drying it at 50–80°C. The KRG extract was prepared from red ginseng water extract, which was extracted three times at 85–90°C for 8 h in circulating hot water. The water content of the pooled extract was 36% of the total weight. KRG was analyzed by HPLC and contained the following major ginsenosides (Rb1, 7.44 mg/g; Rb2, 2.59 mg/g; Rc, 3.04 mg/g; Rd, 0.91 mg/g; Re, 1.86 mg/g; Rf, 1.24 mg/g; Rg1, 1.79 mg/g; Rg2, 1.24 mg/g; and Rg3, 1.39 mg/g) and other minor ginsenosides.
The key cyclophosphamide metabolite 4-HC was purchased from Niomec (Bielefeld, Germany).
2.2. Isolation and culture of follicular keratinocytes
Human occipital scalp skin specimens were obtained from patients undergoing hair transplantation surgery after obtaining informed consent. The Institutional Ethics Committee of the Yonsei University, Wonju College of Medicine, Wonju, Korea, approved all described studies. The study was conducted according to the principles of the Declaration of Helsinki.
For culture of follicular keratinocytes (FKCs), anagen hair follicles were cut off from the hair bulb region and then dermal sheathes were removed from the upper part of the hair follicles. Hair shafts, including part of the outer root sheath, were treated with 0.05% trypsin–EDTA (Invitrogen, Waltham, Massachusetts, USA). The dissociated cells were rinsed in Dulbecco's Modified Eagle's medium supplemented with 10% fetal bovine serum and centrifuged for 5 min at 1,500 rpm. Cells were then resuspended in EpiLife medium (Cascade Biologics, Portland, OR, USA) with EpiLife defined growth supplement (Cascade Biologics) and antibiotics and seeded onto a culture dish. Second-passage FKCs were used in this study.
2.3. Cell viability assay
The cytotoxic effects of KRG on FKCs were determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay [23]. In brief, 1 × 104 cells were seeded in each well containing 100 μL of the growth medium in a 96-well plate. Cells were permitted to adhere for 24 h, and then were treated with serial doses of KRG extract (from 0 μg/mL to 1,000 μg/mL) for 1–2 d. After treatment, the medium in each well was removed and replaced with a phosphate-buffered saline solution containing 5 mg/mL MTT. Then the plate was incubated at 37°C for 4 h. The remaining supernatant was then completely removed and 100 μL of dimethyl sulfoxide was added to each well and mixed thoroughly to dissolve the crystallized formazan. After 10 min of incubation to ensure that all formazan crystals were dissolved, the optical density at 570 nm was determined using an enzyme-linked immunosorbent assay reader. The mean absorbance of the treated group was expressed as the cell viability percentage of the control group's absorbance. Three repeated experiments were performed.
2.4. Human hair follicle organ culture
Human anagen hair follicles were isolated as previously described [24]. Isolated human anagen hair follicles were maintained in Williams E medium (Invitrogen) supplemented with 10 μg/mL insulin (Sigma, St. Louis, MO, USA), 10 ng/mL hydrocortisone (Sigma), 2mM l-glutamine (Invitrogen), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) for 1 d. Isolated anagen hair follicles were cultured in each type of medium for 6 d. In brief, control hair follicles were cultured with vehicle for 6 d. Test groups were pretreated with or without KRG (100 μg/mL or 500 μg/mL) on Day 0. Furthermore, a key cyclophosphamide (chemotherapeutic drug) metabolite, 4-HC (20μM), was added on Day 1 [22]. Western blot analysis and immunofluorescence staining were performed after 2 d of culture. The same experiment was repeated three times.
2.5. Measurement of hair follicle length and morphology
The hair follicle length was defined as the entire length from the base of the hair bulb to the tip of the hair shaft. Measurements were made every 2 d using the measuring scales attached to the objective lens of the microscope until the 6th d of cultivation. The measured values were then statistically analyzed. At the same time, the hair follicle morphology (anagen, early catagen, mid catagen, and late catagen) was observed and the hair cycle score was measured according to the following system: anagen VI, 100; early catagen, 200; mid catagen, 300; and late catagen 400. Experiments were repeated three times.
2.6. Immunofluorescence staining
For immunofluorescence staining of Ki-67, after deparaffinization and rehydration, sections were fixed with 4% paraformaldehyde (Santa Cruz Biotechnology, Santa Cruz, CA, USA) containing 0.1% Triton X-100 (Sigma) for 10 min and equilibrated in phosphate-buffered saline for 15 min at room temperature. After blocking with 4% normal donkey serum, sections were incubated with mouse monoclonal Ki-67 antibody (Abcam, Cambridge, MA, USA) and then incubated again with Alexa Fluor 488-labeled donkey antimouse secondary antibody (Invitrogen). Fluorescent specimens were analyzed with a TCS SPE confocal microscope (Leica Microsystems, Bannockburn, IL, USA).
To evaluate apoptotic cells in hair follicles, terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate-biotin nick-end labeling (TUNEL) was performed using the ApopTag Plus peroxidase in situ apoptosis detection kit (Chemicon, Billerica, MA, USA) according to the manufacturer's instructions. In brief, paraffin sections were digested with 20 μg/mL of proteinase K for 15 min at room temperature and treated with terminal deoxynucleotidyl transferase for 60 min at 37°C. TUNEL-positive cells were visualized by an antidigoxigenin fluorescein antibody. Sections were then counterstained with propidium iodide and visualized on a Leica TCS SPE confocal microscope. The same experiments were repeated three times.
2.7. Western blot analysis
Whole-hair-follicle extracts were isolated using a protein prep kit (Qiagen, Hilden, Germany), incubated for 30 min on ice, and then centrifuged at 12,000 rpm for 10 min to remove any insoluble material. Protein concentration was measured using the Bradford method. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis using a 10% acrylamide gel and then transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked with Tris-buffered saline/Tween-20 containing 5% skim milk for 1 h, incubated overnight with primary antibodies against p53 (Abcam), Bax, Bcl2 (Santa Cruz Biotechnology), and β-actin (Sigma), and then incubated with horseradish-peroxidase-conjugated secondary antibodies. Protein expression was visualized by enhanced chemiluminescence Western blot detection reagents (Santa Cruz Biotechnology). Experiments were repeated three times.
2.8. Statistical analysis
Data handling and drawing were processed using the SPSS version 20.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical analysis was performed using one-way analysis of variance followed by Dunnett multiple comparison tests to compare three or more groups. The Student t test was used to compare two different groups. A p value < 0.05 was considered statistically significant. All data are presented as the mean ± standard deviation of at least three separate experiments.
3. Results
3.1. Cytotoxic effects of KRG on FKCs viability
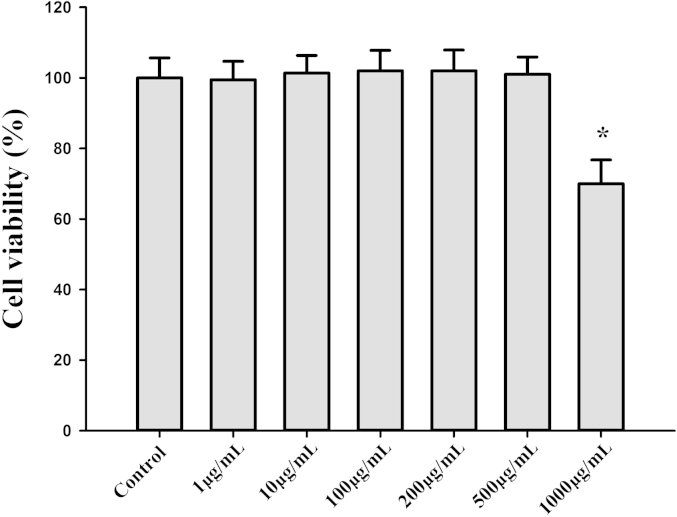
Human FKCs were treated with a serial dose (from 0 μg/mL to 1,000 μg/mL) of KRG extract and its cytotoxic effects were examined. Based on careful titration studies, KRG was found not to affect cell survival or show any significant toxic effect on the FKCs up to a concentration of 500 μg/mL (Fig. 1). The KRG extract, however, showed cytotoxic effects at 1,000 μg/mL (p < 0.05; Fig. 1).
Fig. 1.
Follicular keratinocytes viability assay. Cell viability (%) = (mean absorbency in test wells)/(mean absorbency in control wells) × 100. All the values are shown as mean ± standard deviation. * p < 0.05 versus control cells incubated with media alone.
3.2. KRG reduces 4-HC-induced hair growth inhibition
As a key cyclophosphamide (chemotherapeutic drug) metabolite, 4-HC has been known to produce CIA-like pathogenesis in hair follicle organ culture systems, similar to its in vivo effects [22].
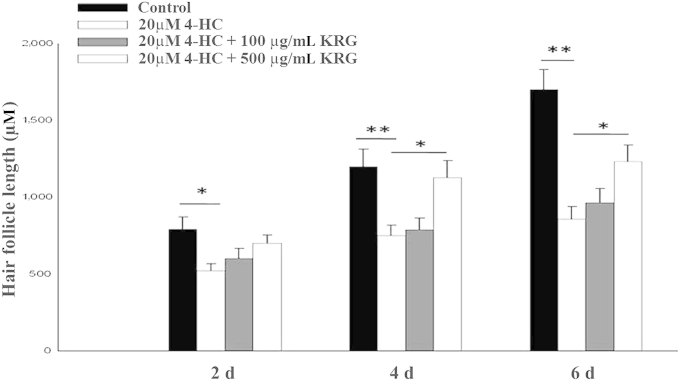
We examined the potential involvement of KRG on 4-HC-induced hair growth inhibition. Hair follicles were incubated with 4-HC alone or with 20μM 4-HC plus KRG extract for 6 d. When human cells were treated with 20μM 4-HC, hair follicle length was significantly reduced after 2 d of culture (p < 0.05; Fig. 2). Pretreatment with 500 μg/mL KRG significantly suppressed 4-HC-induced hair growth inhibition after 4 d of culture. By contrast, pretreatment with 100 μg/mL KRG did not suppress 4-HC-induced hair growth inhibition (Fig. 2).
Fig. 2.
Protective effects of Korean Red Ginseng (KRG) on 4-hydroperoxycyclophosphamide (4-HC)-induced human hair growth inhibition. Human hair follicles were treated with 4-HC alone or 4-HC plus KRG for 6 d. Hair length was measured every 2nd d. All the values are the mean ± standard deviation. *p < 0.05 and **p < 0.01, respectively.
3.3. KRG protects against 4-HC-induced premature catagen development
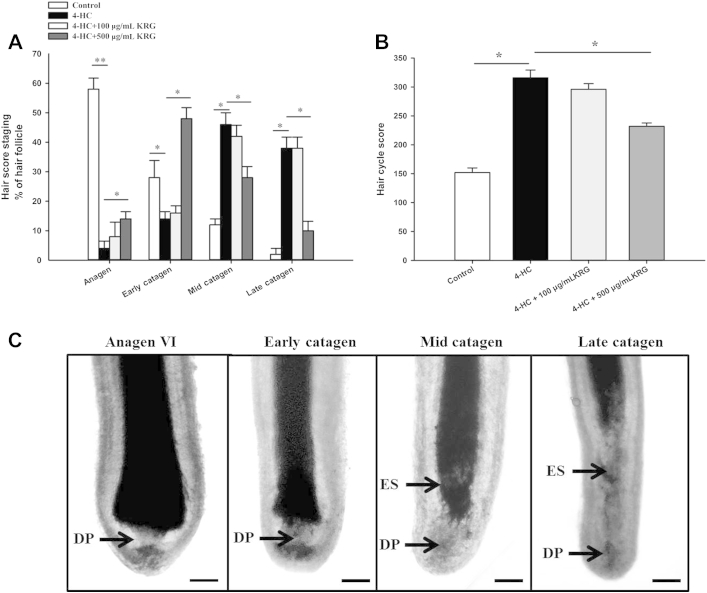
To evaluate whether KRG affects the 4-HC-induced premature catagen-like transformation [22], [25], histological morphology of hair follicle was assessed and the hair cycle score was calculated as described previously [26], [27]. As shown in Fig. 3, only 4% of hair follicles remained in anagen, and > 90% had already approached catagen (14% in early catagen, 46% in mid catagen, and 36% in late catagen; Fig. 3A). Pretreatment with 500 μg/mL KRG significantly (p < 0.05) reduced 4-HC-induced premature catagen development (14% in anagen, 48% in early catagen, 28% in mid catagen, and 10% in late catagen; Fig. 3A). This was confirmed by calculation of the hair cycle score. Hair cycle score was significantly (p < 0.05) increased in the 4-HC-treated hair follicles compared with vehicle controls. The hair cycle score was significantly reduced (p < 0.05) in the KRG-pretreated hair follicle units compared with hair follicles treated with 4-HC alone (Fig. 3B). Approximately 20μM 4-HC accelerated catagen-like regressive development, which was characterized by the upward movement of the hair from the dermal papilla [28], [29] (Fig. 3C).
Fig. 3.
Protective effects of Korean Red Ginseng (KRG) on 4-hydroperoxycyclophosphamide (4-HC)-induced premature catagen development. Human hair follicles were treated with 4-HC alone or 4-HC plus KRG for 6 d. For statistical analysis, anagen VI hair follicle units were assigned a score of 100, and hair follicle units in the early catagen, mid, and late catagen stages were assigned scores of 200, 300, and 400, respectively. The sum of each hair follicle score was then divided by the number of investigated hair follicle units. (A) Analysis of hair cycle staging of each hair follicle (anagen, early catagen, mid catagen, and late catagen). (B) Calculation of the hair cycle score of each hair follicle. (C) The hair cycle stage of each hair follicle was assessed and classified as previously described [26], [27]. In brief, anagen VI (fully developed terminative hair follicles) shows a prominent onion-shaped hair bulb and a narrow and elongated dermal papilla. Early catagen shows a narrow hair bulb, fully opened at the proximal end. Mid catagen shows a partially keratinized presumptive club just above the dermal papilla. Late catagen shows a narrower epithelial strand. All the values are the mean ± standard deviation. Scale bar = 100 μm. *p < 0.05 and **p < 0.01. DP, dermal papilla; ES, epithelial strand.
3.4. KRG suppresses 4-HC-induced inhibition of matrix keratinocyte proliferation and stimulation of matrix keratinocyte apoptosis
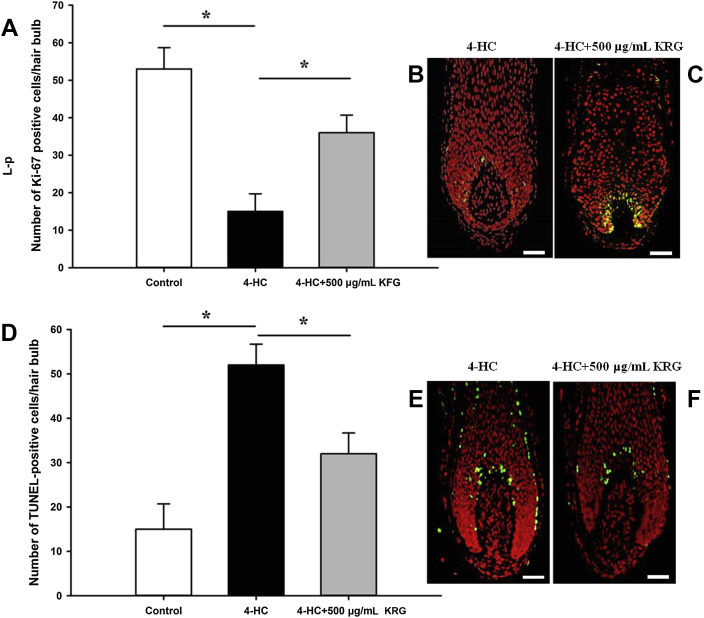
Chemotherapeutic drugs target rapidly proliferating cell populations, such as hair follicles, leading to massive apoptosis in hair matrix keratinocytes, followed by hair loss [10], [30], [31], [32]. Quantitative evaluation of proliferating (Ki-67 positive) and apoptotic (TUNEL positive) hair follicle cells showed that 4-HC significantly (p < 0.05) decreases Ki-67-positive cells and increases TUNEL-positive cells in the hair bulb region. Furthermore, pretreatment with KRG significantly (p < 0.05) suppressed 4-HC-induced inhibition of matrix keratinocyte proliferation and stimulation of matrix keratinocyte apoptosis (Fig. 4).
Fig. 4.
Pretreatment with Korean Red Ginseng (KRG) increases cell proliferation and decreases apoptosis in the hair bulb region. (A–C) Proliferating (Ki-67 positive, green) cells and (D–F) immunodetection of apoptotic cells [terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate-biotin nick-end labeling (TUNEL) positive, green] in the hair bulb region were analyzed. All the values are the mean ± standard deviation. Scale bar = 100 μm. *p < 0.05. Abbreviation4-HC, 4-hydroperoxycyclophosphamide.
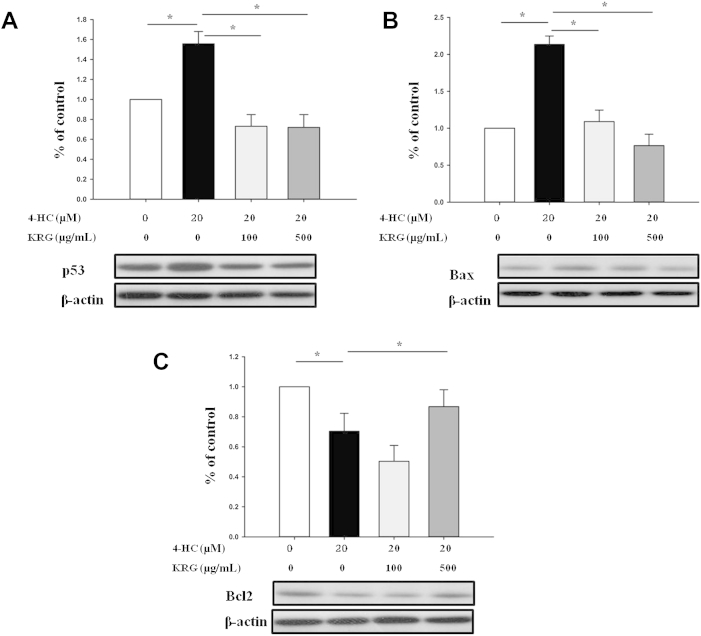
3.5. KRG decreases p53 and proapoptotic Bax expression and increases antiapoptotic Bcl2 expression
The signaling pathways leading to apoptosis of matrix keratinocytes include p53, Bax, Bcl2, and others [10], [33]. To determine whether KRG extract affects the expression of various factors related to apoptosis, Western blot analysis was performed. The 4-HC treatment significantly increased the expression of p53 and Bax proteins, and decreased Bcl2 expression (p < 0.05). Pretreatment with KRG significantly inhibited the expression of these proteins (p < 0.05; Fig. 5).
Fig. 5.
Effects of 4-hydroperoxycyclophosphamide (4-HC) alone or 4-HC plus Korean Red Ginseng (KRG) on the expression of p53, Bcl2, and Bax in human hair follicles. Hair follicles were pretreated with or without KRG (100 μg/mL or 500 μg/mL) on Day 0, and were then treated with 20μM 4-HC on Day 1. After being cultured for 2 d (i.e., after 1 d of treatment with 4-HC), Western blot analysis was performed. HC treatment significantly increased the expression of p53 (A) and Bax proteins (B), and decreased Bcl2 expression (C). All the values are the mean ± standard deviation. *p < 0.05.
4. Discussion
CIA is one of the most distressing side effects for patients undergoing chemotherapy. The incidence of CIA is ∼65% among patients undergoing chemotherapy [5], [7]. However, no efficient preventative pharmacological regimen for CIA has been established to date. Previous research on CIA has been hampered due to a lack of appropriate experimental models that correlate with human tissues [34]. In a recently developed in vitro human hair follicle organ culture model for CIA, it was shown that 4-HC induces apoptosis and then dystrophy in isolated human anagen hair follicles. This is similar to what happens in CIA in vivo and shows characteristic signs such as reduced proliferation and increased apoptosis of hair matrix keratinocytes, hair growth inhibition, and premature catagen induction [22], [34], [35].
KRG is a dietary ingredient that has been used by humans for a long time. Increasing evidence suggests that KRG is a potent regulator of hair growth [17], [18], [19], [20], [21]. Furthermore, KRG can protect against side effects of chemotherapy and radiotherapy [36], [37], [38], [39], [40]. Here, we used an in vitro human hair follicle organ culture model for CIA [22] to examine whether KRG can protect against premature catagen development in response to a key cyclophosphamide metabolite, 4-HC. We also investigated possible mechanisms of protection. This study showed that 4-HC inhibited human hair growth and induced premature catagen development. 4-HC inhibited proliferation and stimulated apoptosis of hair matrix keratinocytes and increased p53 and Bax protein expression while decreasing Bcl2 protein expression. Pretreatment with KRG had a protective effect on 4-HC-induced hair growth inhibition and premature catagen development. In addition, 4-HC-induced inhibition of matrix keratinocyte proliferation and stimulation of matrix keratinocyte apoptosis were successfully suppressed. Moreover, KRG restored 4-HC-induced p53 and Bax/Bcl2 expression.
Activated hair bulb keratinocytes are rapidly proliferating cells and their proliferation is an important event for anagen hair growth. Because chemotherapeutic drugs can directly damage rapidly dividing cells, hair matrix keratinocytes are vulnerable to chemotherapy [41], [42]. In a human hair follicle organ culture model for CIA, KRG protects against premature catagen development and suppresses 4-HC-induced inhibition of matrix keratinocyte proliferation and stimulation of matrix keratinocyte apoptosis. This indicates that the protective effects of KRG on CIA may be mediated through the improvement of hair matrix cell survival.
p53-dependent apoptosis of hair matrix keratinocytes plays a central role in CIA pathogenesis [10]. It has been reported that p53-deficient mice do not show hair loss or apoptosis of keratinocytes after cyclophosphamide administration [43], [44], and these observations also led to the evaluation of inhibitors of p53 signaling for treating CIA [45]. p53 is activated by external and internal stress signals that promote its nuclear accumulation in an active form, inducing either viable cell growth arrest or apoptosis [46]. Transcription-independent p53 activity is involved in many cell death processes and is associated with cytoplasmic or mitochondrial proteins, including members of the Bcl2 family [47], [48], [49]. The Bcl2 family is classified into three classes, namely, antiapoptotic proteins (e.g., Bcl2), proapoptotic proteins (e.g., Bax and Bak), and proapoptotic “BH3-only” proteins [46], [50]. In this study, KRG recovered 4-HC-induced p53 and Bax/Bcl2 expression, indicating that the protective effects of KRG on 4-HC-induced CIA occur at least in part through modulation of p53 and Bax/Bcl2 expression.
In conclusion, KRG may protect against 4-HC-induced premature dystrophy as it occurs in CIA in vivo. Possible mechanisms include the stimulation of hair matrix keratinocyte proliferation and inhibition of hair matrix keratinocyte apoptosis, which are possibly mediated through modulation of p53 and Bax/Bcl2 expression.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This study was supported by a 2013 grant from the Korean Society of Ginseng, funded by the Korea Ginseng Corporation.
References
- 1.Stenn K.S., Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 2.Yeager C.E., Olsen E.A. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432–442. doi: 10.1111/j.1529-8019.2011.01430.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosman S. Cancer and stigma: experience of patients with chemotherapy-induced alopecia. Patient Educ Couns. 2004;52:333–339. doi: 10.1016/S0738-3991(03)00040-5. [DOI] [PubMed] [Google Scholar]
- 4.Lemieux J., Maunsell E., Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17:317–328. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 5.Trüeb R.M. Chemotherapy-induced alopecia. Semin Cutan Med Surg. 2009;28:11–14. doi: 10.1016/j.sder.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Wikramanayake T.C., Amini S., Simon J., Mauro L.M., Elgart G., Schachner L.A., Jimenez J.J. A novel rat model for chemotherapy-induced alopecia. Clin Exp Dermatol. 2012;37:284–289. doi: 10.1111/j.1365-2230.2011.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wikramanayake T.C., Villasante A.C., Mauro L.M., Nouri K., Schachner L.A., Perez C.I., Jimenez J.J. Low-level laser treatment accelerated hair regrowth in a rat model of chemotherapy-induced alopecia (CIA) Lasers Med Sci. 2013;28:701–706. doi: 10.1007/s10103-012-1139-7. [DOI] [PubMed] [Google Scholar]
- 8.Münstedt K., Manthey N., Sachsse S., Vahrson H. Changes in self-concept and body image during alopecia induced cancer chemotherapy. Support Care Cancer. 1997;5:139–143. doi: 10.1007/BF01262572. [DOI] [PubMed] [Google Scholar]
- 9.McGarvey E.L., Baum L.D., Pinkerton R.C., Rogers L.M. Psychological sequelae and alopecia among women with cancer. Cancer Pract. 2001;9:283–289. doi: 10.1046/j.1523-5394.2001.96007.x. [DOI] [PubMed] [Google Scholar]
- 10.Paus R., Haslam I.S., Sharov A.A., Botchkarev V.A. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:e50–e59. doi: 10.1016/S1470-2045(12)70553-3. [DOI] [PubMed] [Google Scholar]
- 11.Li C.P., Li R.C. An introductory note to ginseng. Am J Chin Med (Gard City N Y) 1973;1:249–261. doi: 10.1142/s0192415x73000279. [DOI] [PubMed] [Google Scholar]
- 12.Yun T.K. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song M., Mun J.H., Ko H.C., Kim B.S., Kim M.B. Korean Red Ginseng powder in the treatment of melasma: an uncontrolled observational study. J Ginseng Res. 2011;35:170–175. doi: 10.5142/jgr.2011.35.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M.H., Lee B.H., Jung J.Y., Cheon D.S., Kim K.T., Choi C. Antiviral effect of Korean Red Ginseng extract and ginsenosides on murine norovirus and feline calicivirus as surrogates for human norovirus. J Ginseng Res. 2011;35:429–435. doi: 10.5142/jgr.2011.35.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.Y., Park J.Y., Kang H.J., Kim O.Y., Lee J.H. Beneficial effects of Korean Red Ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: a randomized, double-blind, placebo-controlled trial. Nutr J. 2012;11:47. doi: 10.1186/1475-2891-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuksan V., Sung M.K., Sievenpiper J.L., Stavro P.M., Jenkins A.L., Di Buono M., Lee K.S., Leiter L.A., Nam K.Y., Arnason J.T. Korean Red Ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.H., Jeong K.S., Ryu S.Y., Kim T.H. Panax ginseng prevents apoptosis in hair follicles and accelerates recovery of hair medullary cells in irradiated mice. In Vivo. 1998;12:219–222. [PubMed] [Google Scholar]
- 18.Matsuda H., Yamazaki M., Asanuma Y., Kubo M. Promotion of hair growth by ginseng radix on cultured mouse vibrissal hair follicles. Phytother Res. 2003;17:797–800. doi: 10.1002/ptr.1241. [DOI] [PubMed] [Google Scholar]
- 19.Oh G.N., Son S.W. Efficacy of Korean Red Ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391–395. doi: 10.5142/jgr.2012.36.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu H.J., Yoo M.G., Son S.W. The efficacy of 3% minoxidil vs. combined 3% minoxidil and Korean Red Ginseng in treating female pattern alopecia. Int J Dermatol. 2014;53:e340–e342. doi: 10.1111/ijd.12359. [DOI] [PubMed] [Google Scholar]
- 21.Park G.H., Park K.Y., Cho H.I., Lee S.M., Han J.S., Won C.H., Chang S.E., Lee M.W., Choi J.H., Moon K.C. Red ginseng extract promotes the hair growth in cultured human hair follicles. J Med Food. 2015;18:354–362. doi: 10.1089/jmf.2013.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodó E., Tobin D.J., Kamenisch Y., Bíró T., Berneburg M., Funk W., Paus R. Dissecting the impact of chemotherapy on the human hair follicle: a pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy. Am J Pathol. 2007;171:1153–1167. doi: 10.2353/ajpath.2007.061164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Philpott M.P., Sanders D., Westgate G.E., Kealey T. Human hair growth in vitro: a model for the study of hair follicle biology. J Dermatol Sci. 1994;7:S55–S72. doi: 10.1016/0923-1811(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 25.Kligman A.M. The human hair cycle. J Invest Dermatol. 1959;33:307–316. doi: 10.1038/jid.1959.156. [DOI] [PubMed] [Google Scholar]
- 26.Müller-Röver S., Handjiski B., van der Veen C., Eichmüller S., Foitzik K., McKay I.A., Stenn K.S., Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 27.Ito T., Ito N., Saathoff M., Bettermann A., Takigawa M., Paus R. Interferon-gamma is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br J Dermatol. 2005;152:623–631. doi: 10.1111/j.1365-2133.2005.06453.x. [DOI] [PubMed] [Google Scholar]
- 28.Soma T., Tsuji Y., Hibino T. Involvement of transforming growth factor-beta2 in catagen induction during the human hair cycle. J Invest Dermatol. 2002;118:993–997. doi: 10.1046/j.1523-1747.2002.01746.x. [DOI] [PubMed] [Google Scholar]
- 29.Pi L.Q., Jin X.H., Hwang S.T., Lee W.S. Effects of calcitonin gene-related peptide on the immune privilege of human hair follicles. Neuropeptides. 2013;47:51–57. doi: 10.1016/j.npep.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Paus R., Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 31.Botchkarev V.A. Molecular mechanisms of chemotherapy-induced hair loss. J Investig Dermatol Symp Proc. 2003;8:72–75. doi: 10.1046/j.1523-1747.2003.12175.x. [DOI] [PubMed] [Google Scholar]
- 32.Sharova T.Y., Poterlowicz K., Botchkareva N.V., Kondratiev N.A., Aziz A., Spiegel J.H., Botchkarev V.A., Sharov A.A. Complex changes in the apoptotic and cell differentiation programs during initiation of the hair follicle response to chemotherapy. J Invest Dermatol. 2014;134:2873–2882. doi: 10.1038/jid.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hibino T., Nishiyama T. Role of TGF-beta2 in the human hair cycle. J Dermatol Sci. 2004;35:9–18. doi: 10.1016/j.jdermsci.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Böhm M., Bodó E., Funk W., Paus R. α-Melanocyte-stimulating hormone: a protective peptide against chemotherapy-induced hair follicle damage? Br J Dermatol. 2014;170:956–960. doi: 10.1111/bjd.12759. [DOI] [PubMed] [Google Scholar]
- 35.Bodó E., Kromminga A., Bíró T., Borbíró I., Gáspár E., Zmijewski M.A., van Beek N., Langbein L., Slominski A.T., Paus R. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol. 2009;129:1126–1139. doi: 10.1038/jid.2008.361. [DOI] [PubMed] [Google Scholar]
- 36.Kalkan Y., Kapakin K.A., Kara A., Atabay T., Karadeniz A., Simsek N., Karakus E., Can I., Yildirim S., Ozkanlar S. Protective effect of Panax ginseng against serum biochemical changes and apoptosis in kidney of rats treated with gentamicin sulphate. J Mol Histol. 2012;43:603–613. doi: 10.1007/s10735-012-9412-4. [DOI] [PubMed] [Google Scholar]
- 37.Fu Y.Q., Hua C., Zhou J., Cheng B.R., Zhang J. Protective effects of ginseng total saponins against hepatic ischemia/reperfusion injury in experimental obstructive jaundice rats. Pharm Biol. 2013;51:1545–1551. doi: 10.3109/13880209.2013.802352. [DOI] [PubMed] [Google Scholar]
- 38.Koo H.J., Jang S.A., Yang K.H., Kang S.C., Namkoong S., Kim T.H. Hang do TT, Sohn EH. Effects of red ginseng on the regulation of cyclooxygenase-2 of spleen cells in whole-body gamma irradiated mice. Food Chem Toxicol. 2013;62:839–846. doi: 10.1016/j.fct.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Chang J.W., Park K.H., Hwang H.S., Shin Y.S., Oh Y.T., Kim C.H. Protective effects of Korean Red Ginseng against radiation-induced apoptosis in human HaCaT keratinocytes. J Radiat Res. 2014;55:245–256. doi: 10.1093/jrr/rrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lobina C., Carai M.A., Loi B., Gessa G.L., Riva A., Cabri W., Petrangolini G., Morazzoni P., Colombo G. Protective effect of Panax ginseng in cisplatin-induced cachexia in rats. Future Oncol. 2014;10:1203–1214. doi: 10.2217/fon.13.276. [DOI] [PubMed] [Google Scholar]
- 41.Chon S.Y., Champion R.W., Geddes E.R., Rashid R.M. Chemotherapy-induced alopecia. J Am Acad Dermatol. 2012;67:e37–47. doi: 10.1016/j.jaad.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Paik S.H., Yoon J.S., Ryu H.H., Lee J.Y., Shin C.Y., Min K.H., Jo S.J., Kim K.H., Kwon O. Pretreatment of epidermal growth factor promotes primary hair recovery via the dystrophic anagen pathway after chemotherapy-induced alopecia. Exp Dermatol. 2013;22:496–499. doi: 10.1111/exd.12182. [DOI] [PubMed] [Google Scholar]
- 43.Botchkarev V.A., Komarova E.A., Siebenhaar F., Botchkareva N.V., Komarov P.G., Maurer M., Gilchrest B.A., Gudkov A.V. p53 is essential for chemotherapy-induced hair loss. Cancer Res. 2000;60:5002–5006. [PubMed] [Google Scholar]
- 44.Botchkarev V.A., Komarova E.A., Siebenhaar F., Botchkareva N.V., Sharov A.A., Komarov P.G., Maurer M., Gudkov A.V., Gilchrest B.A. p53 Involvement in the control of murine hair follicle regression. Am J Pathol. 2001;158:1913–1919. doi: 10.1016/S0002-9440(10)64659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J., Lu Z., Au J.L. Protection against chemotherapy-induced alopecia. Pharm Res. 2006;23:2505–2514. doi: 10.1007/s11095-006-9105-3. [DOI] [PubMed] [Google Scholar]
- 46.Haupt S., Berger M., Goldberg Z., Haupt Y. Apoptosis—the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 47.Chipuk J.E., Kuwana T., Bouchier-Hayes L., Droin N.M., Newmeyer D.D., Schuler M., Green D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 48.Moll U.M., Wolff S., Speidel D., Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Coffin A.B., Rubel E.W., Raible D.W. Bax, Bcl2, and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line. J Assoc Res Otolaryngol. 2013;14:645–659. doi: 10.1007/s10162-013-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouillet P., Strasser A. BH3-only proteins—evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]