Abstract
Background
Nephrotoxicity is a common side effect of medications. Panax ginseng is one of the best-known herbal medicines, and its individual constituents enhance renal function. Identification of its efficacy and mechanisms of action against drug-induced nephrotoxicity, as well as the specific constituents mediating this effect, have recently emerged as an interesting research area focusing on the kidney protective efficacy of P. ginseng.
Methods
The present study investigated the kidney protective effect of fermented black ginseng (FBG) and its active component ginsenoside 20(S)-Rg3 against cisplatin (chemotherapy drug)-induced damage in pig kidney (LLC-PK1) cells. It focused on assessing the role of mitogen-activated protein kinases as important mechanistic elements in kidney protection.
Results
The reduced cell viability induced by cisplatin was significantly recovered with FBG extract and ginsenoside 20(S)-Rg3 dose-dependently. The cisplatin-induced elevated protein levels of phosphorylated c-Jun N-terminal kinase (JNK), p53, and cleaved caspase-3 were decreased after cotreatment with FBG extract or ginsenoside 20(S)-Rg3. The elevated percentage of apoptotic LLC-PK1 cells induced by cisplatin treatment was significantly abrogated by cotreatment with FBG and the ginsenoside 20(S)-Rg3.
Conclusion
FBG and its major ginsenoside 20(S)-Rg3, ameliorated cisplatin-induced nephrotoxicity in LLC-PK1 cells by blocking the JNK–p53–caspase-3 signaling cascade.
Keywords: cisplatin, ginsenoside 20(S)-Rg3, mitogen-activated protein kinases, nephrotoxicity, Panax ginseng
1. Introduction
Panax ginseng is one of the best-known herbal medicines and its individual constituents enhance renal function [1]. P. ginseng effectively ameliorated renal dysfunction induced by streptozotocin in rats by attenuating oxidative stress [2]. Korean Red Ginseng not only inhibited the formation of advanced glycation end products and expression of tumor necrosis factor (TNF)-α, but also blocked the mitogen-activated protein kinases (MAPKs) and nuclear factor kappa-light-chain-enhancer of activated B cell-mediated inflammatory pathways in streptozotocin-induced diabetic renal damage [3].
Nephrotoxicity is a common side effect of medications and accounts for approximately 20% of hospital admissions for acute kidney injury [4]. Toxic effects on the kidney related to medications are common and may present as a subtle injury or overt renal failure [5]. Therefore, evaluation of the efficacy of P. ginseng in drug-induced nephrotoxicity, as well as the mechanisms and active constituents involved in this action, have recently emerged as an interesting area of ginseng research focused on its potential efficacy for kidney protection [6].
P. ginseng prevented renal impairment induced by gentamicin, an aminoglycoside antibiotic, in rats [1]. Gentamicin-induced nephrotoxicity is related to oxidative damage. Coadministration with P. ginseng decreased the renal damage induced by gentamicin via the inhibition of free radical formation and restoration of the antioxidant systems [7], [8]. Among the several constituents of ginseng, phenolic acids and flavonoids, which are responsible for the increase in renal blood flow and elimination of free radicals, exhibited protective effects against gentamicin-induced oxidative nephrotoxicity [9].
Cyclosporine, an immunosuppressant drug causes impairment, typical pathologic lesions, and apoptotic cell death in the kidneys [10]. Ginseng protects against cyclosporine-induced renal injury by decreasing the induction of excessive autophagosomes and protein aggregates [11]. Korean Red Ginseng also exhibited protective effects in cyclosporine-induced renal injury via the reduction of renal dysfunction, oxidative stress, and proinflammatory molecules such as induced nitric oxide synthase, cytokines, and transforming growth factor-β1 in rats [10].
Ginsenosides, which are 30-carbon glycosides derived from the triterpenoid dammarane, are major active constituents of P. ginseng. Ginsenosides inhibited the cantharidin-induced cytotoxicity in normal rat kidney cells. Pretreatment with ginsenosides reduced the increases in serum creatinine, urine protein, blood urea nitrogen, and histological changes in rats [12]. These findings may reflect the improvements of renal dysfunction by certain ginsenosides in drug-induced nephrotoxicity.
Research has been conducted to develop methods for increasing the pharmacological efficacy of ginseng by converting the dammarane-based saponins using thermal processing [13]. In line with this notion, black ginseng has been prepared by heat processing and fermentation of raw ginseng. Although some studies of ginseng have focused on its renoprotective effects in diabetes [3], little is known about the effects of black ginseng against nephrotoxicity induced by medications.
The present study sought to investigate the renoprotective effect of black ginseng and its active component ginsenoside 20(S)-Rg3, against cisplatin (a chemotherapy drug)-induced damage in pig kidney (LLC-PK1) cells. In addition, we focused on assessing the role of MAPKs as important mechanistic elements in the kidney protective effects of black ginseng.
2. Materials and methods
2.1. Chemicals and reagents
Ginsenoside 20(S)-Rg3 was purchased from the Ambo Institute (Dajeon, Korea). Cisplatin was purchased from Sigma Aldrich (Seoul, Korea). Dulbecco's modified Eagle's medium was obtained from Cellgro (Manassas, VA, USA). Fetal bovine serum was purchased from Invitrogen Co. (Grand Island, NY, USA). The antibodies for p38 MAPK, phospho-p38, p44/42 MAPK-extracellular signal-regulated kinases, phospho-p44/42, c-Jun-N-terminal kinase (JNK), phospho-JNK, p53, cleaved caspase-3, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the horseradish peroxidase-conjugated antirabbit antibodies were procured from Cell Signaling (Boston, MA, USA). Other chemicals and reagents were of high quality and obtained from commercial sources.
2.2. Preparation of ginseng extracts
Dried powder extract of black ginseng was supplied by the Ginseng By Pharm Co., Ltd., (Wonju, Korea). Four-year-old white ginseng was purchased from a local ginseng market (Geumsan, Korea). The authenticity of the ginseng was determined based on the ingredient profile. The black ginseng was prepared by nine cycles of repeated steaming of the white ginseng at 85°C for 8 h and drying at 50°C for 48 h. To prepare the ginseng extracts, black ginseng was crushed into a powder and extracted once with 10 volumes of distilled water at 80°C for 72 h, and then filtered and chilled. The ginseng extract was fermented with Saccharomyces cerevisiae (Lallemand, Grenaa, Denmark) at 34°C for 25 h. Following fermentation, the black ginseng extract was sterilized at 85°C for 22 h, and then lyophilized. The ginsenosides present in the black ginseng extracts used in this study were Rg2, Rg3, Rh1, Rh2, and Rf at a content of 2.86 μg/mL, 24.52 μg/mL, 12.62 μg/mL, 0.63 μg/mL, and 1.32 μg/mL, respectively [14].
2.3. Renoprotective effect against cisplatin-induced damage in kidney cells
The protective effect against oxidative renal cell damage was evaluated using LLC-PK1 cells [15]. The LLC-PK1 cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 4mM l-glutamine at 37°C in an atmosphere of 5% CO2. The cells were seeded in 96-well culture plates at 1 × 104 cells/well and allowed to adhere for 2 h. Then, the test sample, radical donor 25μM cisplatin, or both were added to the culture medium. Following a 24-h incubation, the medium containing the test sample, radical donor, or both was removed. The cells were incubated in serum-free medium (90 μL/well) and Ez-Cytox reagent (10 μL/well, Itsbio, Seoul, Korea) at 37°C for 2 h. The cell viability was determined by measuring the absorbance at 450 nm using a microplate reader (PowerWave XS; BioTek Instruments, Winooski, VT, USA).
2.4. Western blot analysis
Whole-cell extracts were prepared according to the manufacturer's instructions using a radioimmunoprecipitation assay buffer (Cell Signaling, Danvers, MA, USA) supplemented with 1mM phenylmethylsulfonyl fluoride. The proteins (whole-cell extracts, 20 μg/lane) were separated using electrophoresis in a precast 4–15% Mini-PROTEAN TGX gel (Bio-Rad, Hercules, CA, USA) blotted onto polyvinylidene fluoride (PVDF) membranes and analyzed using epitope-specific primary and secondary antibodies [16]. The bound antibodies were visualized using an enhanced chemiluminescence advance western blotting detection reagents (GE Healthcare, Buckinghamshire, UK) and a Fusion Solo chemiluminescence system (Peqlab Biotechnologie GmbH, Erlangen, Germany).
2.5. Image-based cytometric assay
LLC-PK1 cells were used for an image-based apoptosis assay system. All assays were conducted in accordance with the guidelines for operating the Tali image-based cytometer (Invitrogen, Carlsbad, CA, USA). The cells were treated with samples for 24 h at 37°C under 5 % CO2. The cells were harvested by trypsin treatment using the TrypLE reagent and stained using the Tali apoptosis kit (Invitrogen). The sample was divided and analyzed independently using both the Tali image and flow cytometers following the manufacturer's recommended protocols. The apoptotic portion of the cell population was determined by staining with annexin V-Alexa Fluor 488 conjugate. Propidium iodide (PI) was used to differentiate the dead cells (annexin V positive/PI positive or annexin V negative/PI positive) from the apoptotic cells (annexin V positive/PI negative). The percentages of the cell population reported as viable, apoptotic, and dead by the Tali cytometer were comparable with the data from the same samples independently run on the flow cytometer [17].
2.6. Statistical analysis
Statistical significance was determined using an analysis of variance followed by a multiple comparison test with a Bonferroni adjustment. A p value < 0.05 was considered statistically significant. The analysis was performed using the statistics for the social sciences (SPSS) version 19.0 (SPSS Inc., Chicago, Il, USA).
3. Results and discussion
Cisplatin is one of the most effective and widely used anticancer drugs for the treatment of various solid tumors; however, it causes nephrotoxicity, one of the most well-known and clinically important side-effects [18], [19]. Earlier studies demonstrated that several mechanisms including oxidative stress, DNA damage, and inflammatory responses are closely associated with cisplatin-induced nephrotoxicity [19], [20]. Cisplatin is known to activate p38, extracellular signal-regulated kinases (ERK), and JNK/stress-activated protein kinases in the kidneys both in vitro and in vivo [21]. In addition, genomic DNA is presumably a primary target of cisplatin in tubular cells. The tumor suppressor gene p53 is induced in response to DNA damage, and is implicated in subsequent DNA repair and cell death by apoptosis [22], [23]. In the present study, we sought to identify the kidney protective effect of black ginseng extract and its active ginsenoside 20(S)-Rg3, as well as the mechanisms involved, to determine its therapeutic potential.
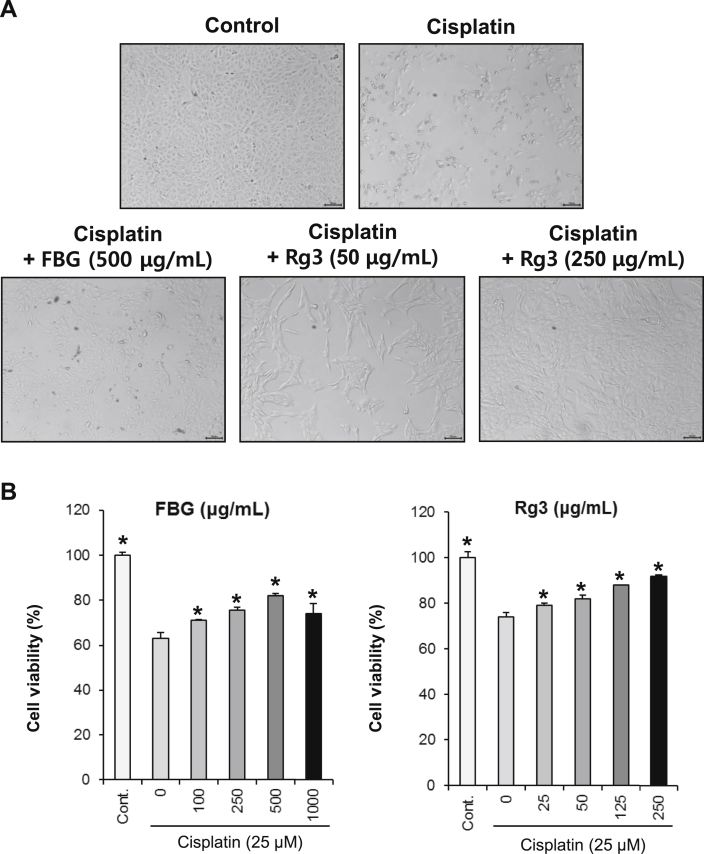
We carried out a cell-based kidney protection assay to evaluate the protective effects of black ginseng extract and ginsenoside 20(S)-Rg3 on LLC-PK1 cells. The kidney cell protection assay condition was established using the LLC-PK1 cell line, which is commonly used in the evaluation of nephrotoxicity [24], [25]. The viability of the LLC-PK1 cells reduced to 60% of the control cell viability after treatment with 25μM of cisplatin. The reduced cell viability induced by cisplatin was significantly recovered by black ginseng and ginsenoside 20(S)-Rg3 dose-dependently (Fig. 1). The black ginseng extract and ginsenoside 20(S)-Rg3 ameliorated the cisplatin-induced nephrotoxicity to control levels at concentrations of 500 μg/mL and 250 μg/mL, respectively.
Fig. 1.
The protective effects of black ginseng and ginsenoside 20(S)-Rg3 on cisplatin-induced renal cell damage. (A) Representative microscopic image of nephroprotective effects of black ginseng and ginsenoside 20(S)-Rg3 following treatment with 25 μM cisplatin. (B) Protection assay of black ginseng and ginsenoside 20(S)-Rg3 in cisplatin-treated (25 μM) LLC-PK1 cells. LLC-PK1 cells were pretreated with various concentrations of black ginseng and ginsenoside 20(S)-Rg3 for 2 h, and then further treated with 25 μM of cisplatin for 24 h. Cell viability was assessed using the MTT assay. *p < 0.05 compared with the cisplatin-treated group. FBG, fermented black ginseng; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
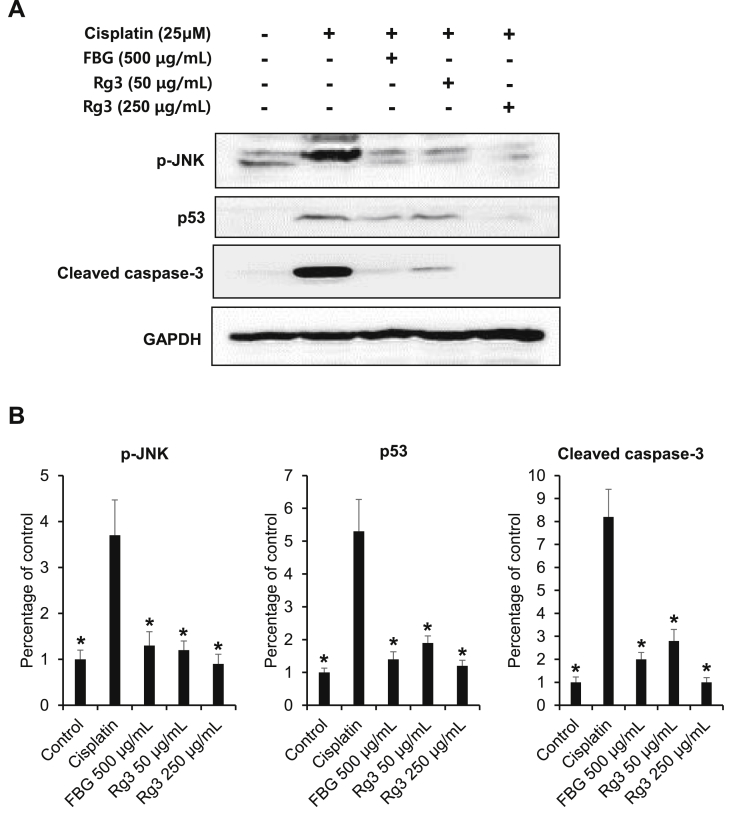
Dysregulation of normal MAPK signaling has been implicated in both acute and chronic kidney disease [26]. In the present study, we sought to determine the role of the MAPKs–p53–caspase apoptotic cascade in mediating the protective effects of black ginseng and ginsenoside 20(S)-Rg3 against oxidative cytotoxicity in renal cells. As shown in Fig. 2, the phosphorylation of JNK was observed 24 h after cisplatin treatment and decreased following treatment with black ginseng and ginsenoside 20(S)-Rg3. However, there were no significant changes in p-ERK and p-p38 (data not shown).
Fig. 2.
The c-Jun N-terminal kinase (JNK)–p53–caspase-3 signaling pathway mediates the protective effect of black ginseng and ginsenoside 20(S)-Rg3 against cytotoxicity in cultured LLC-PK1 cells. (A) Results of the Western blot analysis show the levels of phosphorylated (p)-JNK, JNK, p53, cleaved caspase-3, and GAPDH in LLC-PK1 cells treated with black ginseng and ginsenoside 20(S)-Rg3 with or without cisplatin at different concentrations for 24 h. (B) Quantitative results for p-JNK, p53 and cleaved caspase-3 from Western blot analysis. Whole cell lysates (20 μg)were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred onto PVDF transfer membranes, and probed with the indicated antibodies. Proteins were visualized using an enhanced chemiluminescence detection system. *p < 0.05 compared with the cisplatin-treated group. ERK, extracellular signal-regulated kinases; FBG,fermented black ginseng; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
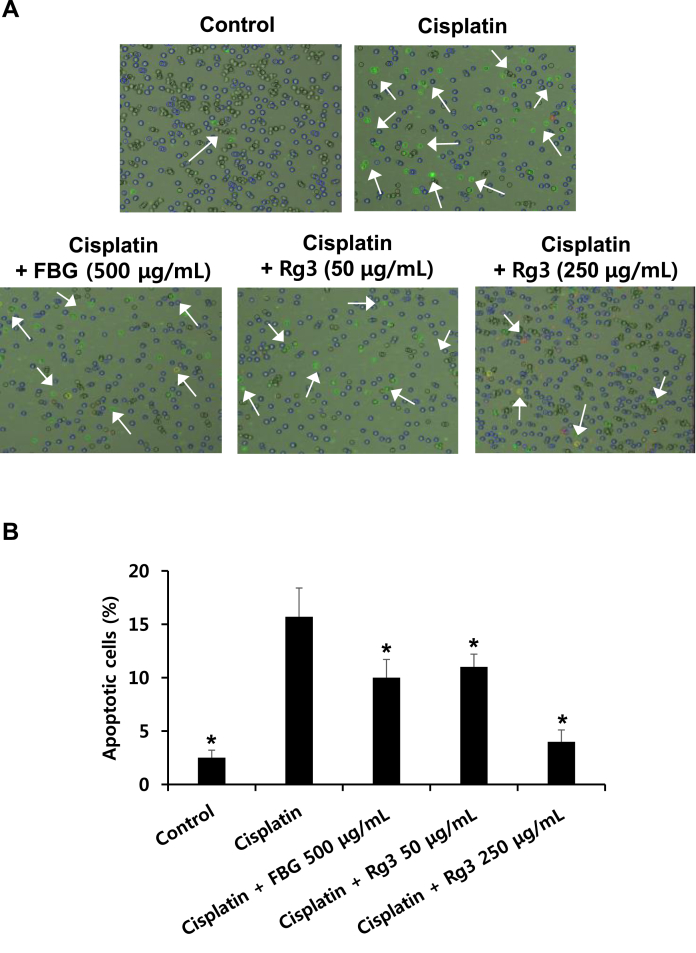
Cisplatin-induced nephrotoxicity is dependent on DNA damage-induced apoptosis [27]. The protein level of p53, which was also markedly increased after treatment with cisplatin, was reduced significantly by a high concentration of black ginseng and ginsenoside 20(S)-Rg3. Similarly, the elevated levels of cleaved caspase-3 also decreased after treatment with black ginseng and ginsenoside 20(S)-Rg3. Fig. 3 shows the effects of black ginseng extract and ginsenoside 20(S)-Rg3 on apoptosis in LLC-PK1 cells. As shown in Fig. 3A, the number of dead and apoptotic cells, which were stained with red and green fluorescence and increased after cisplatin treatment, decreased following cotreatment with black ginseng and more significantly with ginsenoside 20(S)-Rg3. The elevated percentage of apoptotic LLC-PK1 cells induced by cisplatin treatment was markedly decreased following the cotreatment with black ginseng and ginsenoside 20(S)-Rg3 (Fig. 3B).
Fig. 3.
Effects of black ginseng and ginsenoside 20(S)-Rg3 on apoptosis in LLC-PK1 cells. (A) Representative images of apoptosis detection. (B) Percentage of annexin V-positive-stained apoptotic cells. Dead and apoptotic cells were stained with red and green fluorescence. Apoptosis was determined using a Tali image-based cytometer. ∗p < 0.05 compared with the cisplatin-treated group. FBG, fermented black ginseng.
Cisplatin-induced kidney damage has been correlated with the formation of free radicals and oxidative stress, which can activate MAPKs [24], [28], [29]. Treatment with antioxidants and caspase inhibitors can alleviate the effects of cisplatin-related nephrotoxicity. There is considerable evidence showing that the MAPKs, p53, and caspase pathways play important roles in regulation of the apoptosis pathways, as well as the inflammatory process [23], [30]. The present results show that the JNK–p53–caspase-3 signaling cascade plays a critical role in mediating the protective effects of black ginseng and ginsenoside 20(S)-Rg3 against oxidative cytotoxicity in cultured LLC-PK1 cells.
Ginseng showed protective effects against cisplatin-induced nephrotoxicity by alleviating the decreased activities of antioxidant enzymes including superoxide dismutase, glutathione peroxidase, catalase, and glutathione S-transferase as well as the levels of glutathione. This effect consequently reduced the oxidative stress, alterations in biochemical parameters, histological changes, genomic DNA damage, as well as the expression of TNF-α, interleukin-6, and the tumor suppressor p53 [31]. Pretreatment with ginsenoside F11 reduced cisplatin-induced elevation of blood urea nitrogen and creatinine levels, as well as ameliorated the histopathological damage. Further studies showed that F11 suppressed p53 activation, inversed the ratio of B-cell lymphoma-2 associated X protein/B-cell lymphoma-2, and the antioxidative and free radical levels induced by cisplatin, which in turn inhibited tubular cell apoptosis [32]. However, the effect and mechanism of action of ginsenoside 20(S)-Rg3 have not been identified so far.
In conclusion, our results suggest that black ginseng ameliorates nephrotoxicity in LLC-PK1 cells, and ginsenoside 20(S)-Rg3 is likely the major component that mediates this effect through blockade of the JNK–p53–caspase-3 signaling cascade.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was funded by the Gangneung Asan Hospital Biomedical Research Center Promotion Fund. It was also supported by the Korea Institute of Science and Technology Institutional Program (2Z04371).
Contributor Information
Ki Sung Kang, Email: kkang@gachon.ac.kr.
Hyuk-Jai Jang, Email: jhj@gnah.co.kr.
References
- 1.Lee Y.K., Chin Y.W., Choi Y.H. Effects of Korean red ginseng extract on acute renal failure induced by gentamicin and pharmacokinetic changes by metformin in rats. Food Chem Toxicol. 2013;59:153–159. doi: 10.1016/j.fct.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 2.El-Khayat Z., Hussein J., Ramzy T., Ashour M., Oraby F. Protective effect of Panax ginseng against streptozotocin induced renal dysfunction in rats. J Appl Sci Res. 2011;7:1419–1423. [Google Scholar]
- 3.Quan H.Y. Kim do Y, Chung SH. Korean red ginseng extract alleviates advanced glycation end product-mediated renal injury. J Ginseng Res. 2013;37:187–193. doi: 10.5142/jgr.2013.37.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasiri-Toosi Z., Dashti-Khavidaki S., Khalili H., Lessan-Pezeshki M. A review of the potential protective effects of pentoxifylline against drug-induced nephrotoxicity. Eur J Clin Pharmacol. 2013;69:1057–1073. doi: 10.1007/s00228-012-1452-x. [DOI] [PubMed] [Google Scholar]
- 5.Choudhury D., Ahmed Z. Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol. 2006;2:80–91. doi: 10.1038/ncpneph0076. [DOI] [PubMed] [Google Scholar]
- 6.Jeong K.J., Kim G.W., Chung S.H. AMP-activated protein kinase: an emerging target for ginseng. J Ginseng Res. 2014;38:83–88. doi: 10.1016/j.jgr.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karadeniz A., Yildirim A., Simsek N., Turhan H., Kalkan Y., Celebi F. Effect of Panax ginseng on gentamicin sulphate-induced kidney toxicity in rats. Rev Med Vet. 2008;159:215–220. [Google Scholar]
- 8.Kalkan Y., Kapakin K.A., Kara A., Atabay T., Karadeniz A., Simsek N., Karakus E., Can I., Yildirim S., Ozkanlar S. Protective effect of Panax ginseng against serum biochemical changes and apoptosis in kidney of rats treated with gentamicin sulphate. J Mol Histol. 2012;43:603–613. doi: 10.1007/s10735-012-9412-4. [DOI] [PubMed] [Google Scholar]
- 9.Qadir M.I., Tahir M., Lone K.P., Munir B., Sami W. Protective role of ginseng against gentamicin induced changes in kidney of albino mice. J Ayub Med Coll Abbottabad. 2011;23:53–57. [PubMed] [Google Scholar]
- 10.Doh K.C., Lim S.W., Piao S.G., Jin L., Heo S.B., Zheng Y.F., Bae S.K., Hwang G.H., Min K.I., Chung B.H. Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am J Nephrol. 2013;37:421–433. doi: 10.1159/000349921. [DOI] [PubMed] [Google Scholar]
- 11.Lim S.W., Doh K.C., Jin L., Jin J., Piao S.G., Heo S.B., Chung B.H., Yang C.W. Ginseng treatment attenuates autophagic cell death in chronic cyclosporine nephropathy. Nephrology (Carlton) 2014;19:490–499. doi: 10.1111/nep.12273. [DOI] [PubMed] [Google Scholar]
- 12.Xu Q., Jia D., Gao H., Zhang M., He W., Pan S., Liu Y., Li X., Cui J., Yang S. In vitro and in vivo protective effects of gingenosides on acute renal injury induced by cantharidin. J Funct Foods. 2013;5:2012–2018. [Google Scholar]
- 13.Park E.H., Kim Y.J., Yamabe N., Park S.H., Kim H.K., Jang H.J., Kim J.H., Cheon G.J., Ham J., Kang K.S. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J Ginseng Res. 2014;38:22–27. doi: 10.1016/j.jgr.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bak M.J., Jeong W.S., Kim K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int J Mol Med. 2014;34:1516–1522. doi: 10.3892/ijmm.2014.1972. [DOI] [PubMed] [Google Scholar]
- 15.Yokozawa T., Cho E.J., Hara Y., Kitani K. Antioxidative activity of green tea treated with radical initiator 2, 2'-azobis(2-amidinopropane) dihydrochloride. J Agric Food Chem. 2000;48:5068–5073. doi: 10.1021/jf000253b. [DOI] [PubMed] [Google Scholar]
- 16.Ninomiya Y., Cui X., Yasuda T., Wang B., Yu D., Sekine-Suzuki E., Nenoi M. Arsenite induces premature senescence via p53/p21 pathway as a result of DNA damage in human malignant glioblastoma cells. BMB Rep. 2014;47:575–580. doi: 10.5483/BMBRep.2014.47.10.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubey A., Min J.W., Koo H.J., Kim H., Cook T.R., Kang S.C., Stang P.J., Chi K.W. Anticancer potency and multidrug-resistant studies of self-assembled arene-ruthenium metallarectangles. Chemistry. 2013;19:11622–11628. doi: 10.1002/chem.201300870. [DOI] [PubMed] [Google Scholar]
- 18.Li J., Jiang K., Qiu X., Li M., Hao Q., Wei L., Zhang W., Chen B., Xin X. Overexpression of CXCR4 is significantly associated with cisplatin-based chemotherapy resistance and can be a prognostic factor in epithelial ovarian cancer. BMB Rep. 2014;47:33–38. doi: 10.5483/BMBRep.2014.47.1.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh G.S., Kim H.J., Shen A., Lee S.B., Khadka D., Pandit A., So H.S. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press. 2014;12:55–65. doi: 10.5049/EBP.2014.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trujillo J., Chirino Y.I., Molina-Jijón E., Andérica-Romero A.C., Tapia E., Pedraza-Chaverrí J. Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol. 2013;1:448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramesh G., Reeves W.B. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005;289:F166–F174. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 22.Chi S.W. Structural insights into the transcription-independent apoptotic pathway of p53. BMB Rep. 2014;47:167–172. doi: 10.5483/BMBRep.2014.47.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano T., Itoh Y., Matsuo M., Kawashiri T., Egashira N., Oishi R. Involvement of both tumor necrosis factor-alpha-induced necrosis and p53-mediated caspase-dependent apoptosis in nephrotoxicity of cisplatin. Apoptosis. 2007;12:1901–1909. doi: 10.1007/s10495-007-0110-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee C.K., Park K.K., Chung A.S., Chung W.Y. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem Toxicol. 2012;50:2565–2574. doi: 10.1016/j.fct.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.H., Han I.H., Yamabe N., Kim Y.J., Lee W., Eom D.W., Choi P., Cheon G.J., Jang H.J., Kim S.N. Renoprotective effects of Maillard reaction products generated during heat treatment of ginsenoside Re with leucine. Food Chem. 2014;143:114–121. doi: 10.1016/j.foodchem.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 26.Cassidy H., Radford R., Slyne J., O'Connell S., Slattery C., Ryan M.P., McMorrow T. The role of MAPK in drug-induced kidney injury. J Signal Transduct. 2012;2012:463617. doi: 10.1155/2012/463617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pippin J.W., Durvasula R., Petermann A., Hiromura K., Couser W.G., Shankland S.J. DNA damage is a novel response to sublytic complement C5b-9–induced injury in podocytes. J Clin Invest. 2003;111:877–885. doi: 10.1172/JCI15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang K.S., Lee W., Jung Y., Lee J.H., Lee S., Eom D.W., Jeon Y., Yoo H.H., Jin M.J., Song K.I. Protective effect of esculin on streptozotocin-induced diabetic renal damage in mice. J Agric Food Chem. 2014;62:2069–2076. doi: 10.1021/jf403840c. [DOI] [PubMed] [Google Scholar]
- 29.Song K.I., Park J.Y., Lee S., Lee D., Jang H.J., Kim S.N., Ko H., Kim H.Y., Lee J.W., Hwang G.S. Protective effect of tetrahydrocurcumin against cisplatin-induced renal damage: in vitro and in vivo studies. Planta Med. 2015;81:286–291. doi: 10.1055/s-0035-1545696. [DOI] [PubMed] [Google Scholar]
- 30.Francescato H.D., Costa R.S., Júnior F.B., Coimbra T.M. Effect of JNK inhibition on cisplatin-induced renal damage. Nephrol Dial Transplant. 2007;22:2138–2148. doi: 10.1093/ndt/gfm144. [DOI] [PubMed] [Google Scholar]
- 31.Yousef M.I., Hussien H.M. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem Toxicol. 2015;78:17–25. doi: 10.1016/j.fct.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Kong L., Zhang J., Yu G., Lv G., Zhang F., Chen X., Tian J., Fu F. The pseudoginsenoside F11 ameliorates cisplatin-induced nephrotoxicity without compromising its anti-tumor activity in vivo. Sci Rep. 2014;4986:4. doi: 10.1038/srep04986. [DOI] [PMC free article] [PubMed] [Google Scholar]