Abstract
Maternal occupational pesticide exposure during pregnancy and/or paternal occupational pesticide exposure around conception have been suggested to increase risk of leukemia in the offspring. With a view to providing insight in this area we pooled individual level data from 13 case-control studies participating in the Childhood Leukemia International Consortium (CLIC). Occupational data were harmonized to a compatible format. Pooled individual analyses were undertaken using unconditional logistic regression. Using exposure data from mothers of 8,236 cases, and 14,850 controls, and from fathers of 8,169 cases and 14,201 controls the odds ratio (OR) for maternal exposure during pregnancy and the risk of acute lymphoblastic leukemia (ALL) was 1.01 (95% confidence interval (CI) 0.78, 1.30) and for paternal exposure around conception 1.20 (95% 1.06, 1.38). For acute myeloid leukemia (AML), the OR for maternal exposure during pregnancy was 1.94 (CI 1.19, 3.18) and for paternal exposure around conception 0.91 (CI 0.66, 1.24.) based on data from 1,329 case and 12,141 control mothers, and 1,231 case and 11,383 control fathers. Our finding of a significantly increased risk of AML in the offspring with maternal exposure to pesticides during pregnancy is consistent with previous reports. We also found a slight increase in risk of ALL with paternal exposure around conception which appeared to be more evident in children diagnosed at the age of five years or more and those with T cell ALL which raises interesting questions on possible mechanisms.
Keywords: pesticide, occupation, leukemia, childhood, pooled analysis, meta-analysis
Introduction
Little is known about the etiology of childhood leukemia and its main sub-types, acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) but it is likely that they are attributable to a mixture of genetic and environmental factors,1 which may vary by disease sub-type, or for ALL, by immunophenotype. Most cases occur before the age of five years, although T cell ALL is seen mainly in slightly older children. Some of the most common chromosomal translocations seen in both sub-types of ALL2, 3 and AML4 may be of prenatal origin, suggesting a role for parental exposures. Individual studies rarely have the power to investigate potential risk factors by sub-type and/or immunophenotype, especially for uncommon exposures. To help overcome this, we pooled individual data from studies in the Childhood Leukemia International Consortium (CLIC), a multi-national collaboration of case-control studies of childhood leukemia.5 The focus of these analyses was parental occupational exposures to pesticides.
The term ‘pesticide’ covers a large, heterogeneous group of chemicals used to control insects, weeds, fungi and other pests. The active ingredients of each chemical may have different mutagenic, carcinogenic or immunotoxic properties. More than 20 individual pesticides have been classified as, at least ‘probable or possible’ human carcinogens by the International Agency for Research on Cancer.6 Exposure of the father before conception could result in germ cell damage, while maternal exposure during pregnancy can result in fetal exposure, as demonstrated by pesticide residuals found in umbilical cord blood and meconium.7 Prenatal exposure to certain insecticides, has been associated with translocations found in children with AML.8, 9 Propoxur, has been associated with t(8;21) translocations in cord blood9 and permethrin has been associated with a 11q23 translocation in a case report of congenital AML,8 thus suggesting that maternal pesticide exposure around pregnancy could result in chromosome translocations in the offspring. Maternal pre-natal occupational pesticide exposures have been examined in two recent meta-analyses,10, 11 with both reporting an increased risk of all leukemias, as well as of leukemia sub-types. However, the estimates for ALL and AML were based on five or fewer studies because most studies in the overall analyses did not report risk by leukemia sub-type, and none reported by immunophenotype. Pre-conceptional paternal occupational exposure has also been suggested as a risk factor in individual studies of ALL12 and AML.13
The aim of the current analyses was to investigate whether parental occupational pesticide exposure in the prenatal period increased the risk of ALL or AML in the offspring. We also aimed to investigate whether the relationship varied by immunophenotype of ALL. For these analyses, we used all CLIC studies that had relevant data available in 2012, that is, 13 studies (12 with ALL cases and 10 with AML cases) that were conducted in North America, Europe and Australasia over a 30 year period.
Methods
Original data were requested from each of the participating studies including demographics, disease sub-types, potential covariates, variables used for control selection or matching and occupational pesticide exposure assessments for both parents. A summary of study design and participant details, including inclusion criteria, has already been published5 and the characteristics of each study as well as participation fractions (based on information available from published studies or obtained directly from study personnel) are listed in Table 1. Definition of the participation fraction varied across studies. In most cases, the studies were conducted on a nationwide or region-wide basis and thus they included a mixture of urban and rural subjects. All studies were approved by the relevant institutional or regional ethical committees.
Table 1.
Selected characteristics including occupational exposure assessment of the 13 studies in the CLIC pooled analyses of parental occupational pesticide exposure and the risk of leukemia in the offspring
| Country, Study (years of case accrual) |
Method of occupational assessment |
Case | Control | Time period(s) of interesta | Scope of assessment |
Final exposure variableb |
Prevalence of
‘High likelihood of pesticide exposure’ amongst controls |
Source of conversion tool to other Occupational Classifications (where applicable) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Participationc | Nd | Source | Participationc | Nd | Mothers | Fathers | ||||||
| 1. Use of an Occupational Classification
System |
|||||||||||||
| France, ADELE (1993–1999) | ISCO 1988 | Hospitals | 95% | ALL: 240 AML: 36 |
Hospitals (same as cases) | 99% | 288 | Extracted from work history (start & end year of
each job) 1. Main year before conception (defined as the year of the mid point of the year before conception) 2. Main year of pregnancy (defined as the year of the midpoint of the pregnancy) |
All jobs held in time periods | 4 level 1. ‘High likelihood of pesticide exposure’ 2. ‘Moderate likelihood of exposure’ 3. ‘Limited likelihood of exposure’ 4 = ‘No or minimal likelihood of pesticide exposure’ |
0.4 | 1.7 | Correspondence Table ISCO 08 to ISCO 8819 |
| Greece, NARECHEM (1993–1994) | ISCO 1988 | Nationwide hospital cancer registry | 100% | ALL; 140 AML: 13 |
Hospital | 96% | 300 | 1.One year before birth 2.During pregnancy |
All jobs held in time periods | As above | 2.9 | 9.8 | Correspondence Table ISCO 08 to ISCO 8819 |
| France ESCALE (2003–2004) | ISCO 1968 | Population-based cancer registry (nationwide) | 91% | ALL: 648 AML: 101 |
Population quotas by age, sex, region (nationwide) | 71% | 1681 | 1.During pregnancye | Main job in time period | As above | 0.8 | 4.3 | Correspondence Table ISCO 68 to ISCO 8820 |
| Greece, NARECHEM (1996–2010) | ISCO 1968 | Nationwide hospital cancer registry | 83% | ALL: 964 AML: 113 |
Hospital | 96% | 1085 | 1. One year before birth 2. During pregnancy |
All jobs held in time periods | As above | 1.1 | 3.0 | Correspondence Table ISCO 68 to ISCO 8820 |
| Germany, GCCR (1988–1994) |
Germany, Bundesgentur fur Abeit Wirtschaftsklasse 1988 | Population-based cancer registry (nationwide) | 82% | ALL: 751 AML: 130 |
Population-based registry (community based but complete nationwide coverage) | 71% | 2458 | 1. Conception 2. During pregnancy |
Main job in time periods | As above | 1.2 | 3.0 | Correspondence Table to ISCO 88 obtained from Federal Statistical Office, Germany21 |
| UK, UKCCS (1991–1996) | UK, Standard Occupational Classification 1990 | Population-based tailored referral systems | 93% | ALL: 1461 AML: 248 |
GP registries (nationwide) | 64% | 3448 | 1. Around conception 2. During pregnancy |
All jobs held in time periods | As above | 0.3 | 2.4 | Correspondence Table to ISCO 88 obtained from Office for National Statistics, UK22 |
| US, NCCLS (1995–2008) | US, Census Occupational Classification Codes,1990 |
Hospitals | 86% | ALL: 840 AML: 145 |
Birth registry (state wide) | 68% | 1226 | Extracted from work history (start and end month, year of
each job) 1. Year before conception 2. During pregnancy |
All jobs held in time periods | As above | 2.0 | 8.3 | Correspondence Tables obtained from the National Crosswalk Center23 between 1990 Census to 2000 Census codes and 2000 Census codes to ISCO 88 |
| US, COG-E15 (1989–1993) | US, Department of Labor Dictionary of Occupational Titles (4th ed., rev. 1991) | Children’s Cancer Group clinical trials | 87% | ALL: 1914 | RDD | 70% | 1987 | Extracted from work history (start and end month, year of
each job) 1. Year before conception 2. During pregnancy |
All jobs held in time periods | As above | 1.1 | 4.2 | Correspondence Tables obtained from the National Crosswalk Center23 between DOT to 2000 Census codes and 2000 Census codes to ISCO 88 |
| 2. Pesticide exposure already
assigned |
|||||||||||||
| Australia, Aus-ALL (2003–2007) | Answers to initial structured questionnaire and follow-up job specific interview reviewed by expert14 | Hospitals (nationwide) | 75% | ALL: 389 | RDD | 64% of agreed controls | 876 | 1. One year before birth | All jobs held in time period | 3 level: 1 = medium/high exposure 2 = low exposure 4 = not exposed |
0.2 | 5.5 | |
| Canada, Quebec (1980–2000) | Answers to initial structured interview and follow-up job specific questions reviewed by expert12, 16 | Hospitals (province wide) | 93% | ALL: 790 | Health Insurance file population-based registry (province-wide) | 86% | 790 | 1. Two years before conception 2. During pregnancy |
All jobs held in time periods | 2 level: 1 = exposed 4 = not exposed |
4.4 | 7.9 | |
| Italy, SETIL (1998–2001) | Answers to initial structured interview and follow-up job specific questions reviewed by experts24 (restricted to agricultural pesticides) | Nationwide clinical database | 91% | ALL: 601 AML: 32 |
Population-based National Health Service Registry | 69% | 1044 | 1. One year before conception 2. During pregnancy |
All jobs held in time periods | 2 level: 1 = exposed 4 = not exposed |
0.4 | 1.9 | |
| New Zealand, NZCCS (1990–1993) | Exposure assignment based on detailed questionnaire and interview about pesticide exposures in each job | Registry (nationwide) | 92% | ALL: 97 AML: 22 |
Birth registry (nationwide) | 69% | 303 | 1. Two years before birth 2. During pregnancy |
All jobs held in time periods | 2 level: 1 = exposed 4 = not exposed |
1.3 | 18.0 | |
| 3. Pesticide exposure data collected, but
exposure not assigned |
|||||||||||||
| US, COG-E14 (1988–1993) | Detailed questionnaire about each type of pesticide use in each job | Children’s Cancer Group clinical trials | 76% | AML: 517 | RDD | 72% | 610 | Extracted from work history (start and end month, year of
each job) 1. Year before conception 2. During pregnancy |
All jobs held in time periods | 4 level: Tertiles of exposuref 1 = ‘High’ 2 = ‘Medium’ 3 = ‘Low’ 4 = unexposed |
0.3 | 5.2 | |
The time periods of interest were 1. Around conception for the father and 2. During pregnancy for the mother.
In the final pooling process, a 4 level variable was used, but levels 2 and or 3 were empty for studies with less than 4 categories.
Participation fractions are based on information available from published studies or obtained directly from study personnel. Definition of the participation fraction may vary across studies.
Occupational histories were available for more than 90% of parents. The numbers of mothers and fathers with occupational histories are in Supplementary Table 2.
In France ESCALE, paternal exposure during pregnancy was used as a proxy for paternal exposure at conception as these data were not available.
Based on tertiles of the total time any pesticide was in the air the subject or on the skin/clothing during time period among exposed control mothers and fathers.
ISCO: International Standard Classification for Occupation
RDD: random digit dialing
Original occupational exposure data
The time periods of interest were the year before conception for fathers and during the pregnancy for the mother. However, the included studies had data for differing periods around conception or only during pregnancy in some (Table 1). In four studies, data for jobs in the time periods were extracted from the provided work history.
Occupational data were provided in three main formats (Table 1); 1) Eight studies (France: Adele and Escale; Greece Nationwide Registry for Childhood Haematogical Malignancies (NARECHEM) 1993–1994 and 1996–2011; Germany; United Kingdom Childhood Cancer Study (UKCCS); US Northern California Childhood Leukemia Study (NCCLS); US Children’s Oncology Group (COG)-E15 provided jobs coded using an occupational coding system, which needed to have pesticide exposure assigned; 2) Four studies provided data in which jobs in the relevant time periods had already been assessed for pesticide exposure and exposure assigned (Australia, Canada, Italy, New Zealand) and 3) One study (US COG-E14) provided detailed pesticide questionnaire data which needed to be collated to a single exposure variable.
Development of a Job Exposure Matrix (JEM)
The assessments from two of the studies, Australia14 and Canada15, were used to develop a JEM to assign the likelihood of pesticide exposure for the studies with job title codes. Both of these studies use the expert assessment method to assess occupational pesticide exposure.16 In this method a full job history is taken, and job specific questionnaires are asked about each relevant job (for example an orchardist would be asked the Farmer questions while a sports field manager would be asked the Gardener questions). The answers to these questions are reviewed on an individual level by experts, such as industrial hygienists or chemists, who determine whether the person was likely to be exposed to pesticides in that job. For each job title in International Standard Classifications of Occupation (ISCO)-2008 (08)17 we determined what proportion of the jobs in the Australian data were assessed as being exposed to pesticides. All job codes were then assigned to a category relating to the certainty of pesticides exposure as follows; 1) Job codes where 70% or more people (males and females combined) with the ISCO-08 code had been assessed as exposed to pesticides (‘High likelihood of pesticide exposure’); 2) Job codes where 25% ≤ 70% were assessed as exposed (‘Moderate likelihood of exposure’); 3) Job codes where 10 ≤25% were exposed (‘Limited likelihood of exposure’) and 4) Job codes where less than 10% were exposed (‘No or minimal likelihood of pesticide exposure’ (Reference Group)). ISCO-08 jobs codes that were rare or not used in the Australian dataset were identified and these were assigned an exposure category by an occupational epidemiologist (LF). Modifications to the exposure categories were made after doing similar comparisons of expert assessment and jobs coding from the Canadian study.18 The final exposure codes in the JEM were then assigned to equivalent ISCO-88 codes and hence to jobs in the other occupational classification systems using conversion tools (Table 1)19–23. In the case of ‘many to one’ or ‘one to many’ matches to job codes across systems, a judgment was made of the exposure category that best fitted the original job code description. Rather than assigning an exposure category to all codes in each of the coding systems, other than ISCO-08 or ISCO-88, matches were only found for those that appeared in any of the datasets. A full list of the job codes which were categorized as highly likely to be occupationally exposed in each of the occupational coding systems is found in Supplementary Table 1. Finally, we compared the job titles in the ‘High likelihood of pesticide exposure’ category to a list of jobs involving possible pesticide exposure based on a literature search of the topic. Because of likely heterogeneity across studies in pesticide usage patterns, we did not attempt to differentiate between types of pesticides.
Harmonisation of occupational data from other studies
Among the four studies where pesticide exposure had already been assigned, three (Canada, Italy and New Zealand) had assigned pesticide exposure as yes or no. These categories in the Italian study were derived from the assessment of the probability and intensity of exposure,24 while those in the New Zealand data were derived from detailed assessment. In order to pool with the studies for which we used the JEM, we coded exposed subjects the same as the ‘High likelihood of pesticide exposure’ category and non-exposed the same as the ‘No or minimal likelihood of pesticide exposure’ category. In the Australian study which assigned more levels of exposure, we coded those with ‘probable high/medium exposure’ the same as the ‘High likelihood of pesticide exposure’ category, and ‘probable low exposure and possible low/medium/high exposure’ the same as the ‘Moderate likelihood of exposure’ category and those assessed as not exposed the same as the ‘No or minimal likelihood of pesticide exposure’ category (Table 1).
US COG-E14 had collected data about exposure to individual pesticides. These data were collated into a single ‘any pesticide exposure’ for each time period, with four levels of exposure (in order to be comparable to the studies for which we used the JEM), based on the information about total contact time with pesticides.
Statistical analyses
Two analytic approaches were taken which both used the final exposure measure. Firstly, study-specific odds ratios (ORs) of ALL and AML and exposure to pesticides were estimated and included in meta-analyses in order to identify heterogeneity between the studies. Secondly, as main approach, individual data were pooled in a single dataset and the pooled ORs estimated. Because the final exposure measure was an imprecise approximation of occupational exposure, the main focus of both the meta-analysis and pooled analyses was to contrast the OR between Exposure Category 1 (‘High likelihood of pesticide exposure’) to the Reference Category 4 (‘No or minimal likelihood of pesticide exposure’) for both the study specific and pooled analyses. While those with other exposure categories were included in the analyses, a trend across categories response was not investigated and results from ‘Moderate likelihood of exposure’ and ‘Limited likelihood of exposure’ categories are only shown in a supplementary table. ALL and AML were analyzed separately and where possible, subgroup analysis were undertaken by ALL immunophenotypes and by type of occupational assessment.
The Escale study only had paternal exposure data for the time period during pregnancy so this was used as a proxy for before conception exposure.
Generating and meta-analyzing study-level ORs from individual-level data and meta-analyses from published studies
Unconditional logistic regression (SAS version 9.2, SAS Institute Inc, Cary, NC, USA) was used to estimate ORs and 95 percent confidence intervals (95% CIs) for occupational pesticide exposures in mothers during pregnancy and for fathers before conception. All models included child’s age and sex and additional study-specific matching variables. This approach was used to optimize the number of available cases and controls.25 The following variables were considered a priori to be potential confounders or independent competing factors (birth order, birth weight (in studies where data were readily available), ethnicity, maternal age group and education (for maternal analyses) and paternal age group and education (for paternal analyses)). Maternal and paternal education were the only common socio-economic level indicators that were available in all studies, albeit in different formats. Factors that were independently associated with both the exposure and outcome were retained in the final models. The study-specific ORs were combined in a meta-analysis in Stata version 11.2 (StataCorp LP, College Station Texas, USA, 2009), using the random effects model (to acknowledge the between study heterogeneity, such as in terms of study designs, occupational assessment methods, and pesticide use across countries and time26). Summary ORs, 95% CIs, I2 statistics (a measure of the variation across studies that is not due to chance)27 and forest plots were produced. Studies without any cases or controls in the ‘High likelihood of pesticide exposure’ were not included in the meta-analyses.
Finally, we identified additional published papers or other theses that had been included in recent meta-analyses11, 28 (supplemented by information from one of the authors (personal communication, D Wigle April 2013)) of maternal occupational pesticide exposure and the risk of ALL29–34 or AML13, 29, 31 or paternal exposure and risk of ALL12, 30, 32–38 or AML13, 36–38 of studies not being part of CLIC The search strategies of all these meta-analyses can be found in the supplementary material. Using one of these search strategies,10 we searched PubMed (National Library of Medicine, Bethesda, MD) to identify papers of maternal occupational pesticide exposure and the risk of ALL14, 39, or AML39 or paternal exposure of both sub-types40 published January 2009 to 31 October 2013. We extracted the relevant OR from each of these studies to calculate summary ORs (as described above) with the results from the individual CLIC studies, after excluding studies which had an overlap of data with the CLIC studies,12, 14, 30, 38, 41 did not specify which parent was exposed35, 36 or did not provide an overall pesticide exposure variable.34 Another published study37 was excluded as it was a subset of the UK National Registry of Childhood Tumours (NRCT) study.40 One of the CLIC studies, the UKCCS, was also a subset of the NRCT study and was thus excluded from the meta-analyses of the CLIC and other published studies.
Pooled analyses of individual-level data
Unconditional logistic regression (SAS version 9.2, SAS Institute Inc, Cary, NC, USA) was used to estimate pooled OR and 95% CI for occupational pesticide exposures in mothers during pregnancy and for fathers before conception. All models included the child’s age and sex, year of birth group (grouped into five approximately equal time periods), ethnicity (Caucasian, European or White versus the rest) and a variable denoting the study of origin. The following variables were tested to determine whether they were independently associated with both the exposure and outcome: Birth order, birth weight (for the subset of studies where data were readily available), the relevant parent’s age group and education (recoded into three groups: secondary education not completed, completed secondary education, and tertiary education), and study-specific matching variables (by allocating all the other studies the same dummy value for each variable); of these, only maternal or paternal education was retained. As children with Down syndrome have higher rates of leukemia than other children, analyses were repeated, excluding these children.
Results
Data were obtained from 12 studies for 8,835 ALL cases and from 10 studies for 1,357 AML cases (Table 2). There were 15,486 controls from studies with ALL cases and 12,443 from those with AML cases. The same controls were used for ALL and AML cases if the original study included both types of leukemia. Most studies recruited children under the age of 15, except one study that included children up to the age of 10 years (Italy) and one study of AML that included children up to the age of 18 years (US COG-E14). Maternal occupational data were available for 93.2% of ALL cases, 95.9% ALL controls, 97.9% of AML cases, 97.6% of AML controls and paternal occupational data for 92.5%, 91.7 %, 90.6%, and 91.5%, respectively (Table 2). These rates reflect missing occupational data from the original studies, for example, most studies had fewer fathers participating than mothers. The table showing the demographic characteristics of the total sample and the individual studies is provided as Supplementary Table 2.
Table 2.
Key demographic characteristics of participants in the 13 studies in the CLIC pooled analyses of parental occupational pesticide exposure and the risk of leukemia in the offspring
| ALL (12 studies) | AML (10 studies) | |||||||
|---|---|---|---|---|---|---|---|---|
| Case (n= 8835) | Controla (n = 15486) | Case (n= 1357) | Controlb (n= 12443) | |||||
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Boy | 4972 | 56.3 | 8634 | 55.8 | 713 | 52.5 | 6928 | 55.7 |
| Girl | 3863 | 43.7 | 6852 | 44.2 | 644 | 47.5 | 5515 | 44.3 |
| Age (years)c | ||||||||
| 0–1 | 958 | 10.8 | 2272 | 14.7 | 376 | 27.7 | 2000 | 16.1 |
| 2–4 | 4109 | 46.5 | 6156 | 39.8 | 259 | 19.1 | 4610 | 37.0 |
| 5–9 | 2570 | 29.1 | 4507 | 29.1 | 313 | 23.1 | 3526 | 28.3 |
| 10–14 | 1198 | 13.6 | 2551 | 16.5 | 342 | 25.2 | 2231 | 17.9 |
| 15–17 | 0 | 0.0 | 0 | 0.0 | 67 | 4.9 | 76 | 0.6 |
| Year of birth | ||||||||
| 1970–1978 | 294 | 3.3 | 395 | 2.6 | 151 | 11.1 | 289 | 2.3 |
| 1979–1987 | 2555 | 28.9 | 4318 | 27.9 | 426 | 31.4 | 2943 | 23.7 |
| 1988–1996 | 3927 | 44.5 | 7226 | 46.7 | 578 | 42.6 | 6350 | 51.0 |
| 1997–2005 | 1936 | 21.9 | 3394 | 21.9 | 175 | 12.9 | 2710 | 21.8 |
| 2006–2011 | 123 | 1.4 | 153 | 1.0 | 27 | 2.0 | 151 | 1.2 |
| Child has Down Syndrome | ||||||||
| Yes | 103 | 1.2 | 6 | 0.0 | 89 | 6.6 | 4 | 0.0 |
| Maternal education | ||||||||
| Did not finish secondary education | 2327 | 26.3 | 3950 | 25.5 | 322 | 23.7 | 3479 | 28.0 |
| Completed secondary education | 3859 | 43.7 | 6619 | 42.7 | 664 | 48.9 | 5098 | 41.0 |
| Tertiary education | 2588 | 29.3 | 4756 | 30.7 | 358 | 26.4 | 3706 | 29.8 |
| Missing | 61 | 0.7 | 161 | 1.0 | 13 | 1.0 | 160 | 1.3 |
| Paternal education | ||||||||
| Did not finish secondary education | 2420 | 27.4 | 4266 | 27.5 | 349 | 25.7 | 3847 | 30.9 |
| Completed secondary education | 3341 | 37.8 | 5400 | 34.9 | 559 | 41.2 | 4095 | 32.9 |
| Tertiary education | 2579 | 29.2 | 4757 | 30.7 | 348 | 25.6 | 3708 | 29.8 |
| Missing | 495 | 5.6 | 1063 | 6.9 | 101 | 7.4 | 793 | 6.4 |
| Maternal occupational pesticide exposure data during pregnancy available | 8236 | 93.2 | 14850 | 95.9 | 1329 | 97.9 | 12141 | 97.6 |
| Paternal occupational pesticide exposure data around conception available | 8169 | 92.5 | 14201 | 91.7 | 1231 | 90.7 | 11383 | 91.5 |
| Occupational pesticide exposure data available for both parents | 7679 | 86.9 | 13704 | 88.5 | 1210 | 89.2 | 11184 | 89.9 |
Includes controls from all studies with ALL cases (that is, all studies except US, COG-E15).
Includes controls from all studies with AML cases (that is, all studies except Australia, Aus-ALL, Canada, Quebec and US, US, COG-E14)).
Age groups are based on the child’s age at the censoring date. For case, this was the date at diagnosis and for controls, it was the date that the study investigators nominated (either the date of recruitment or the date of the questionnaire return).
Meta-analyses of CLIC studies
Twelve CLIC studies were included in the meta-analysis of parental occupational pesticide exposures and the risk of ALL in the offspring. There were 8,236 cases and 14,850 controls in the meta-analysis of maternal exposure and 8,157 cases and 14,201 controls in that of paternal exposures. Further details about each study are in Supplementary Table 3. The summary OR for maternal exposure and the risk of ALL in the offspring was 1.03 (95% CI 0.77, 1.38) with little evidence of heterogeneity among the ORs (Table 3). The summary OR for paternal occupational exposure and the risk of ALL in the offspring was 1.22 (95% CI 0.94, 1.58) with high heterogeneity (I2 = 68.7%) (Table 3). When the paternal analyses were stratified by the type of occupational data, high heterogeneity was only seen in studies for which coded job titles were used to assign occupational assessment (summary OR 1.28, 95% CI 0.89, 1.85, I2 = 77.5%) and not among studies where pesticide assessment was based on more detailed questions or assessment (summary OR 1.15, 95% CI 0.84, 1.57, I2 = 20.1%) (results not otherwise shown).
Table 3.
Summary ORs from Meta-analyses of parental occupational exposures to pesticides and the risk of leukaemia in the offspring
| Leukemia Type |
Mother during pregnancy | Father around conception | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study N |
Total N Case/control |
Summary OR (95% CI)a, b |
I2 | Maximum percentage difference when individual studies removed in turn |
Study n |
Total N Case/control |
Summary OR (95% CI)a, b |
I2 | Maximum percentage difference when individual studies removed in turn |
|
| ALL | 12 | 8236/14850 | 1.03 (0.77, 1.38) | 11.2 | 8.4 | 12 | 8157/14201 | 1.22 (0.94, 1.58) | 68.7 | 7.8 |
| B Cell | 12 | 6529/14850 | 1.04 (0.78, 1.38) | 0.0 | 7.7 | 12 | 6449/14201 | 1.14 (0.85, 1.54) | 71.4 | 10.1 |
| T Cell | 7c | 526/10726 | 1.66 (0.88, 3.14) | 0.0 | 25.8 | 10c | 784/13681 | 1.86 (1.34, 2.58) | 5.4 | 10.3 |
| AML | 5c | 895/5428 | 2.69 (1.49, 4.86) | 0.0 | 23.9 | 8c | 1184/10863 | 1.12 (0.72, 1.70) | 32.2 | 10.8 |
The random effects model was used to calculate the summary OR
OR comparing Category 1 (High likelihood of pesticide exposure) to Reference Category 4 (No or minimal likelihood of pesticide exposure)
Studies without any cases in Category 1 (High likelihood of pesticide exposure) were not included in the meta-analysis.
When individual studies were omitted in turn from the meta-analyses, the summary estimate changed by less than eight percent (OR scale). The summary estimates were higher for T cell ALL than B cell ALL (Table 3) for both maternal and paternal exposures, but the estimates for T cell ALL were based on smaller numbers of cases.
As only studies with any cases in the ‘High likelihood of pesticide exposure’ category were included in the AML meta-analyses, 895 cases and 5,428 controls from five studies were included for maternal exposures, and 1,184 cases and 10,863 controls from eight studies for paternal exposures. Further details about each study are in Supplementary Table 4. The summary ORs for maternal and paternal occupational pesticide exposures and the risk of AML in the offspring were 2.69 (95% 1.49, 4.86) and 1.12 (95% CI 0.72, 1.70), respectively with little or low heterogeneity among the ORs (Table 3). When individual studies were removed one by one, the summary estimates for maternal exposure changed by up to 26% while those for paternal exposure changed by less than 11%.
Meta-analyses of CLIC studies together with previously published papers
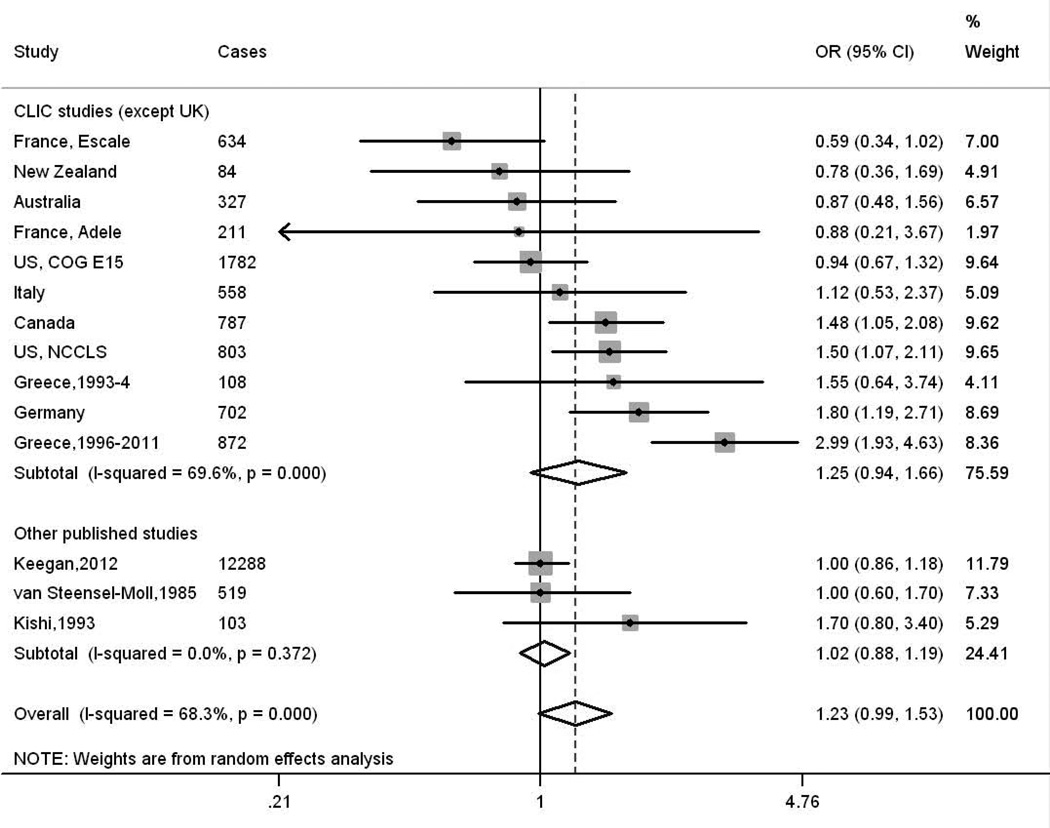
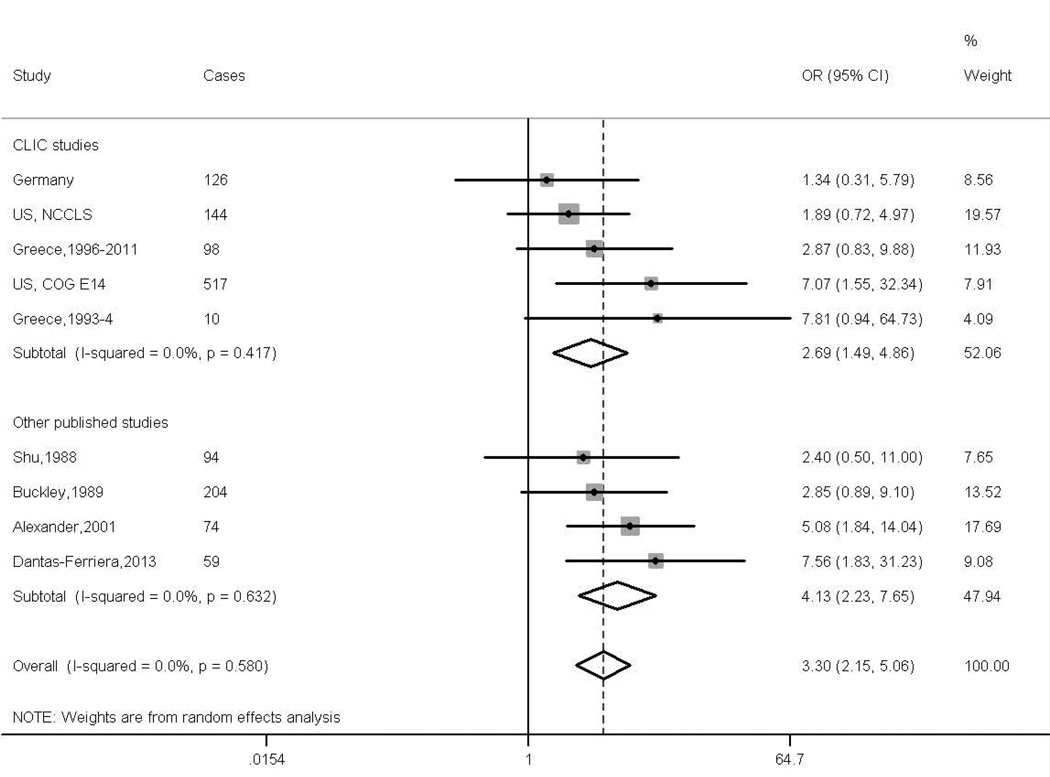
For the combined meta-analyses of the CLIC data and previous published papers of ALL we added estimates from six additional studies for the investigation of maternal exposure29, 31–33, 39, 42 and three for those of paternal exposure32, 33, 40, 40, while those of AML contained estimates from an additional four studies for maternal exposure13, 29, 31, 39 and three for paternal exposure,13, 31, 40 but excluded one of the CLIC studies from the paternal analyses. The resulting summary ORs for maternal exposures during pregnancy and paternal exposures around conception and the risk of ALL in the offspring were 1.35 (95% CI 0.96, 1.89, I2 =43.0%) (Supplementary Figure 1) and 1.23 (95% CI 0.99, 1.53 I2 =68.3%) (Figure 1) respectively. The summary estimates for maternal and paternal exposures respectively and the risk of AML in the offspring were 3.30 (95% CI 2.15, 5.06, I2 =0.0%) (Figure 2) and 1.14 (95% CI 0.88, 1.49 I2 =24.0%) (Supplementary Figure 2).
Figure 1.
Figure 2.
Pooled analyses of individual data from CLIC studies
The analyses for ALL included 12 studies (8,236 case mothers, 14,850 control mothers, 8,169 case fathers and 14,201 control fathers). No association was seen with maternal occupational pesticide exposure during pregnancy and the risk of ALL (OR 1.01, 95% CI 0.78, 1.30) (Table 4). There was no difference in the OR when the analyses were stratified by type of occupational assessment, or immunophenotype (Table 4).
Table 4.
Pooled OR (95% CI) for the association between parental occupational exposures to pesticides and the risk of leukaemia in the offspring: Overall and by subgroups
| Maternal exposures during pregnancy | Paternal exposures around conception | |||||
|---|---|---|---|---|---|---|
| Total N Case/Controls |
% in
High likelihood of exposure category |
ORa,b (95% CI) | Total N Case/Controls |
% in
High likelihood of exposure category |
ORb,c (95% CI) | |
| 1. ALL | ||||||
| Overall | 8236/14850 | 1.3/1.1 | 1.01 (0.78, 1.30) | 8169/14201 | 5.5/4.3 | 1.20 (1.06, 1.38) |
| Age at diagnosis | ||||||
| 0–1 years | 880/2192 | 1.1/0.6 | 1.20 (0.50, 2.88) | 895/2156 | 4.8/3.3 | 1.18 (0.77, 1.79) |
| 2–4 years | 3832/5906 | 0.9/1.0 | 0.74 (0.48, 1.14) | 3842/5666 | 4.9/4.5 | 1.02 (0.83, 1.24) |
| 5 or more years | 3524/6752 | 1.8/1.2 | 1.18 (0.84, 1.67) | 3432/6379 | 6.4/4.3 | 1.38 (1.13, 1.67) |
| Interaction p value = 0.33 | Interaction p value = 0.07 | |||||
| Immunophenotype | ||||||
| B-lineage cases | 6529/14850 | 1.3/1.1 | 1.00 (0.76, 1.31) | 6448/14201 | 5.6/4.3 | 1.19(1.03, 1.37) |
| T-lineage cases | 841/14850 | 1.4/1.1 | 1.07 (0.58, 1.97) | 818/14201 | 6.1/4.3 | 1.42 (1.04, 1.94) |
| Type of occupational assessment | ||||||
| Expert assessmentd | 1755/2676 | 2.1/1.5 | 0.90 (0.57, 1.43) | 1672/2510 | 7.0/4.8 | 1.24 (0.95, 1.63) |
| Assessment based on coded job titlese | 6384/11871 | 1.1/0.9 | 1.06 (0.78, 1.45) | 6413/11407 | 5.0/3.8 | 1.22 (1.05, 1.43) |
| 2. AML | ||||||
| Overall | 1329/12141 | 1.8/0.8 | 1.94 (1.19, 3.18) | 1231/11383 | 4.5/4.0 | 0.91 (0.66,1.24) |
| Age at diagnosis | ||||||
| Less than 5 years | 627/6491 | 1.6/0.7 | 1.92(0.86, 4.28)d | 585/6114 | 3.9/3.8 | 0.84 (0.52, 1.37)e |
| 5 or more years | 702/5650 | 2.0/1.0 | 1.96 (1.02, 3.77)d | 646/5269 | 5.0/4.2 | 0.97 (0.64, 1.49)e |
| Interaction p value =0.52 | Interaction p value = 0.72 | |||||
| Type of occupational assessment | ||||||
| Assessment not based on coded job titlesf | 569/1929 | 1.9/0.5 | 3.83 (1.29, 11.34) | 511/1776 | 5.3/5.4 | 1.05 (0.62, 1.77) |
| Assessment based on coded job titlesg | 760/10212 | 1.7/0.9 | 1.63 (0.90, 2.98) | 1201/10409 | 4.3/4.2 | 0.85 (0.62, 1.18) |
Adjusted for age, sex, birth year group, ethnicity, study and maternal education
OR comparing Category 1 (High likelihood of pesticide exposure) to Reference Category 4 (No or minimal likelihood of pesticide exposure)
Adjusted for age, sex, birth year group, ethnicity, study and paternal education
Australia (Aus-ALL), Canada (Quebec), Italy(SETIL) (Agricultural pesticides only)
France (ADELE & ESCALE), Greece (NARECHEM 1993–1994 & 1996–2011), Germany (GCCR), UK (UKCCS), US (COG -E15). See Table 1 for details of the Occupational coding system.
Italy(SETIL) (Agricultural pesticides only), New Zealand (NZCCS), US (COG (COG-E14).
France (ADELE & ESCALE), Greece (NARECHEM 1993–1994 & 1996–2011), Germany (GCCR), UK (UKCSS). See Table 1 for details of the Occupational coding system.
The OR for paternal occupational pesticide exposure and the risk of ALL in the offspring was 1.20 (95% CI 1.06, 1.38) (Table 4). The risk of ALL related to exposure appeared to be stronger in children diagnosed at five years or older than for those diagnosed earlier (p value for the interaction 0.07). When the analyses were stratified by both immunophenotype and age at diagnosis, the ORs for B cell and T cell ALL were 1.04 (95% CI 0.85, 1.25) and 1.16 (95% CI 0.65, 2.09) in children aged under five years, and 1.39 (95% CI 1.12, 1.71) and 1.55 (95% CI 1.07, 2.25), respectively in children aged five years or more (results not shown). There was little difference in the OR when the analyses were stratified by type of occupational assessment (Table 4).
The analyses for AML included 10 studies (1,329 case mothers, 12,141 control mothers, 1,231 case fathers and 11,383 control fathers). The OR for maternal occupational exposure during pregnancy was 1.94 (95% 1.19, 3.18) (Table 4). While there was little variation by age at diagnosis, the OR varied by whether the exposure assessment was based on job codes or another method. One study (US, COG-E14) contributed nearly 50% of the cases for this analysis; when this study was excluded, the resulting pooled OR was 1.51 (95% CI 0.83, 2.74). No association was seen with paternal occupational exposure around the time of conception (OR 0.91, 95% CI 0.66, 1.24), or when these analyses were stratified by type of occupational assessment or age at diagnosis (Table 4).
When all the analyses for ALL and AML were rerun excluding children with Down syndrome (103 ALL cases and six controls, 89 AML cases and four controls), there was little change in the results and there was also little difference when they were stratified by the birth year group (data not shown). The estimates for paternal exposure and for maternal exposure changed little when adjusted for the exposure level of the other parent. Few cases (0.9 % of ALL cases and 0.7% of AML cases) and 0.6% of controls had both parents in the ‘High likelihood of pesticide exposure’ group. The ORs for ALL and AML in the offspring with both parents being exposed compared to both parents being unexposed were 1.29 (95% CI 0.90, 1.85) and 1.43 (95% CI 0.67, 3.08), respectively (results not tabulated).
Discussion
Our findings suggest that it may be important to investigate occupational exposure to pesticides by sub-type of leukemia as the findings for ALL were different from those for AML for both maternal and paternal exposure. For maternal occupational exposures to pesticides during pregnancy, we found a significantly increased risk of AML using pooled data from 10 international case-control studies in the offspring, although the findings lacked precision when the largest study was excluded, while no increased risk of ALL was found using data from 12 studies. For paternal occupational pesticide exposures around the time of conception, we found about a 20% increased risk of childhood ALL in the analyses of pooled data from 12 international case-control studies, but no association with AML using data from 10 studies.
Our observations for maternal exposure and the risk of AML are consistent with previously published studies13, 29, 31, 39 with additional support provided by studies implicated the use of pesticides by the mother in the home environment during pregnancy as a risk factor for AML in the offspring.13, 43
The finding for maternal exposure and ALL is at odds with the elevated risk reported in two previous meta-analyses.10, 11 and a recent Brazilian study.39 The disparity with other previous literature appears to be due mainly to the findings of four studies, which all had <200 cases and reported ORs for exposures during pregnancy of greater than 2.5.29, 31, 33, 39 One of these was conducted in China31 and another in Japan33 The definition of pesticide exposure was based on maternal recall of pesticide exposure in the Chinese study,31 while in the hospital–based Japanese study,33 it was defined as working in an agricultural industry. The other two studies29, 39 were restricted to ALL diagnosed either before 18 months29 or two years of age.39 The first of these was an international study obtaining information about maternal occupational exposures during an interview, but may have included non-occupational exposures in some countries.29 The other was a hospital-based case-control Brazilian study in which mothers were asked about exposure to agricultural pesticides.39 On the other hand, all the CLIC studies were conducted in predominantly Caucasian populations and included children under 15 years and the exposure was restricted to occupational exposures, thus the difference in the findings could be explained by a mixture of factors; differing distributions of cytogenetic sub-types between the populations,44 or age groups45 as susceptibility to pesticides could be restricted to certain cytogenetic sub-types, or related to the definitions of exposure. The findings could also reflect differences in the types of pesticides used and the protection measures used during pesticide application. However, we have insufficient information to speculate on these issues.
It is biologically plausible that maternal occupational pesticide exposure could increase the risk of either sub-type of leukemia in the offspring as there is evidence that some pesticides cross the placental blood barrier, thus resulting in fetal exposure.7 However, to the best of our knowledge, while there have been several case reports of translocations associated with AML being found in either in cord blood or soon after delivery, following maternal insecticide exposure during pregnancy,8, 9 there are no such evidence for translocations associated with ALL.
For paternal exposures, the increased risk is consistent with two32, 33 of three previous studies,34, 35, 42 as well as those of the participating Canadian study, 12 but different from the UK NRCT study42 which reported an OR of 1.00 (95% CI 0.86, 1.33)
Our findings for paternal exposure and the risk of ALL in the offspring raise some interesting issues as the association appears stronger in older children and those with T cell ALL. T cell ALL generally occurs between the ages of six to eight years of age while precursor B cell (the vast majority of B cell lineage cases) is mainly seen in children aged less than five years. Despite this, there is still evidence that T cell ALL can originate in the prenatal period,3 and thus damage to paternal germ cells could play a role, albeit with a long latency.
However, our findings also suggested the association between paternal pesticide exposure and B cell ALL was more pronounced in older children. Another possible explanation for our findings is that paternal occupational pesticide exposure around conception is a proxy measure for either paternal exposure during the child’s early years or for exposure of the child by living on or close to a farm, but we do not have the data to investigate this theory. Exposed parents can track pesticides back into the home such as on shoes and on hands,46 or homes and play areas can be contaminated by air drift from pesticide spraying,47 thus higher levels of pesticide residues have been detected on children’s hands and in house dust in farmhouses than others.46 In one of the CLIC studies, US NCCLS, children who lived in homes where chlorthal (a potentially carcinogenic agricultural herbicide) was detected in carpet dust had an increased risk of ALL.48
Consistent with previous studies,13, 40 we found no association between paternal occupational pesticide exposure and the risk of AML in the offspring in the pooled analyses.
The major strength of our investigation was the large sample size, which allowed us to investigate exposure by sub-type of leukemia in more detail than in previous individual studies; and access to the original data allowing better harmonization of exposure variables and their categorization as compared to literature-based meta-analyses.
However, there were also major limitations with respect to our investigations. Notably, the occupational exposure data were available in many forms. In order to harmonize the data, a crude measure of exposure was developed. For most studies, we had only job title information coded in different formats. It is unlikely that all people with the same job code would have the same level of exposure, or that pesticide exposure levels would have been similar across all the study populations (North America, Europe and Australasia) and over time (30 years). The size of farms, the crops grown and the animals raised would have varied across studies and the types and extent of pesticide handled may have varied by gender. The proportion of controls classed as exposed to pesticides varied by study, which may reflect true differences in exposure to pesticides, for example between countries, or weaknesses in the exposure measure. Despite these limitations, the estimates obtained for ALL using studies that had used coded job titles were similar to the three studies (Australia, Canada and Italy) that used expert occupational assessment. No such comparison was possible for AML as only the Italian study included cases of AML. This study only assigned exposure to agricultural pesticides, but this was considered an adequate proxy for any occupational pesticide exposure.49 In addition, most of the job titles in our ‘High likelihood of pesticide exposure’ category were agriculture or farm-related.
Our study has the same limitation as most previous research of this topic, that is, the inability to define exposure by type of pesticide. By combining all pesticide exposure into a single measure of general pesticide exposure, we may have diluted the effect of different pesticides and introduced non-differential misclassification. If we could have defined pesticides more specifically, we may still not have not been able to address the issue of exposure to multiple types of pesticides as exposed individuals commonly use more than one type.12
We developed the JEM using data from Australia and Canada, but applied it to US and European studies, which raises questions about the validity of the JEM in other settings. However, to the best of our knowledge, our choices of ‘High likelihood of exposure’ job codes are in line with other published literature.
In all of the studies, data were collected using structured questionnaires that focused on jobs instead of exposures in attempts to minimize recall bias. Nonetheless, this would not remove the potential for cases to think more deeply about jobs held.
Although our investigation focused on the exposure time-windows of around conception or pregnancy, occupational exposure is likely to have extended over a wider time period. Among mothers, there was a high correlation (Spearman P 0.884) with maternal exposures around conception and during pregnancy with over 98.5 % of women having the same exposure code in studies with both time periods. This also means that the risks we observed may also apply to a broader period of time, such as before conception or during the child’s early years. Most occupational pesticide exposures occur in farming or agriculture,49 and the children of exposed parents may have been exposed around the home to agricultural pesticides, such as through spray drift.47 Another limitation was that there were few parents, especially women in the ‘High likelihood of pesticide exposure’ category, which made some estimates imprecise, despite the sample size.
In conclusion, we found an increased risk of AML in the offspring following maternal occupational pesticide exposure during pregnancy. We also found that the risk of ALL increased slightly with paternal occupational pesticide exposure around conception. More information is needed by pesticide type and about the use of protective measures during application before any recommendations are made in relation to pesticide use in the workforce and the risk of childhood leukemia.
Supplementary Material
Novelty and Impact statement.
Parental occupational pesticide exposure before birth may be a risk factor for childhood leukemia. Using pooled individual level occupational pesticide exposure data from 13 case- control studies (over 8,000 acute lymphoblastic leukemia (ALL) cases and 14,000 controls, and 1,200 acute myeloid leukemia (AML) cases and 12,000 controls), we found an increased risk of AML with maternal exposure during pregnancy and a slightly increased risk of ALL with paternal exposure around conception.
Acknowledgements
We would like to thank our dear colleague and friend, Patricia Buffler, who passed away before the submission of this manuscript. She was a founding member and Chair of CLIC as well as the driving force behind the NCCLS. She provided unconditional support to finding the causes of childhood leukemia, and her scientific leadership and guiding forces within CLIC will be remembered.
The Aus-ALL consortium conducted the study and the Telethon Institute for Child Health Research (TICHR), University of Western Australia, was the coordinating centre. Bruce Armstrong (Sydney School of Public Health), Elizabeth Milne (TICHR), Frank van Bockxmeer (Royal Perth Hospital), Michelle Haber (Children’s Cancer Institute Australia), Rodney Scott (University of Newcastle), John Attia (University of Newcastle), Murray Norris (Children’s Cancer Institute Australia), Carol Bower (TICHR), Nicholas de Klerk (TICHR), Lin Fritschi (WA Institute for Medical Research, WAIMR), Ursula Kees (TICHR), Margaret Miller (Edith Cowan University), Judith Thompson (WA Cancer Registry) were the research investigators, Helen Bailey (TICHR) was the project coordinator and Alison Reid (WAIMR) performed the occupational analyses. The clinical Investigators were: Frank Alvaro (John Hunter Hospital, Newcastle); Catherine Cole (Princess Margaret Hospital for Children, Perth); Luciano Dalla Pozza (Children’s Hospital at Westmead, Sydney); John Daubenton (Royal Hobart Hospital, Hobart); Peter Downie (Monash Medical Centre, Melbourne); Liane Lockwood, (Royal Children’s Hospital, Brisbane); Maria Kirby (Women’s and Children’s Hospital, Adelaide); Glenn Marshall (Sydney Children’s Hospital, Sydney); Elizabeth Smibert (Royal Children’s Hospital, Melbourne); Ram Suppiah, (previously Mater Children’s Hospital, Brisbane).
GCCR: The German study was conducted by the nationwide German Childhood Cancer Registry (GCCR) at the Institute of Medical Biostatistics, Epidemiology and Informatics at the Johannes Gutenberg-University Mainz; researchers involved were Drs Jörg Michaelis (head), Peter Kaatsch, Uwe Kaletsch, Rolf Meinert, Anke Miesner and Joachim Schüz.
NARECHEM Greek Pediatric Hematology Oncology Clinicians: Margarita Baka MD: Department of Pediatric Hematology –Oncology, “Pan.&Agl. Kyriakou” Children’s Hospital, Athens, Greece, Thivon & Levadeias, Goudi; Maria Moschovi MD: Hematology-Oncology Unit, First Department of Pediatrics, Athens University Medical School, “Aghia Sophia” General Children's Hospital, Athens, Greece, Thivon & Papadiamantopoulou, Goudi, 11527 Athens, Greece; Sophia Polychronopoulou MD: Department of Pediatric Hematology-Oncology, “Aghia Sophia” General Children's Hospital, Athens, Greece, Thivon & Papadiamantopoulou, Goudi, 11527 Athens, Greece; Emmanuel Hatzipantelis MD, PhD: Pediatric Hematology Oncology Unit, 2nd Pediatric Department of Aristotle University, AHEPA General Hospital, Thessaloniki, Greece, 1 St. Kyriakidi, 54636 Thessaloniki, Greece; Ioanna Fragandrea MD: Pediatric Oncology Department, Hippokration Hospital, Thessaloniki, Greece; Eftychia Stiakaki MD: Department of Pediatric Hematology-Oncology, University Hospital of Heraklion, Heraklion, Greece; Nick Dessypris, MSc, PhD and Evanthia Bouka, MPH: Department of Hygiene, Epidemiology and Medical Statistics, Athens University Medical School, 11527 Athens, Greece; Ioannis Matsoukis MD: Department of Hygiene, Epidemiology and Medical Statistics, Athens University Medical School, 11527 Athens, Greece.
The SETIL (Italian Multicentric Epidemiological Study on Risk Factors of Childhood Leukaemia, Non Hodgkin Lymphoma and Neuroblastoma) Working Group:
Corrado Magnani and Alessandra Ranucci (Cancer Epidemiology Unit, CPO Piedmont Novara); Lucia Miligi, Alessandra Benvenuti, Patrizia Legittimo and Angela Veraldi (Occupational and Environmental Unit ,ISPO, Firenze); Antonio Acquaviva (AOU Siena); Maurizio Aricò, Alma Lippi and Gabriella Bernini (AOU Meyer, Firenze); Giorgio Assennato (ARPA, Bari); Stefania Varotto and Paola Zambon (Università di Padova); Pierfranco Biddau and Roberto Targhetta (Ospedale Microcitemico, Cagliari); Luigi Bisanti and Giuseppe Sampietro (ASL di Milano); Francesco Bochicchio, Susanna Lagorio, Cristina Nuccetelli, Alessandro Polichetti and Serena Risica, (ISS, Roma); Santina Cannizzaro and Lorenzo Gafà (LILT, Ragusa); Egidio Celentano (ARSan, Napoli); Pierluigi Cocco (Università di Cagliari); Marina Cuttini (IRCCS Burlo Garofolo, Trieste); Francesco Forastiere,Ursula Kirchmayer and Paola Michelozzi (Dipartimento Epidemiologia Regione Lazio, Roma); Erni Guarino (INT Napoli); Riccardo Haupt (Istituto Giannina Gaslini, Genova); Franco Locatelli (Università di Pavia and AO Bambin Gesù, Roma); Lia Lidia Luzzatto (ASL 1 , Torino); Giuseppe Masera (Università Milano Bicocca, Monza); Pia Massaglia (Università di Torino); Stefano Mattioli and Andrea Pession (Università di Bologna); Domenico Franco Merlo and Vittorio Bocchini (IST, Genova); Liliana Minelli and Manuela Chiavarini (Università degli Studi di Perugia);Margherita Nardi (AOU Pisa); Paola Mosciatti and Franco Pannelli (Università di Camerino);Vincenzo Poggi (AORN Santobono – Pausilipon, Napoli);Alessandro Pulsoni (Sapienza University, Roma); Carmelo Rizzari (AO San Gerardo, Monza); Roberto Rondelli (Policlinico S.Orsola, Bologna); Gino Schilirò (Università di Catania); Alberto Salvan (IASI-CNR, Roma); Maria Valeria Torregrossa and Rosaria Maria Valenti, (Università degli Studi di Palermo);Alessandra Greco, Gian Luca DeSalvo and Daniele Monetti (IOV-IRCCS, Padova); Claudia Galassi (San Giovanni Battista Hospital, Torino); Veronica Casotto (IRCCS Burlo Garofolo, Trieste); Gigliola de Nichilo (ASL BT, SPRESAL Barletta); Alberto Cappelli, (Accademia dei Georgofili, Florence).
The New Zealand Childhood Cancer Study was co-ordinated at the University of Otago, where the study team included JD Dockerty, GP Herbison (who helped prepare data for this pooled analysis), DCG Skegg and JM Elwood. The names of the interviewers, secretaries, research assistants, clinicians, pathologists and cancer registry staff who contributed are listed in earlier publications from the NZ study.
The UKCCS was conducted by 12 teams of investigators (ten clinical and epidemiological and two biological) based in university departments, research institutes, and the National Health Service in Scotland. Its work is coordinated by a management committee. Further information can be found on the web-site www.ukccs.org.
COG: The E14 and E15 cohorts of the Children’s Oncology Group was identified by CCG (Children’s Cancer Group) principle and affiliate member institutions. Further information can be found on the web-site: http://www.curesearch.org/.
The NCCLS thanks the families for their participation and the clinical investigators at the following collaborating hospitals for help in recruiting patients: University of California Davis Medical Center (Dr. J. Ducore), University of California San Francisco (Drs. M. Loh and K. Matthay), Children's Hospital of Central California (Dr. V. Crouse), Lucile Packard Children's Hospital (Dr. G. Dahl), Children's Hospital Oakland (Dr. J. Feusner), Kaiser Permanente Roseville (former Sacramento; Drs. K. Jolly and V. Kiley), Kaiser Permanente Santa Clara (Drs. C. Russo, A. Wong, and D. Taggar), Kaiser Permanente San Francisco (Dr. K. Leung), and Kaiser Permanente Oakland (Drs. D. Kronish and S. Month). Finally, the NCCLS thanks the entire study staff and former University of California, Berkeley Survey Research Center for their effort and dedication.
The French authors would like to thank all of the Société Française de lutte contre les Cancers de l’Enfant et de l’Adolescent (SFCE) principal investigators: André Baruchel (Hôpital Saint-Louis/Hôpital Robert Debré, Paris), Claire Berger (Centre Hospitalier Universitaire, Saint-Etienne), Christophe Bergeron (Centre Léon Bérard, Lyon), Jean-Louis Bernard (Hôpital La Timone, Marseille), Yves Bertrand (Hôpital Debrousse, Lyon), Pierre Bordigoni (Centre Hospitalier Universitaire, Nancy), Patrick Boutard (Centre Hospitalier Régional Universitaire, Caen), Gérard Couillault (Hôpital d’Enfants, Dijon), Christophe Piguet (Centre Hospitalier Régional Universitaire, Limoges), Anne-Sophie Defachelles (Centre Oscar Lambret, Lille), François Demeocq (Hôpital Hôtel-Dieu, Clermont-Ferrand), Alain Fischer (Hôpital des Enfants Malades, Paris), Virginie Gandemer (Centre Hospitalier Universitaire – Hôpital Sud, Rennes), Dominique Valteau-Couanet (Institut Gustave Roussy, Villejuif), Jean-Pierre Lamagnere (Centre Gatien de Clocheville, Tours), Françoise Lapierre (Centre Hospitalier Universitaire Jean Bernard, Poitiers), Guy Leverger (Hôpital Armand-Trousseau, Paris), Patrick Lutz (Hôpital de Hautepierre, Strasbourg), Geneviève Margueritte (Hôpital Arnaud de Villeneuve, Montpellier), Françoise Mechinaud (Hôpital Mère et Enfants, Nantes), Gérard Michel (Hôpital La Timone, Marseille), Frédéric Millot (Centre Hospitalier Universitaire Jean Bernard, Poitiers), Martine Münzer (American Memorial Hospital, Reims), Brigitte Nelken (Hôpital Jeanne de Flandre, Lille), Hélène Pacquement (Institut Curie, Paris), Brigitte Pautard (Centre Hospitalier Universitaire, Amiens), Stéphane Ducassou (Hôpital Pellegrin Tripode, Bordeaux), Alain Pierre-Kahn (Hôpital Enfants Malades, Paris), Emmanuel Plouvier (Centre Hospitalier Régional, Besançon), Xavier Rialland (Centre Hospitalier Universitaire, Angers), Alain Robert (Hôpital des Enfants, Toulouse), Hervé Rubie (Hôpital des Enfants, Toulouse), Stéphanie Haouy (Hôpital Arnaud de Villeneuve, Montpellier), Christine Soler (Fondation Lenval, Nice), and Jean-Pierre Vannier (Hôpital Charles Nicolle, Rouen).
The Canada, Québec Study was conducted in the province over a twenty year period in all university-affiliated pediatric centers hospitals designated to diagnose and treat pediatric cancers, under the direction of Claire Infante-Rivard. Main support collaborators were Alexandre Cusson, Marcelle Petitclerc and Denyse Hamer. We thank all families for their generous participation
Funding
The work reported in this paper by Helen Bailey was undertaken during the tenure of a Postdoctoral Fellowship from the International Agency for Research on Cancer, partially supported by the European Commission FP7 Marie Curie Actions, - People- Co-funding of regional, national and international programmes (COFUND). The CLIC administration, annual meetings, and pooled analyses are partially supported by the National Cancer Institute, NCI, USA (grant R03CA132172), National Institute of Environmental Health Sciences, NIEHS, USA (grants P01 ES018172 and R13 ES021145-01), the Environmental Protection Agency, EPA, USEPA, USA (grant RD83451101), and the Children with Cancer, CwC, UK (Award No. 2010/097).
Aus-ALL was supported by the Australian National Health and Medical Research Council (Grant ID 254539).
The Canadian study was funded by The National Cancer Institute of Canada; Grant numbers: #014113, #010735-CERN #RFA0405; The Medical Research Council of Canada; Grant number: MOP 37951; The Fonds de la recherche en santé du Québec; Grant number: #981141; The Bureau of Chronic Disease Epidemiology, Canada; Health and Welfare Canada; The Leukemia Research Fund of Canada; and the National Health and Research Development Program, Ottawa.
ADELE Grant sponsors: INSERM, the French Ministère de l’Environnement, the Association pour la Recherche contre le Cancer, the Fondation de France, the Fondation Jeanne Liot, the Fondation Weisbrem-Berenson, the Ligue Contre le Cancer du Val de Marne, the Ligue Nationale Contre le Cancer.
ESCALE Grant sponsors: INSERM, the Fondation de France, the Association pour la Recherche sur le Cancer (ARC), the Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS), the AgenceFrançaise de Sécurité Sanitaire de l’Environnement et du Travail (AFSSET), the association Cent pour sang la vie, the Institut National du Cancer (INCa), the Agence Nationale de la Recherche (ANR), the Cancéropôle Ile-de-France;
The German study (GCCR) was supported by a grant from the Federal Ministry of the Environment, Nuclear Safety and Nature Preservation.
NARECHEM, is supported in part by the National and Kapodistrian University, Athens, Greece.
The SETIL study was financially supported by research grants received by AIRC (Italian Association on Research on Cancer), MIUR (Ministry for Instruction, University and Research), Ministry of Health, Ministry of Labour, Piedmont Region.
The New Zealand Childhood Cancer Study was funded by the Health Research Council of NZ, the NZ Lottery Grants Board, the Otago Medical School (Faculty Bequest Funds), the Cancer Society of NZ, the Otago Medical Research Foundation, and the A.B. de Lautour Charitable Trust.
The Northern California Childhood Leukemia Study (NCCLS) is supported by the National Institutes of Health (NIH), USA (grants P01 ES018172, R01 ES09137, and P42-ES04705), Environmental Protection Agency (USEPA), USA (grant RD83451101), and the CHILDREN with CANCER (CwC), UK (former Children with Leukaemia) for data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, USEPA, or the CwC.
The United Kingdom Childhood Cancer Study (UKCCS) is sponsored and administered by Leukaemia and Lymphoma Research. The researchers are independent from the funders.
COG: The E14 and E15 cohorts of the Children’s Oncology Group were funded by National Institutes of Health (NIH), USA (Grants R01CA049450 (E14) and R01CA048051 (E15)) and The Children’s Cancer Research Fund, Minneapolis, MN
Abbreviations
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- Aus-ALL
Australian Study of Causes of Acute Lymphoblastic Leukaemia in Children
- CI
Confidence interval
- CLIC
Childhood Leukemia International Consortium
- COG
Childhood Oncology Group (Children’s Cancer Group)
- ESCALE
Epidemiological Study on childhood Cancer and Leukemia
- GCCR
German Childhood Cancer Registry
- ISCO
International Standard Classification for Occupation
- JEM
Job Exposure Matrix
- NARECHEM
Nationwide Registration for Childhood Haemotological Malignancies
- NCCLS
Northern California Childhood Leukemia Study (USA)
- NEC
Not else classified
- NZCCS
New Zealand Childhood Cancer Study
- OR
Odds ratio
- RDD
random digit dialling
- UKCCS
United Kingdom Childhood Cancer Study
Footnotes
Authorship: All authors are principal investigators, co-investigators or designated collaborators of participating CLIC studies
Reference List
- 1.McNally RJ, Parker L. Environmental factors and childhood acute leukemias and lymphomas. Leuk Lymphoma. 2006;47(4):583–598. doi: 10.1080/10428190500420973. [DOI] [PubMed] [Google Scholar]
- 2.Gruhn B, Taub JW, Ge Y, Beck JF, Zell R, Hafer R, Hermann FH, Debatin KM, Steinbach D. Prenatal origin of childhood acute lymphoblastic leukemia, association with birth weight and hyperdiploidy. Leukemia. 2008;22(9):1692–1697. doi: 10.1038/leu.2008.152. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi-Ishimae M, Eguchi M, Kempski H, Greaves M. NOTCH1 mutation can be an early, prenatal genetic event in T-ALL. Blood. 2008;111(1):376–378. doi: 10.1182/blood-2007-02-074690. [DOI] [PubMed] [Google Scholar]
- 4.McHale CM, Wiemels JL, Zhang L, Ma X, Buffler PA, Feusner J, Matthay K, Dahl G, Smith MT. Prenatal origin of childhood acute myeloid leukemias harboring chromosomal rearrangements t(15;17) and inv(16) Blood. 2003 Jun 1;101(11):4640–4641. doi: 10.1182/blood-2003-01-0313. [DOI] [PubMed] [Google Scholar]
- 5.Metayer C, Milne E, Clavel J, Infante-Rivard C, Petridou E, Taylor M, Schuz J, Spector LG, Dockerty JD, Magnani C, Pombo-de-Oliveira MS, Sinnett D, Murphy M, Roman E, Monge P, Ezzat S, Mueller BA, Scheurer ME, Armstrong BK, Birch J, Kaatsch P, Koifman S, Lightfoot T, Bhatti P, Bondy ML, Rudant J, O'Neill K, Miligi L, Dessypris N, Kang AY, Buffler PA. The Childhood Leukemia International Consortium. Cancer Epidemiol. 2013 Feb 8; doi: 10.1016/j.canep.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer. Occupational exposures in insecticide application, and some pesticides. [AccessedSeptember 9, 2009];IARC Monographs in the evaluation of carcinogenic risks in humans. 1991 53 53Available at: URL: http://monographs.iarc.fr/ENG/Monographs/vol53/index.php. [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrea EM, Bielawski DM, Posecion NC, Corrion M, Villanueva-Uy E, Bernardo RC, Jin Y, Janisse JJ, Ager JW. Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environ Res. 2009;109(1):116–122. doi: 10.1016/j.envres.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borkhardt A, Wilda M, Fuchs U, Gortner L, Reiss I. Congenital leukaemia after heavy abuse of permethrin during pregnancy. Arch Dis Child Fetal Neonatal Ed. 2003 Sep;88(5):F436–F437. doi: 10.1136/fn.88.5.F436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaFiura KM, Bielawski DM, Posecion NC, Ostrea EM, Matherly LH, Taub JW, Ge Y. Association between prenatal pesticide exposures and the generation of leukemia-associated t(8;21) Pediatr Blood Cancer. 2007;49(5):624–628. doi: 10.1002/pbc.21283. [DOI] [PubMed] [Google Scholar]
- 10.Van Maele-Fabry G, Lantin A, Hoet P, Lison D. Childhood leukaemia and parental occupational exposure to pesticides: a systematic review and meta-analysis. Cancer Causes Control. 2010;21(6):787–809. doi: 10.1007/s10552-010-9516-7. [DOI] [PubMed] [Google Scholar]
- 11.Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117(10):1505–1513. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Infante-Rivard C, Sinnett D. Preconceptional paternal exposure to pesticides and increased risk of childhood leukaemia. Lancet. 1999;354(9192):1819. doi: 10.1016/S0140-6736(05)70586-9. [DOI] [PubMed] [Google Scholar]
- 13.Buckley JD, Robison LL, Swotinsky R, Garabrant DH, LeBeau M, Manchester P, Nesbit ME, Odom L, Peters JM, Woods WG. Occupational exposures of parents of children with acute nonlymphocytic leukemia: a report from the Childrens Cancer Study Group. Cancer Res. 1989 Jul 15;49(14):4030–4037. [PubMed] [Google Scholar]
- 14.Glass DC, Reid A, Bailey HD, Milne E, Fritschi L. The Australian Study of Causes of Acute Lymphoblastic Leukaemia in Children Consortium. Risk of childhood acute lymphoblastic leukaemia following parental occupational exposure to pesticides. Occup Environ Med. 2012;69(11):846–849. doi: 10.1136/oemed-2011-100250. [DOI] [PubMed] [Google Scholar]
- 15.Infante-Rivard C, Siemiatycki J, Lakhani R, Nadon L. Maternal exposure to occupational solvents and childhood leukemia. Environ Health Perspect. 2005;113(6):787–792. doi: 10.1289/ehp.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siemiatycki J, Day NE, Fabry J, Cooper JA. Discovering carcinogens in the occupational environment: a novel epidemiologic approach. J Natl Cancer Inst. 1981;66(2):217–225. [PubMed] [Google Scholar]
- 17.International Labour Office. [AccessedMay 18, 2012];ISCO 08 International Standard Classification of Occupation. Volume 1 http://www ilo org/wcmsp5/groups/public/---dgreports/---dcomm/---publ/documents/publication/wcms_172572 pdf2012. [Google Scholar]
- 18.Department of Manpower and Immigration. Canadian Classification and Dictionary of Occupations 1971, Volume 1: Classifications and definitions. Ottawa, Canada: Information Canada; 1971. [Google Scholar]
- 19.International Labour Office. [AccessedMay 17, 2012];Correspondence table ISCO-08 to ISCO-88. http://www ilo org/public/english/bureau/stat/isco/isco08/index htm 2013. [Google Scholar]
- 20.International Labour Office. ISCO-88 International Standard Classification of Occupations. Geneva: International Labour Office; 1990. [Google Scholar]
- 21.Federal Statistical Office Germany. [AccessedOctober 9, 2012];Klassifikation de Berufe- Kld-92-mit der Signierung für den Mikrozenus in Verbindung mit dem Umsteigerschüssel für die Internationale Standardklassifikation der berufe zur Anwendung in der Europäischen Gemeinschaft - ISCO COM. https://www destatisde/DE/Methoden/DemografischeRegionaleStandards/Umsteigerschluessel_KldB92_ISCO88COM xls?__blob=publicationFile 1992;Available at: URL: https://www.destatis.de/DE/Methoden/DemografischeRegionaleStandards/Umsteigerschluessel_KldB92_ISCO88COM.xls?__blob=publicationFile. [Google Scholar]
- 22.Classifications and Harmonisation Unit Office for National Statistics (UK) OOSS User Guide 1990:01.2 Mapping of Standard Occupational Classification 1990 (SOC 1990) to International Standard Classification Of Occupations European Community version (ISCO-88 Com) Classifications and Harmonisation Unit,Office for National Statistics (UK) 1990
- 23.The National Crosswalk Service Center (NCSC) [AccessedNovember 19, 2012];2012 http://www xwalkcenter org/ Available at: URL: http://www.xwalkcenter.org/. [Google Scholar]
- 24.Miligi L, Benvenuti A, Mattioli S, Salvan A, Tozzi GA, Ranucci A, Legittimo P, Rondelli R, Bisanti L, Zambon P, Cannizzaro S, Kirchmayer U, Cocco P, Celentano E, Assennato G, Merlo DF, Mosciatti P, Minelli L, Cuttini M, Torregrossa V, Lagorio S, Haupt R, Risica S, Polichetti A, Magnani C. Risk of childhood leukaemia and non-Hodgkin's lymphoma after parental occupational exposure to solvents and other agents: the SETIL Study. Occup Environ Med. 2013 Jun 1;70(9):648–655. doi: 10.1136/oemed-2012-100951. [DOI] [PubMed] [Google Scholar]
- 25.Breslow NE, Day NE. International Agency for Research on Cancer ed. Statistical Methods in Cancer Research, Volume I - The analysis of case-control studies. IARC Scientific Publications No. 32 ed. Lyon: International Agency for Research on Cancer; 1980. Conditional Logistic Regression for Matched Sets. [PubMed] [Google Scholar]
- 26.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. Br Med J. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Maele-Fabry G, Lantin AC, Hoet P, Lison D. Residential exposure to pesticides and childhood leukaemia: A systematic review and meta-analysis. Environment International. 2011;37(1):280–291. doi: 10.1016/j.envint.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Alexander FE, Patheal SL, Biondi A, Brandalise S, Cabrera ME, Chan LC, Chen Z, Cimino G, Cordoba JC, Gu LJ, Hussein H, Ishii E, Kamel AM, Labra S, Magalhaes IQ, Mizutani S, Petridou E, de Oliveira MP, Yuen P, Wiemels JL, Greaves MF. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61(6):2542–2546. [PubMed] [Google Scholar]
- 30.McKinney PA, Fear NT, Stockton D. Parental occupation at periconception: findings from the United Kingdom Childhood Cancer Study. Occup Environ Med. 2003;60(12):901–909. doi: 10.1136/oem.60.12.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shu XO, Gao YT, Brinton LA, Linet MS, Tu JT, Zheng W, Fraumeni JF., Jr A population-based case-control study of childhood leukemia in Shanghai. Cancer. 1988;62(3):635–644. doi: 10.1002/1097-0142(19880801)62:3<635::aid-cncr2820620332>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 32.van Steensel-Moll HA, Valkenburg HA, van Zanen GE. Childhood leukemia and parental occupation. A register-based case-control study. Am J Epidemiol. 1985;121(2):216–224. doi: 10.1093/oxfordjournals.aje.a113992. [DOI] [PubMed] [Google Scholar]
- 33.Kishi R, Katakura Y, Yuasa J, Miyake H. Association of parents' occupational exposure to cancer in children. A case-control study of acute lymphoblastic leukemia. Sangyo Igaku. 1993 Nov;35(6):515–529. doi: 10.1539/joh1959.35.515. [DOI] [PubMed] [Google Scholar]
- 34.Danila RN. An epidemiological study of ALL in children less than 16 years – an evaluation of potential risk factors with emphasis on farm exposures. Minnesota, USA: University of Minnesota; 1989. [Google Scholar]
- 35.Abadi-Korek I, Stark B, Zaizov R, Shaham J. Parental Occupational Exposure and the Risk of Acute Lymphoblastic Leukemia in Offspring in Israel. J Occup Environ Med. 2006;48(2):165–174. doi: 10.1097/01.jom.0000183343.81485.7c. [DOI] [PubMed] [Google Scholar]
- 36.Kristensen P, Andersen A, Irgens LM, Laake P, Bye AS. Incidence and risk factors of cancer among men and women in Norwegian agriculture. Scand J Work Environ Health. 1996;22(1):14–26. doi: 10.5271/sjweh.104. [DOI] [PubMed] [Google Scholar]
- 37.Pearce MS, Hammal DM, Dorak MT, McNally RJ, Parker L. Paternal occupational exposure to pesticides or herbicides as risk factors for cancer in children and young adults: a case-control study from the North of England. Archives of Environmental and Occupational Health. 2006;61(3):138–144. doi: 10.3200/AEOH.61.3.138-144. [DOI] [PubMed] [Google Scholar]
- 38.Wen WQ, Shu XO, Steinbuch M, Severson RK, Reaman GH, Buckley JD, Robison LL. Paternal military service and risk for childhood leukemia in offspring. Am J Epidemiol. 2000;151(3):231–240. doi: 10.1093/oxfordjournals.aje.a010198. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira JD, Couto AC, Pombo-de-Oliveira MS, Koifman S. In utero pesticide exposure and leukemia in Brazilian children < 2 years of age. Environ Health Perspect. 2013 Feb;121(2):269–275. doi: 10.1289/ehp.1103942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keegan TJ, Bunch KJ, Vincent TJ, King JC, O'Neill KA, Kendall GM, MacCarthy A, Fear NT, Murphy MF. Case-control study of paternal occupation and childhood leukaemia in Great Britain, 1962–2006. Br J Cancer. 2012 Oct 23;107(9):1652–1659. doi: 10.1038/bjc.2012.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinbuch M. The role of environmental exposures in the etiology of childhood acute myeloid leukemia. Ohio, USA: Ohio State University; 1994. [Google Scholar]
- 42.Infante-Rivard C, Mur P, Armstrong B, varez-Dardet C, Bolumar F. Acute lymphoblastic leukaemia among Spanish children and mothers' occupation: a case-control study. J Epidemiol Community Health. 1991;45(1):11–15. doi: 10.1136/jech.45.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudant J, Menegaux F, Leverger G, Baruchel A, Nelken B, Bertrand Y, Patte C, Pacquement H, Verite C, Robert A, Michel G, Margueritte G, Gandemer V, Hemon D, Clavel J. Household exposure to pesticides and risk of childhood hematopoietic malignancies: The ESCALE study (SFCE) Environ Health Perspect. 2007;115(12):1787–1793. doi: 10.1289/ehp.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen B, Wang YY, Shen Y, Zhang WN, He HY, Zhu YM, Chen HM, Gu CH, Fan X, Chen JM, Cao Q, Yang G, Jiang CL, Weng XQ, Zhang XX, Xiong SM, Shen ZX, Jiang H, Gu LJ, Chen Z, Mi JQ, Chen SJ. Newly diagnosed acute lymphoblastic leukemia in China (I): abnormal genetic patterns in 1346 childhood and adult cases and their comparison with the reports from Western countries. Leukemia. 2012 Jul;26(7):1608–1616. doi: 10.1038/leu.2012.26. [DOI] [PubMed] [Google Scholar]
- 45.Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339(9):605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 46.Lu CS, Fenske RA, Simcox NJ, Kalman D. Pesticide exposure of children in an agricultural community: Evidence of household proximity to farmland and take home exposure pathways. Environ Res. 2000;84(3):290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- 47.Weppner S, Elgethun K, Lu CS, Hebert V, Yost MG, Fenske RA. The Washington aerial spray drift study: Children's exposure to methamidophos in an agricultural community following fixed-wing aircraft applications. Journal of Exposure Science and Environmental Epidemiology. 2006;16(5):387–396. doi: 10.1038/sj.jea.7500461. [DOI] [PubMed] [Google Scholar]
- 48.Metayer C, Colt JS, Buffler PA, Reed HD, Selvin S, Crouse V, Ward MH. Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia. J Expo Sci Environ Epidemiol. 2013 Jan 16; doi: 10.1038/jes.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacFarlane E, Glass D, Fritschi L. Is farm-related job title an adequate surrogate for pesticide exposure in occupational cancer epidemiology? Occup Environ Med. 2009 Aug;66(8):497–501. doi: 10.1136/oem.2008.041566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.