Abstract
Background
There has been an increasing interest in use of mineralocorticoid receptor antagonists (MRAs) in patients with heart failure with preserved ejection fraction (HFPEF). However, a comprehensive evaluation of MRA effects on left ventricular (LV) structure and function in these patients is lacking. In this meta‐analysis, we evaluated the effects of MRAs on LV structure and function among patients with diastolic dysfunction or HFPEF.
Methods & Results
Randomized, controlled clinical trials evaluating the efficacy of MRAs in patients with diastolic dysfunction or HFPEF were included. The primary outcome was change in E/e’, a specific measure of diastolic function. Secondary outcomes included changes in other measures of diastolic function, LV structure, surrogate markers for myocardial fibrosis (carboxy‐terminal peptide of procollagen type I [PICP] and amino‐terminal peptide of pro‐collagen type‐II [PIIINP]), blood pressure, and exercise tolerance. In the pooled analysis, MRA use was associated with significant reduction in E/e’ (weighted mean difference [WMD] [95% confidence interval {CI}]: −1.68 [−2.03 to −1.33]; P<0.0001) and deceleration time (WMD [95% CI]: −12.0 ms [−23.3 to −0.7]; P=0.04) as compared with control, suggesting and improvement in diastolic function. Furthermore, blood pressure and levels of PIIINP and PICP were also significantly reduced with MRA therapy with no significant change in LV mass or dimensions.
Conclusion
MRA therapy in patients with asymptomatic diastolic dysfunction or HFPEF is associated with significant improvement in diastolic function and markers of cardiac fibrosis without a significant change in LV mass or dimensions.
Keywords: diastolic dysfunction, heart failure with preserved ejection fraction, mineralocorticoid receptor antagonist
Heart failure (HF) with preserved ejection fraction (HFPEF) is a significant public health problem with increasing prevalence, high morbidity and mortality, and lack of effective proven therapies.1, 2 Although substantial progress has been made in the management and outcomes of heart failure with reduced ejection fraction (HFREF), outcomes associated with HFPEF remain unchanged.1, 2 Well‐established pharmacological therapies for HFREF have failed to improve prognosis in HFPEF.3, 4, 5, 6 This has largely been attributed to the phenotypic variability and the complex pathophysiological mechanisms underlying the development of HFPEF.7
Left ventricular (LV) diastolic dysfunction (LVDD) and LV hypertrophy (LVH) are frequently implicated in the pathogenesis of HFPEF8 and are independently associated with heightened risk for HF hospitalization and cardiovascular (CV) mortality in HFPEF.9, 10 Progression of LVDD has been shown to play an important role in development of symptomatic HF in patients with hypertensive heart disease.11, 12 As a result, there has been a significant interest in therapies that could ameliorate LVDD and adverse LV remodeling in order to potentially prevent or treat HFPEF. Mineralocorticoid receptor antagonists (MRAs), such as spironolactone and epleronone, are one such class of drugs that have been shown to reduce LVDD, LVH, and myocardial fibrosis in animal models.13, 14 Furthermore, MRAs have been shown to improve CV outcomes in subjects with HFREF.15, 16, 17, 18, 19 Several studies have evaluated the efficacy and safety of MRAs in patients with asymptomatic LVDD20, 21, 22, 23, 24 as well as established HFPEF.25, 26, 27, 28, 29, 30
Recently, the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial showed that use of spironolactone did not significantly reduce the overall incidence of adverse CV outcomes among HFPEF patients. However, there was a beneficial effect of spironolactone observed in patients with higher baseline N‐terminal pro‐B‐type natriuretic peptide (NT‐ProBNP) levels and in patients enrolled in the Americas.31, 32 A better understanding of the effects of MRA therapy on LV structure and function is imperative to delineate the potential mechanisms underlying these favorable effects of MRAs. Against this background, the present meta‐analysis was done to assess the impact of MRA therapy on echocardiographic parameters, surrogate measures for myocardial fibrosis (markers of collagen turnover such as carboxy‐terminal peptide of procollagen type I [PICP] and amino‐terminal peptide of pro‐collagen type II [PIIINP]), and surrogate clinical outcomes (exercise capacity and serum potassium levels) in patients with HFPEF or asymptomatic LVDD.
Methods
Search Strategy
A search of PubMed, EMBASE, the Cochrane Controlled Trial registry, and US Clinical Trials databases was performed using these key terms: diastolic dysfunction; heart failure; diastolic heart failure; heart failure with normal ejection fraction; heart failure with preserved ejection fraction; aldosterone receptor antagonist, blocker, blockade, blocking agent; canrenoate; potassium canrenoate; canrenoic acid; spironolactone; aldoacotne; Inspra; eplerenone; human. No language limitations were imposed in the search strategy. From these lists, published clinical trials investigating the effects of MRAs on LV structure and function among patients with HFPEF or hypertension (HTN) without clinical HF were identified. The references cited in the included articles were also examined for additional studies. The search strategy, study selection, and analysis were carried out in accord with the PRISMA statement for systematic reviews.33
Study Selection
All selected abstracts and titles were scanned independently by 2 reviewers (A.P. and S.G.) to identify articles for potential inclusion. Studies included in this analysis were required to have: (1) randomized, controlled trial (RCT) design; (2) a study population with either symptomatic HFPEF or HTN without clinical HF; (3) described method of HF or LVDD diagnosis with reported ejection fraction >45%; (4) data on baseline symptom status of study participants (HF symptoms versus asymptomatic with diastolic dysfunction); (5) clearly defined intervention and control groups; and (6) echocardiographic outcome data available, such as measures of LVDD and LV dimension. Studies involving subjects with recent myocardial infarction or known valvular heart disease were excluded.
Data Extraction and Risk of Bias Assessment
Clinical, echocardiographic, and outcome data were extracted from individual studies and entered into a data extraction form. This included information about study design, patient characteristics (age, sex, ejection fraction, and functional status), intervention strategy (drug used and dose), length of follow‐up, and measures of individual outcome results at baseline and follow‐up. For multiple studies published from a single data set, the largest study with primary findings was included in the analysis. Risk of bias was assessed using the Cochrane risk of bias assessment tool.34 Discrepancies between reviewers were resolved through discussion and consensus.
Outcome Variables
Changes in E/e’, a specific measure of diastolic function, was assessed as the primary outcome in this study. Secondary outcomes analyzed in this study include changes in other measures of diastolic function (E/A and deceleration time [DT]), indexed LV mass, LV end diastolic diameter, serum biomarkers of collagen turnover (PICP and PIIINP), 6‐minute walk distance (6‐MWD) test, and serum potassium.
Statistical Analysis
Statistical analysis was conducted using Revman software (version 5.2; Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2008). Quantitative outcomes changing from baseline to follow‐up were summarized and compared by weighted mean difference (WMD) with 95% confidence interval (CI) between treatment group and control group using fixed and random‐effects models. Heterogeneity between studies was assessed using I2 statistic and publication bias with Begg's funnel plot method.35 For outcomes with significant heterogeneity (I2>50%), the random‐effects model is reported in the text and figures; for all others, the fixed‐effects models are reported. Predefined subgroup analyses were conducted a priori for studies with HFPEF versus asymptomatic LVDD patients for all outcomes. All P values were 2‐tailed with statistical significance specified at 0.05 and CI computed at 95% level.
Results
Eleven RCTs that enrolled 942 participants were included in the present meta‐analysis, with a weighted mean duration of follow‐up of 9.1±3.0 months (Figure 1). Six trials included patients with symptomatic HFPEF, whereas the other 5 included patients with asymptomatic LVDD and/or LVH. The characteristics of the included studies are summarized in Table. Spironolactone was used in 8 of the included trials, whereas eplerenone was used in 2 studies and canrenone was used in 1 trial. Seven studies were conducted in a double‐blind fashion comparing MRA against placebo. Four studies compared MRAs against usual care without placebo administration in the control group. All 11 studies included baseline and follow‐up echocardiographic examination of study participants. In all studies, the timing of outcome follow‐up coincided with the completion of drug intervention.
Figure 1.
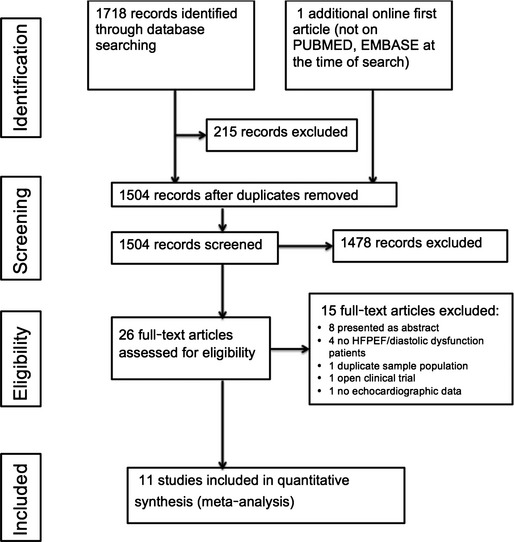
Summary of study identification and selection. HFPEF indicates heart failure with preserved ejection fraction.
Table 1.
Characteristics of Trials Included in the Meta‐Analysis
| Author, Year | Follow‐up (Months) | Intervention Group | Control Group | Clinical Characteristics | Ejection Fraction | Patients Treatment/Control (% Women) | Mean Age, Years (SD) |
|---|---|---|---|---|---|---|---|
| Sato 200220 | 15 months |
Spironolactone 25 mg/day+ACE inhibitor |
ACE inhibitor only | Essential Hypertension, LVH | >50% | 10/10 (45%) | 53 |
| Grandi 200224 | 6 months |
Canerenone 50 mg/day+ACE inhibitor |
ACE inhibitor only | Essential HTN, LVH and LVDD | Normal | 17/17 (38%) | 56 |
| Roonsitrong 200521 | 4 months |
Spironolactone 25 mg/day |
Placebo | Mild diastolic dysfunction | >50% | 15/15 (78%) | 71 |
| Kosmala 201122 | 6 months |
Spironolactone 25 mg/day |
Placebo | Metabolic syndrome | >50% | 40/39 (54%) | 59 |
| Kosmala 201323 | 6 months |
Spironolactone 25 mg/day |
Placebo | BMI >30+diastolic dysfunction | >50% | 58/55 (62%) | 58 |
| Mottram 200426 | 6 months |
Spironolactone 25 mg/day |
Placebo | Hypertension, diastolic heart failure (NYHA class II) | >50% | 15/15 (63%) | 62 |
| Liu 200629 | 6 months |
Spironolactone Mean dose: 47 mg/day |
Usual care | Diastolic heart failure (NYHA class II/III) | >50% | 40/38 (80%) | NA |
| Mak 200927 | 12 months |
Eplerenone 25 to 50 mg/daily |
Usual care | HFPEF+evidence of diastolic dysfunction | >45% | 24/20 (54%) | 80 |
| Deswal 201125 | 6 months |
Eplerenone 25 to 50 mg/daily |
Placebo | HFPEF (NYHA class II/III) | >50% | 21/23 (7%) | 70 |
| Edlemann 201328 | 12 months |
Spironolactone 25 mg/day |
Placebo | HFPEF (NYHA class II/III) | >50% | 213/209 (52%) | 67 |
| Kurrelmeyer 201430 | 6 months |
Spironolactone 25 mg/day |
Placebo | HFPEF (NYHA class II/III) | >50% | 24/24 (100%) | 71 |
ACE indicates angiotensin‐converting enzyme; BMI, body mass index; HFPEF, heart failure with preserved ejection fraction; HTN, hypertension; LVDD, left ventricular diastolic dysfunction; LVH, left ventricular hypertrophy; NA, not available; NYHA, New York Heart Association.
Quality Assessment
Cochrane risk of bias assessment tool was used to perform quality assessment. During quality assessment, random sequence generation was observed in all studies. Double‐blind design was used in 7 studies. Incomplete outcome data or selective reporting of results were not observed in any of the selected studies. Details of risk of bias assessment are given in Figure 2. We did not observe a significant risk of publication bias for the primary outcome in the included studies (Begg's test correlation coefficient: 0.19; P=0.85).
Figure 2.
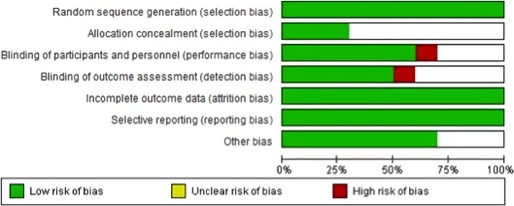
Risk of bias assessment for studies included in the meta‐analysis.
Effect of MRAs on Diastolic Function
Overall pooled estimates and subgroup (HFPEF and asymptomatic LVDD) effect estimates for measures of diastolic function are shown in Figure 3. E/e’, the primary outcome, was reported at baseline and follow‐up in 6 studies. In the pooled analysis of all available studies, MRA use was asso‐ciated with a significant reduction in E/e’ compared with control (WMD [95% CI]: −1.68 [−2.03 to −1.33]; P<0.00001), suggesting improvement in diastolic function. This beneficial effect of MRA on E/e’ was also evident across HFPEF (n=4 studies; WMD [95% CI]: −1.85 [−2.24 to −1.46]; P<0.00001) and asymptomatic LVDD subgroups (n=2 studies; WMD [95% CI]: −0.96 [−1.77 to −0.15]; P=0.02; Figure 3A).
Figure 3.
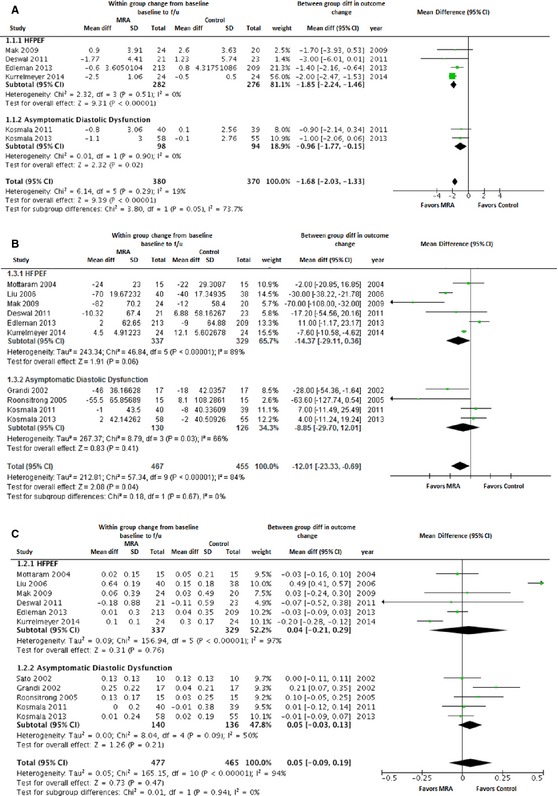
Forest plot showing the pooled difference between the mineralocorticoid receptor antagonist therapy group and the control group for changes in measures of left ventricular diastolic function from baseline to follow‐up. Results are presented for the pooled analyses of all available studies as well as subgroups of studies with HFPEF and asymptomatic diastolic dysfunction. A, E/E’; (B) deceleration time; (C) E/A. Results are presented for the pooled analyses of all available studies as well as the subgroups of studies with HFPEF and asymptomatic diastolic dysfunction. CI, indicates confidence interval; HFPEF, heart failure with preserved ejection fraction; MRA, mineralocorticoid receptor antagonist.
Among other measures of diastolic function, 10 studies reported DT. In the pooled analysis of all available studies, there was a significant, but modest, reduction in DT with MRA use (WMD [95% CI]: −12.0 ms [−23.3 to −0.7]; P=0.04). In subgroup analyses, MRA use was associated with a modest reduction in DT with a trend toward significance in the HFPEF group (WMD [95% CI]: −14.4 ms [−29.1 to 0.4]; P=0.06), but not in asymptomatic LVDD subgroup (Figure 3B). E/A at baseline and follow‐up was also reported in 11 studies. There was no significant change in E/A with use of MRA compared to control group in the overall pooled analysis (WMD [95% CI]: 0.05 [−0.09 to 0.19]; P=0.47) as well as the HFPEF and asymptomatic LVDD subgroups (Figure 3C).
Effect of MRAs on LV Size and Mass
Overall pooled estimates and subgroup (HFPEF and asymptomatic LVDD) effect estimates for LV end‐diastolic diameter and indexed LV mass are shown in Figure 4. LV end‐diastolic diameter was reported at baseline and follow‐up in 6 studies. In the pooled analysis of all available studies, MRA use was not associated with any significant change in LV end‐diastolic diameter (WMD [95% CI]: −0.51 mm [−1.3 to 0.28]; P=0.21). Among HFPEF patients (n=3), there was a modest reduction in LV end‐diastolic diameter with a trend toward significance with MRA use (WMD [95% CI]: −1.04 mm [−2.11 to 0.04]; P=0.06; Figure 4A). Four studies (3 HFPEF and 1 asymptomatic LVDD) reported indexed LV mass at baseline and follow‐up. In the pooled analysis, indexed LV mass did not change significantly with use of MRA compared to the control group (WMD [95% CI]: 0.60 g/m2 [−7.75 to 8.95]; P=0.90; Figure 4B).
Figure 4.
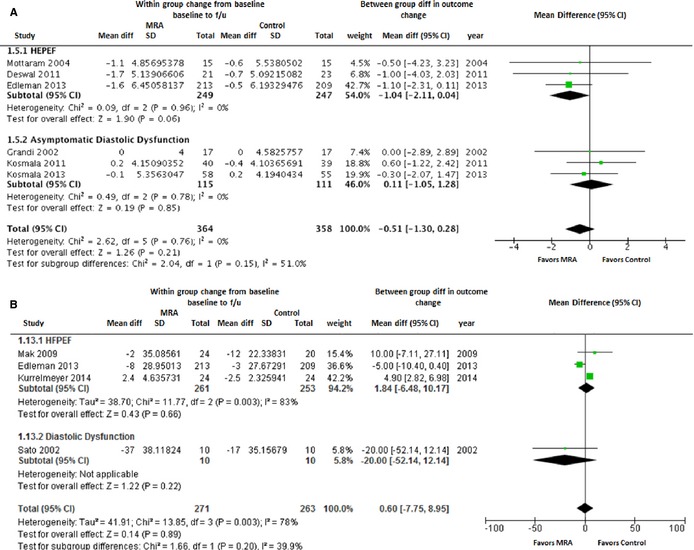
Forest plot showing the pooled difference between the mineralocorticoid receptor antagonist therapy group and the control group for changes in left ventricular end diastolic diameter (A) and left ventricular mass index (B) from baseline to follow‐up. Results are presented for the pooled analyses of all available studies as well as the subgroups of studies with HFPEF and asymptomatic diastolic dysfunction. CI, indicates confidence interval; HFPEF, heart failure with preserved ejection fraction; MRA, mineralocorticoid receptor antagonist.
Effect of MRAs on Markers of Myocardial Fibrosis
Five studies provided information of surrogate markers of cardiac fibrosis, such as PIIINP and PICP. In the pooled analysis, there was a significant reduction in PIIINP with use of MRAs in the overall cohort (WMD [95% CI]: −0.90 μg/L [−1.38 to −0.43]; P=0.0002) as well as in the HPFEF and asymptomatic LVDD subgroups (Figure 5A). Similar reduction in PICP levels was also observed with MRA use in the overall pooled analysis (WMD [95% CI]: −17.6 ng/mL [−31.2 to −4.0]; P=0.01; Figure 5B).
Figure 5.
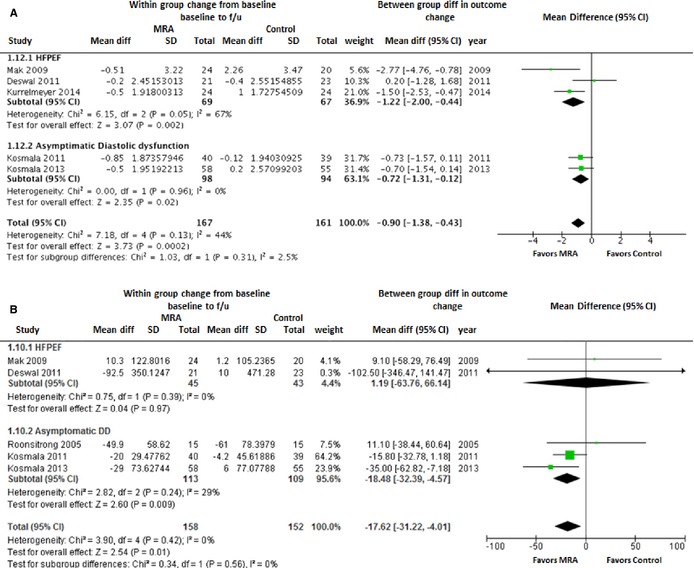
Forest plot showing the pooled difference between the mineralocorticoid receptor antagonist therapy group and the control group for changes in measures of collagen turnover (surrogate for myocardial fibrosis) from baseline to follow‐up. A, PIIINP; (B) PICP. Results are presented for the pooled analyses of all available studies as well as the subgroups of studies with HFPEF and asymptomatic diastolic dysfunction. CI, indicates confidence interval; HFPEF, heart failure with preserved ejection fraction; MRA, mineralocorticoid receptor antagonist; PICP, carboxy‐terminal peptide of procollagen type I; PIIINP, amino‐terminal peptide of procollagen type II.
Effect of MRAs on Blood Pressure
In the pooled analysis of all available studies with BP measurements, MRA use was associated with significant reduction in systolic blood pressure (SBP; WMD [95% CI]: −3.0 mm Hg [−5.5 to −0.6]; P=0.02) as well as diastolic blood pressures (DBP; WMD [95% CI]: −1.7 mm Hg [−2.7 to −0.6]; P=0.002). In subgroup analyses, the reduction in SBP and DBP with MRA use was significant in asymptomatic LVDD patients, but not the HFPEF patients (Figure 6).
Figure 6.
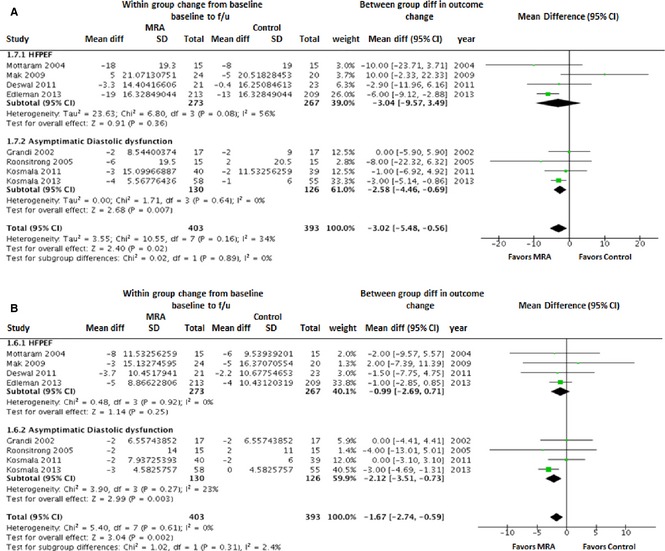
Forest plot showing the pooled difference between the mineralocorticoid receptor antagonist therapy group and the control group for changes in systolic blood pressure (A) and diastolic blood pressure (B). Results are presented for the pooled analyses of all available studies as well as the subgroups of studies with HFPEF and asymptomatic diastolic dysfunction. CI, indicates confidence interval; HFPEF, heart failure with preserved ejection fraction; MRA, mineralocorticoid receptor antagonist.
Clinical Safety and Efficacy of MRA Therapy
Exercise capacity (performance on 6‐MWD test) was assessed in 3 HFPEF studies. There was no significant change in 6‐MWD with use of MRA as compared to the control group (WMD [95% CI]: −8.2 m [−16.7 to 0.2]; P=0.51; Figure 7A). Serum potassium levels increased significantly with MRA use as compared to the control group in the overall cohort (WMD [95% CI]: 0.22 mmol/L [0.17 to 0.27]; P<0.00001) as well as HFPEF and asymptomatic LVDD subgroups (Figure 7B).
Figure 7.
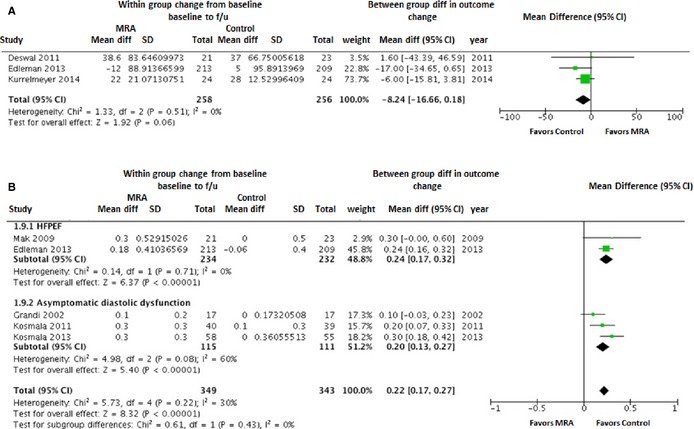
Forest plot showing the pooled difference between the mineralocorticoid receptor antagonist therapy group and the control group for changes in 6‐minute walk test (A) and serum potassium levels (B). Serum potassium levels results are presented for the pooled analyses of all available studies as well as the subgroups of studies with HFPEF and asymptomatic diastolic dysfunction. CI, indicates confidence interval; HFPEF, heart failure with preserved ejection fraction; MRA, mineralocorticoid receptor antagonist.
Discussion
The principal findings of this meta‐analysis indicate that MRA therapy is associated with an improvement in diastolic function, blood pressure, and markers of myocardial fibrosis without any significant changes in indexed LV mass or LV cavity size. To our knowledge, this is the largest, most comprehensive evaluation of the impact of MRA therapy on cardiac structure and function in patients with or at risk for HFPEF.
Mineralocorticoids have been associated with cardiac fibrosis and LVH resulting in myocardial stiffness and diastolic dysfunction.36, 37 A recent meta‐analysis of trials on use of MRA in HFREF showed favorable reverse LV remodeling along with improvements in systolic and diastolic function.38 We observe a similar effect of MRA on biomarkers of collagen turnover and cardiac fibrosis in the current study. We also observed that MRA use was associated with a significant improvement in E/e’, a specific measure of LV diastolic function,39 modest improvement in DT, and no change in E/A. Assessment of diastolic dysfunction is frequently challenging, and typically several indices are necessary for optimal identification.39, 40 The observed heterogeneity in the effect of MRA on different measures of diastolic function could be related to the differences in the outcome definitions. The E/A is most commonly reported in most studies, but is unreliable as a single measure of diastolic dysfunction owing to the nonlinear relationship with dysfunction. Furthermore, both E/A and DT are load dependent and may be influenced by the volume status of the patient on the day of examination. From a physiological standpoint, as myocardial relaxation becomes abnormal, the rate of decrease in the LV pressure is slower and DT becomes longer, but LV filling pressure is not necessarily elevated. E/e’ ratio remains normal as long as left atrial pressure remains normal.41 A reduction in blood pressure with MRA use, as observed in our analysis, would also be expected to lower LV filling pressures and improve the E/e’ ratio.42
We did not observe any significant changes in LV mass or LV end diastolic dimension with use of MRA in the overall study population. Increased LV mass and LV hypertrophy have been identified as significant predictors of adverse clinical outcomes among HFPEF patients.9 Lack of significant effect on LV mass or size in the present study could be related to type II error owing to small sample size or relatively short follow‐up duration. Future large studies with longer follow‐up duration are needed to better understand the biological effect of MRA on LV mass and size.
Despite favorable effects of MRA therapy on diastolic function and myocardial fibrosis, no significant change in 6‐MWD was observed on pooled analysis. This could also be owing to a type II error given that 6‐MWD was only reported in 3 studies. Furthermore, changes in exercise capacity may lag behind improvements in surrogate serological measures of myocardial fibrosis and diastolic function. It is plausible that the lack improvement in these symptoms despite significant reductions in markers of myocardial fibrosis could be a function of the relatively short duration of follow‐up among the included studies.
The recently published TOPCAT trial evaluated the clinical safety and efficacy of MRA in HFPEF.31 Although the trial did not reach statistical significance for the primary endpoint, there were several interesting findings that point toward efficacy of MRA in HFPEF. First, there was a significant reduction in the secondary outcome of HF hospitalization among patients treated with MRAs. Second, participants enrolled in the trial based on elevated NT‐ProBNP levels seemed to benefit significantly with use of MRAs.31 Finally, the recently published regional post‐hoc analyses demonstrated significant clinical improvements among patients from the Americas.32 A beneficial effect on diastolic function observed in our study could potentially explain some of the clinical benefits of MRA among HFPEF patients that were observed by TOPCAT investigators and others.43
There are several limitations of this study. First, because it is a meta‐analysis, the validity of our results is dependent on the validity of the studies included. We did not include patient‐level data. Second, the overall sample size of the study population is relatively small. Thus, it is possible that type II error may be responsible for some of the negative findings. Third, all measures of diastolic function and LV geometry were not consistently reported in all the included clinical trials. Similarly, the measures of exercise capacity (6‐MWD test) were reported in only 3 trials, whereas peak oxygen consumption, a gold standard for measuring exercise capacity, was reported in only 1 study. Fourth, significant heterogeneity was observed on pooled analysis of included studies for most outcomes. This could be related to the differences in the study design with respect to the choice of MRA (spironolactone in 8 studies versus eplerenone/canrenone in 3 studies), lack of consistent use of placebo with usual therapy in the comparator arm (usual therapy+placebo in 7 studies, usual therapy only in 4 studies), and duration of treatment. Also, the mean follow‐up duration in these clinical trials was relatively short (<1 year). Finally, none of the included trials reported clinical outcomes, such as HF hospitalizations and mortality.
In conclusion, our meta‐analysis indicates that MRA therapy in patients with HFPEF and impaired diastolic function may exert a beneficial effect on diastolic dysfunction and myocardial fibrosis. Further studies with long‐term follow‐up are needed to determine whether these favorable changes in diastolic function can translate into significant clinical improvement in these patients.
Disclosures
Dr Amil M. Shah receives research support from Novartis, Gilead, Actelion. All other authors have no disclosures relevant to this manuscript.
Acknowledgments
The authors thank Kazeen Abdullah (Division of Cardiology, UTSW Medical Center, Dallas, TX) and Amitasha Sinha (Department of Internal Medicine, Sinai Hospital, Baltimore, MD) for their help in data collection and manuscript preparation. This study was not supported by any grant or research funding.
(J Am Heart Assoc.2015;4:e002137 doi: 10.1161/JAHA.115.002137)
References
- 1. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 2. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 3. Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler‐echocardiographic study (SWEDIC). Eur J Heart Fail. 2004;6:453–461. [DOI] [PubMed] [Google Scholar]
- 4. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; Investigators IP . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J; Investigators C, Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 6. Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction. A meta‐analysis. J Am Coll Cardiol. 2011;57:1676–1686. [DOI] [PubMed] [Google Scholar]
- 7. Rose‐Jones LJ, Rommel JJ, Chang PP. Heart failure with preserved ejection fraction: an ongoing enigma. Cardiol Clin. 2014;32:151–161, ix–x. [DOI] [PubMed] [Google Scholar]
- 8. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. [DOI] [PubMed] [Google Scholar]
- 9. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circ Heart Fail. 2014;7:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE; Investigators IP . Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. [DOI] [PubMed] [Google Scholar]
- 11. Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular‐vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brilla CG, Matsubara LS, Weber KT. Anti‐aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol. 1993;25:563–575. [DOI] [PubMed] [Google Scholar]
- 14. Lacolley P, Safar ME, Lucet B, Ledudal K, Labat C, Benetos A. Prevention of aortic and cardiac fibrosis by spironolactone in old normotensive rats. J Am Coll Cardiol. 2001;37:662–667. [DOI] [PubMed] [Google Scholar]
- 15. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; Group E‐HS . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.21073363 [Google Scholar]
- 16. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post‐Acute Myocardial Infarction Heart Failure E, Survival Study I . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 18. Bangalore S, Kumar S, Messerli FH. When conventional heart failure therapy is notenough: angiotensin receptor blocker, direct renin inhibitor, or aldosterone antagonist? Congest Heart Fail. 2013;19:107–115. [DOI] [PubMed] [Google Scholar]
- 19. Lavie CJ, DiNicolantonio JJ, O'Keefe JH, Ventura HO. Aldosterone antagonists: evidence‐based yet underutilized effective heart failure therapy. Congest Heart Fail. 2013;19:105–106. [DOI] [PubMed] [Google Scholar]
- 20. Sato A, Hayashi M, Saruta T. Relative long‐term effects of spironolactone in conjunction with an angiotensin‐converting enzyme inhibitor on left ventricular mass and diastolic function in patients with essential hypertension. Hypertens Res. 2002;25:837–842. [DOI] [PubMed] [Google Scholar]
- 21. Roongsritong C, Sutthiwan P, Bradley J, Simoni J, Power S, Meyerrose GE. Spironolactone improves diastolic function in the elderly. Clin Cardiol. 2005;28:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kosmala W, Przewlocka‐Kosmala M, Szczepanik‐Osadnik H, Mysiak A, O'Moore‐Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on LV function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imaging. 2011;4:1239–1249. [DOI] [PubMed] [Google Scholar]
- 23. Kosmala W, Przewlocka‐Kosmala M, Szczepanik‐Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart. 2013;99:320–326. [DOI] [PubMed] [Google Scholar]
- 24. Grandi AM, Imperiale D, Santillo R, Barlocco E, Bertolini A, Guasti L, Venco A. Aldosterone antagonist improves diastolic function in essential hypertension. Hypertension. 2002;40:647–652. [DOI] [PubMed] [Google Scholar]
- 25. Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (RAAM‐PEF). J Card Fail. 2011;17:634–642. [DOI] [PubMed] [Google Scholar]
- 26. Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. [DOI] [PubMed] [Google Scholar]
- 27. Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy NF, Patle AK, Baugh JA, McDonald KM. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54:1674–1682. [DOI] [PubMed] [Google Scholar]
- 28. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B; Aldo DHFI . Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA. 2013;309:781–791. [DOI] [PubMed] [Google Scholar]
- 29. Liu G, Ji ZG, Liu KS. Effects of spironolactone in treatment of elderly hypertensive patients with diastolic heart failure. Chin J New Drugs Clin Remedies. 2006;8:567–570. [Google Scholar]
- 30. Kurrelmeyer KM, Ashton Y, Xu J, Nagueh SF, Torre‐Amione G, Deswal A. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2014;20:560–568. [DOI] [PubMed] [Google Scholar]
- 31. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 32. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 33. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The prisma statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods G, Cochrane Statistical Methods G . The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edelmann F, Tomaschitz A, Wachter R, Gelbrich G, Knoke M, Dungen HD, Pilz S, Binder L, Stahrenberg R, Schmidt A, Marz W, Pieske B. Serum aldosterone and its relationship to left ventricular structure and geometry in patients with preserved left ventricular ejection fraction. Eur Heart J. 2012;33:203–212. [DOI] [PubMed] [Google Scholar]
- 38. Li X, Qi Y, Li Y, Zhang S, Guo S, Chu S, Gao P, Zhu D, Wu Z, Lu L, Shen W, Jia N, Niu W. Impact of mineralocorticoid receptor antagonists on changes in cardiac structure and function of left ventricular dysfunction: a meta‐analysis of randomized controlled trials. Circ Heart Fail. 2013;6:156–165. [DOI] [PubMed] [Google Scholar]
- 39. Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler‐conductance catheterization study. Circulation. 2007;116:637–647. [DOI] [PubMed] [Google Scholar]
- 40. Gaasch WH, Little WC. Assessment of left ventricular diastolic function and recognition of diastolic heart failure. Circulation. 2007;116:591–593. [DOI] [PubMed] [Google Scholar]
- 41. Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two‐dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–506. [DOI] [PubMed] [Google Scholar]
- 42. Kazik A, Wilczek K, Polonski L. Management of diastolic heart failure. Cardiol J. 2010;17:558–565. [PubMed] [Google Scholar]
- 43. van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B‐type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. [DOI] [PubMed] [Google Scholar]


