Abstract
Background
Risk prediction is a critical step in patient selection for aortic valve replacement (AVR), yet existing risk scores incorporate very few echocardiographic parameters. We sought to evaluate the incremental predictive value of a complete echocardiogram to identify high‐risk surgical candidates before AVR.
Methods and Results
A cohort of patients with severe aortic stenosis undergoing surgical AVR with or without coronary bypass was assembled at 2 tertiary centers. Preoperative echocardiograms were reviewed by independent observers to quantify chamber size/function and valve function. Patient databases were queried to extract clinical data. The cohort consisted of 432 patients with a mean age of 73.5 years and 38.7% females. Multivariable logistic regression revealed 3 echocardiographic predictors of in‐hospital mortality or major morbidity: E/e’ ratio reflective of elevated left ventricular (LV) filling pressure; myocardial performance index reflective of right ventricular (RV) dysfunction; and small LV end‐diastolic cavity size. Addition of these echocardiographic parameters to the STS risk score led to an integrated discrimination improvement of 4.1% (P<0.0001). After a median follow‐up of 2 years, Cox regression revealed 5 echocardiographic predictors of all‐cause mortality: small LV end‐diastolic cavity size; LV mass index; mitral regurgitation grade; right atrial area index; and mean aortic gradient <40 mm Hg.
Conclusions
Echocardiographic measures of LV diastolic dysfunction and RV performance add incremental value to the STS risk score and should be integrated in prediction when evaluating the risk of AVR. In addition, findings of small hypertrophied LV cavities and/or low mean aortic gradients confer a higher risk of 2‐year mortality.
Keywords: aortic stenosis, aortic valve replacement, echocardiography, outcomes
Introduction
Aortic stenosis (AS) is one of the most common valvular heart diseases with a pooled prevalence of 12.4% in older adults.1 Severe AS has been identified as a major cause of morbidity and mortality and, if untreated, can result in debilitating clinical symptoms, progressive heart failure, and sudden cardiac death. The economic impact of AS on the health care system is substantial.2 Traditionally, surgical aortic valve replacement (SAVR) has been the mainstay of treatment for severe AS, but has been associated with 3.2% to 5.6% perioperative mortality when performed without and with concomitant coronary artery bypass graft surgery (CABG), respectively.3, 4 In high‐risk elderly patients, the risk of mortality approaches 10%, and 1 of 3 of these patients’ experiences a major morbidity.3, 4 A contributing factor to the adverse outcomes observed is that many of the elderly patients considered for SAVR are frail and have multiple chronic conditions.5
The emergence of therapeutic alternatives to surgery, such as transcatheter aortic valve replacement (TAVR), has provided a treatment alternative for a significant number of patients with severe AS and high surgical risk, usually defined as a predicted risk of mortality >8% to 10%.6 Approximately 290 000 elderly patients at high surgical risk could potentially be treated with TAVR in Europe and North America.1 Hence, the significance of operative risk prediction has increased dramatically owing to its importance in guiding therapeutic decision making in patients with severe AS. Clinicians and researchers rely on risk scores such as The Society of Thoracic Surgeons (STS) Predicted Risk and the European System for Cardiac Operative Risk Evaluation (EuroSCORE) to estimate risk, although it is recognized that these scores do not capture a number of important variables.
At the present time, risk scores integrate very few echocardiographic variables in their calculations, typically limited to left ventricular (LV) ejection fraction (LVEF), mitral regurgitation (MR) grade, and pulmonary artery systolic pressure (PASP). This represents a missed opportunity to enhance risk prediction given the wealth of hemodynamic, functional, and structural data available in routinely performed preoperative echocardiograms. Recently, our group demonstrated the incremental prognostic value of preoperative echocardiograms in patients undergoing isolated CABG.7 Thus, we expanded this hypothesis to patients undergoing SAVR to determine the incremental prognostic value of the echocardiogram in addition to the STS risk score to predict short‐ and long‐term mortality and major morbidity.
Methods
Study Design
A cohort of consecutive patients undergoing SAVR with or without CABG at 2 tertiary referral hospitals in the United States and Canada was assembled. Preoperative echocardiograms were analyzed by 2 independent observers who were blinded to the outcomes. A comprehensive echocardiographic protocol was quantitatively measured, reflecting right‐ and left‐sided chamber size and geometry, systolic and diastolic function of the right and left ventricles, and valvular hemodynamics. Clinical variables were extracted from patient databases and vital status from the Social Security Registry. The study was approved by the institutional review board before commencement, and the manuscript was prepared in accord with the Strengthening of Reporting of Observational Studies in Epidemiology guidelines.8
Setting
The cohort consisted of consecutive patients who underwent isolated SAVR or combined SAVR+CABG at the Massachusetts General Hospital between 2008 and 2010 (Harvard University, Boston, MA) and at the Jewish General Hospital between 2010 and 2012 (McGill University, Montreal, Quebec, Canada). Both hospitals are academic centers with access to TAVR (although TAVR was in its early stages of adoption and limited to research protocols during the time frame of this study). Patients were followed from the time of their index admission to hospital discharge and subsequently followed forward for vital status up to February 2013.
Participants
The inclusion criteria were: (1) severe AS defined as a peak velocity ≥4 m/s, mean gradient ≥40 mm Hg, aortic valve area (AVA) <1.0 cm2, or indexed AVA <0.6 cm2/m2 by Doppler echocardiography in combination with 2‐dimensional echocardiographic appearance of severe valvular AS; (2) SAVR performed with or without CABG; (3) preoperative transthoracic echocardiogram (TTE) performed at the study center ≤3 months before surgery. Patients who had concomitant mitral or tricuspid valve surgery, greater than moderate aortic regurgitation, previous valvuloplasty or valve replacement, or complex congenital heart disease were excluded. Patient who had surgical intervention on the aorta were not excluded. Because echocardiographic images were reanalyzed for the purpose of this study, patients were excluded if their digital echocardiographic images could not be retrieved or were uninterpretable.
Predictor Variables
A comprehensive list of quantitative parameters of left‐ and right‐sided chamber size, geometry, systolic and diastolic function, and valvular function was established a priori and then measured from the preoperative echocardiograms. The acquisitions, measurements, and normal reference limits adhered to the guidelines of the American Society of Echocardiography (ASE).9, 10, 11, 12, 13 Right and left atrial areas were measured at end‐systole in the apical 4‐chamber view; left atrial height was also measured to calculate left atrial volume using the single‐plane area‐length method. Right ventricular (RV) area was measured at end‐diastole and end‐systole in the apical 4‐chamber view to calculate fractional area change (FAC) as: [RV end‐diastolic area−RV end‐systolic area]/[RV end‐diastolic area]. Tricuspid valve closure to opening time was measured by continuous‐wave Doppler of the tricuspid regurgitation and/or pulsed Doppler of the tricuspid inflow. Pulmonic ejection time was measured from pulsed Doppler profiles obtained at the distal RV outflow tract. RV myocardial performance index (MPI), an index of RV efficiency reflecting both systolic and diastolic function, was calculated as: [Tricuspid valve closure opening time−Pulmonic ejection time]/[Pulmonic ejection time]. PASP was measured by continuous‐wave Doppler of the tricuspid regurgitation with 10 mm Hg added for right atrial pressure. The choice of parameters was confined to those that could be measured from the routine echocardiography protocol at both institutions; for example, tricuspid annular plane systolic excursion and/or velocity were not included in our panel because they were not routinely acquired in all patients.
LVEF and LV volumes were measured using the biplane Simpson's method. LV mass was measured using the Devereux method. LV diastolic function was graded as normal, impaired relaxation, pseudonormal filling, or restrictive filling according to the ASE's recommended multiparametric approach that integrates pulsed Doppler of the mitral and pulmonary vein inflow, tissue Doppler of the medial and lateral annulus, and left atrial volume. Valvular regurgitation was graded as normal, mild, moderate, or severe according to the ASE's recommended cutoffs for color Doppler jet area, vena contracta diameter, and proximal isovelocity shell area, where applicable. Measures of AS severity included peak velocity, peak and mean gradients, dimensionless index calculated as [LV outflow tract (LVOT) velocity/Peak velocity], AVA using velocity time integrals rather than velocities, and AVA indexed to body surface area.
Measurements were performed in duplicate by 2 independent echocardiography readers and arbitrated by a third senior reader. All readers were cardiologists trained in echocardiography. To ensure accuracy and consistency of measurement techniques, readers participated in focused training sessions and quality audits throughout the study. Readers were blinded to the clinical data and outcomes when interpreting the echocardiograms.
Outcome Variables
The primary outcome was a composite of in‐hospital mortality or major morbidity defined according to the STS as any one of the following: all‐cause death, stroke, renal failure (Risk, Injury, Failure, Loss of kidney function, and End‐stage kidney disease [RIFLE] classification ≥3), prolonged ventilation ≥48 hours, or need for reoperation.14 The secondary outcome was long‐term all‐cause mortality defined as death from any cause occurring from the time of cardiac surgery to the end of follow‐up. There were no patients lost to follow‐up for the primary or secondary outcomes.
Data Sources
Echocardiograms were acquired with the Philips IE33, Sonos 7500, or GE Vivid 7 machines and analyzed on the Xcelera workstation (Philips Medical Systems, Andover, MA) at the Massachusetts General Hospital; and with the GE Vivid 7 machine and EchoPAC workstation (GE, Milwaukee, WI) at the Jewish General Hospital. Clinical data, including in‐hospital outcomes, were extracted from the STS Adult Cardiac Surgery Database and vital status was extracted from the Social Security Death Index by way of the Research Patient Data Registry (Partners Healthcare, Boston, MA) at the Massachusetts General Hospitals; and from the ChartMaxx electronic medical record at the Jewish General Hospital.
Statistical Analyses
Echocardiography variables were preserved in their continuous form15 and standardized such that the resulting odds ratios (ORs) represented the increase in odds per SD in that variable. To test the appropriateness of entering a continuous variable in a logistic regression model, each echocardiographic variable (x‐axis) was plotted against the logit of the primary outcome (y‐axis) and the resulting graphical plot was inspected for linearity. When a nonlinear relationship was observed, the variable was transformed into ordinal form.
For the primary outcome, multivariable logistic regression with Akaike information criterion (AIC)‐based model selection was used to identify the optimal echocardiographic parameters to predict in‐hospital mortality or major morbidity after adjusting for the STS predicted risk of mortality or major morbidity (STS‐PROMM). For the secondary outcome, multivariable Cox regression with AIC‐based model selection was used to identify the optimal echocardiographic parameters to predict all‐cause mortality after adjusting for the Society of Thoracic Surgeons predicted risk of mortality (STS‐PROM). The AIC model selection procedure approximates a backward‐forward selection procedure with a P‐value cutoff of ≈0.15, hence the optimal predictors may have confidence intervals (CIs) that cross unity.
Model performance statistics were calculated for STS‐PROM (or STS‐PROMM) alone, and then for STS‐PROM (or STS‐PROMM) plus the echocardiographic variables. These statistics included the c‐statistic and integrated discrimination improvement (IDI).16 All analyses were performed with the STATA 13 statistical software package (StataCorp LP, College Station, TX).
Results
A total of 1144 patients underwent SAVR at both sites during the study period, of which 432 were eligible and included in the cohort (Figure). The main exclusion criterion was not having an available echocardiogram ≤3 months before surgery; excluded patients who did not have an available echocardiogram were similar to included patients in terms of mean age (73.9 vs. 73.5 years), proportion of females (41.1% vs. 38.7%), STS‐PROM (5.5% vs. 5.3%), and observed in‐hospital mortality (3.2% vs. 3.2%). The median time from echocardiography to surgery was 13 days (interquartile range [IQR], 6–43). The median time from surgery to end of follow‐up was 728 days (IQR, 354–1063).
Figure 1.
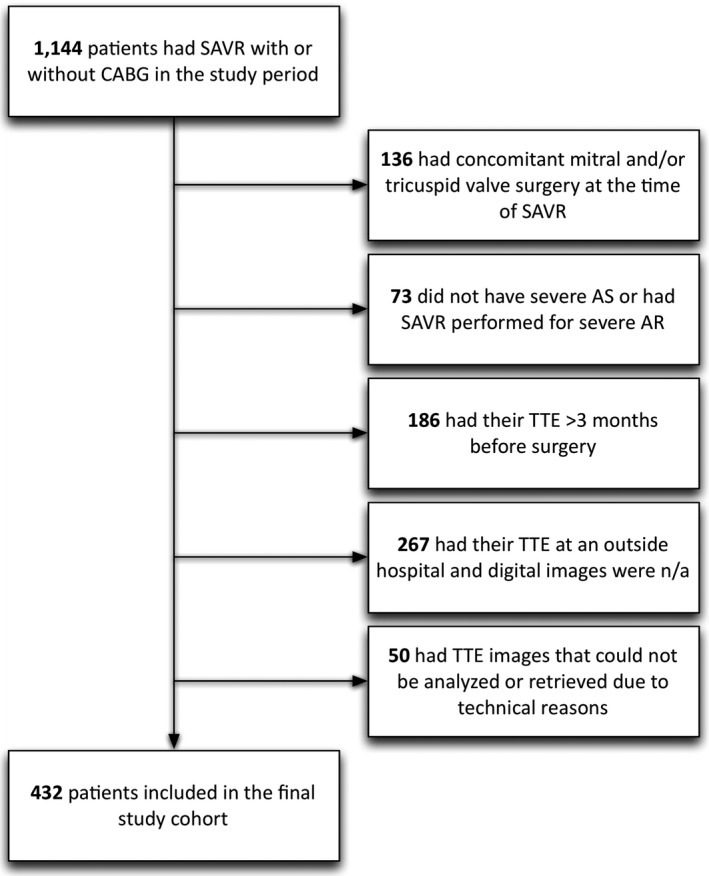
Flow diagram. Of 1144 patients that underwent SAVR with or without CABG, 432 met the selection criteria and were included in the cohort. Excluded patients who did not have an available echocardiogram were similar to included patients in terms of mean age (73.9 vs. 73.5 years), proportion of females (41.1% vs. 38.7%), STS‐PROM (5.5% vs. 5.3%), and observed in‐hospital mortality (3.2% vs. 3.2%). AR indicates aortic regurgitation; AS, aortic stenosis; CABG, coronary artery bypass graft surgery; SAVR, surgical aortic valve replacement; STS‐PROM, Society of Thoracic Surgeons predicted risk of mortality; TTE, transthoracic echocardiography.
Baseline clinical characteristics are shown in Table 1 and in‐hospital outcomes are shown in Table 2. The mean age of the cohort was 73.5±10.3 years, with 38.7% females. The surgical procedure was isolated SAVR in 49.3% and combined SAVR/CABG in 50.7%. The mean STS‐PROM was 5.3±5.1%, and STS‐PROMM was 25.7±14.1%. There were 45 deaths and 88 composite adverse events observed (20.4% in‐hospital mortality or major morbidity). Adverse event rates were similar at both study centers. Those who had an adverse event were more likely to have advanced age, female sex, chronic kidney disease, and recently decompensated heart failure.
Table 1.
Clinical Characteristics
| Overall (n=432) | In‐Hospital Mortality or Major Morbidity | ||
|---|---|---|---|
| Yes (n=88) | No (n=344) | ||
| Age, y | 73.5±10.3 | 75.5±10.5 | 73.0±10.2 |
| Female, % | 167 (38.7) | 41 (46.6) | 126 (36.6) |
| BMI, kg/m2 | 28.8±6.0 | 28.7±6.7 | 28.8±5.8 |
| Diabetes, % | 157 (36.3) | 35 (39.8) | 122 (35.5) |
| Chronic renal disease, % | 185 (42.8) | 51 (58.0) | 134 (39.0) |
| Chronic lung disease, % | 77 (17.8) | 21 (23.9) | 56 (16.3) |
| Peripheral vascular disease, % | 72 (16.7) | 13 (14.8) | 59 (17.2) |
| Cerebrovascular disease, % | 74 (17.1) | 16 (18.2) | 58 (16.9) |
| Previous myocardial infarction, % | 143 (33.1) | 36 (40.9) | 107 (31.1) |
| Atrial fibrillation, % | 104 (24.1) | 24 (27.3) | 80 (23.3) |
| Prior cardiac surgery, % | 56 (13.0) | 11 (12.5) | 45 (13.1) |
| Heart failure within 2 weeks, % | 205 (47.5) | 54 (61.4) | 151 (33.9) |
| Charlson comorbidity index | 2.2±1.5 | 2.6±1.5 | 2.0±1.5 |
| STS‐PROM | 5.3±5.1 | 8.0±6.9 | 4.6±4.3 |
| Aortic prosthesis size, mm | 22.4±2.1 | 22.3±2.2 | 22.4±2.1 |
| Concomitant coronary bypass, % | 219 (50.7) | 54 (61.4) | 165 (48.0) |
| Cardiopulmonary bypass time, min | 140.6±59.6 | 160.4±74.2 | 135.5±54.2 |
BMI indicates body mass index; STS‐PROM, Society of Thoracic Surgeons predicted risk of mortality.
Table 2.
In‐Hospital Outcomes
| Overall (n=432) | |
|---|---|
| Mortality or major morbidity, % | 88 (20.4) |
| Mortality, % | 14 (3.2) |
| Stroke, % | 18 (4.2) |
| Acute kidney injury (RIFLE ≥3), % | 25 (5.8) |
| Prolonged intubation (≥48 hours), % | 32 (7.7) |
| Reoperation, % | 47 (10.9) |
RIFLE indicates Risk, Injury, Failure, Loss of kidney function, and End‐stage kidney disease.
Preoperative echocardiographic parameters are shown in Table 3. AS severity was reflected by AVA 0.75±0.22 cm2, indexed AVA 0.39±0.11 cm2/m2, and peak and mean gradients of 79.6 and 52.7 mm Hg, respectively. One hundred ten patients had severe AS with low mean gradient <40 mm Hg, of which 10.2% had low LVEF <35%. The prevalence of RV systolic dysfunction (abnormal FAC) was 14.4%, whereas subclinical RV dysfunction (abnormal RV MPI with normal RV FAC) was 45.2%. The prevalence of LV hypertrophy was 52.3% and small LV end‐diastolic volume index (LVEDVi) was 9.3%.
Table 3.
Preoperative Echocardiographic Parameters
| Overall (n=432) | In‐Hospital Mortality or Major Morbidity | ||
|---|---|---|---|
| Yes (n=88) | No (n=344) | ||
| LVOT diameter, cm | 2.1±0.2 | 2.1±0.2 | 2.1±0.2 |
| LVOT velocity, cm/s | 93.4±21.8 | 91.8±22.1 | 93.8±21.8 |
| Peak velocity, cm/s | 439.3±47.3 | 426.7±84.7 | 442.5±74.7 |
| Peak gradient, mm Hg | 79.6±27.0 | 75.7±29.3 | 80.6±26.3 |
| Mean gradient, mm Hg | 52.7±18.6 | 50.0±20.2 | 53.4±18.2 |
| Mean gradient | |||
| <40 mm Hg, % | 110 (25.5) | 26 (29.6) | 84 (24.5) |
| 40 to 69 mm Hg, % | 246 (57.1) | 49 (55.7) | 197 (57.4) |
| ≥70 mm Hg, % | 75 (17.4) | 13 (14.8) | 62 (18.1) |
| Dimensionless index | 0.22±0.07 | 0.22±0.07 | 0.22±0.06 |
| Aortic valve area, cm2 | 0.75±0.22 | 0.76±0.24 | 0.74±0.21 |
| Aortic valve area index, cm2/m2 | 0.39±0.11 | 0.41±0.12 | 0.39±0.11 |
| Ascending aorta diameter, cm | 3.2±0.5 | 3.2±0.5 | 3.2±0.5 |
| Bicuspid aortic valve, % | 43 (10.5) | 6 (7.2) | 37 (11.3) |
| LA volume index, mL/m2 | 44.0±17.7 | 43.2±17.8 | 44.2±17.7 |
| LV end‐diastolic volume index, mL/m2 | 59.5±20.2 | 57.4±20.7 | 60.0±20.1 |
| LVEF, % | 57.8±14.3 | 56.1±14.9 | 58.3±14.1 |
| LVEF | |||
| <35%, % | 44 (10.2) | 10 (11.4) | 34 (9.9) |
| 35% to 54%, % | 94 (21.9) | 27 (30.7) | 67 (19.6) |
| 55% to 74%, % | 261 (60.7) | 45 (51.1) | 216 (63.2) |
| ≥75%, % | 31 (7.2) | 6 (6.8) | 57 (7.3) |
| LV MPI | 0.27±0.16 | 0.29±0.16 | 0.27±0.16 |
| LV mass index, g/m2 | 114.7±32.7 | 113.1±35.3 | 115.1±32.1 |
| Mean e’ | 6.0±2.0 | 5.5±1.7 | 6.2±2.0 |
| Mean E/e’ | 18.1±8.5 | 21.2±9.9 | 17.3±8.0 |
| RA area index, cm/m2 | 9.4±2.9 | 9.7±2.9 | 9.4±2.8 |
| RV end‐diastolic area index, cm/m2 | 8.5±2.2 | 8.7±2.2 | 8.5±2.2 |
| RV fractional area change, % | 47.0±11.6 | 46.8±13.8 | 47.1±11.0 |
| RV MPI | 0.41±0.22 | 0.48±0.23 | 0.39±0.21 |
| Pulmonary arterial systolic pressure, mm Hg | 42.1±13.3 | 44.3±13.7 | 41.5±13.1 |
| Mitral regurgitation | |||
| None or trivial, % | 158 (36.9) | 32 (36.4) | 126 (37.1) |
| Mild, % | 203 (47.4) | 39 (44.3) | 164 (48.2) |
| Moderate, % | 65 (15.2) | 16 (18.2) | 49 (14.4) |
| Severe, % | 2 (0.5) | 1 (1.1) | 1 (0.3) |
| Tricuspid regurgitation | |||
| None or trivial, % | 227 (53.1) | 38 (43.2) | 178 (54.1) |
| Mild, % | 164 (38.3) | 39 (44.3) | 125 (38.0) |
| Moderate, % | 34 (7.9) | 10 (11.4) | 24 (7.3) |
| Severe, % | 3 (0.7) | 1 (1.1) | 2 (0.6) |
LA indicates left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; MPI, myocardial performance index; RA, right atrium; RV, right ventricle.
In the logistic regression model to predict in‐hospital mortality or major morbidity, 3 echocardiographic parameters were independently predictive after adjusting for STS‐PROMM (Table 4): E/e’ (standardized OR, 1.40; 95% CI, 1.03, 1.78), RV MPI (OR, 1.25; 95% CI, 0.94, 1.65), and LVEDVi (OR, 0.82; 95% CI, 0.54, 1.00). Model performance was significantly improved by addition of these 3 echocardiographic parameters to the STS‐PROMM, resulting in an IDI of 4.1% (P<0.001) and an increase in c‐statistic from 0.70 for STS‐PROMM alone to 0.74 for STS‐PROMM plus echocardiographic parameters. The IDI was driven mainly by the new model predicting a higher risk in patients that experienced an adverse event.
Table 4.
Optimal Echocardiographic Predictors for In‐Hospital Mortality/Morbidity
| Unadjusted | Adjusted | |
|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| STS‐PROMM | 1.003 (1.002, 1.003) | 1.003 (1.002, 1.004) |
| LVEF | ||
| <35% | 1.41 (0.65, 3.06) | |
| 35% to 55% | 1.93 (1.12, 3.35) | |
| 55% to 75% | Referent | |
| >75% | 1.15 (0.45, 2.97) | |
| LV end‐diastolic volume index | 0.82 (0.98, 1.01) | 0.82 (0.54, 1.00) |
| E/e’ | 1.51 (1.18, 1.92) | 1.40 (1.03, 1.78) |
| LV mass index | 1.00 (0.72, 1.38) | |
| MR grade | 1.10 (0.84, 1.45) | |
| LA volume index | 1.00 (0.70, 1.19) | |
| RV FAC | 0.97 (0.77, 1.23) | |
| RV end‐diastolic area index | 1.16 (0.91, 1.47) | |
| RV MPI | 1.44 (1.14, 1.82) | 1.25 (0.94, 1.65) |
| PASP | 1.30 (0.87, 1.68) | |
| TR grade | 1.32 (0.99, 1.75) | |
| RA area index | 1.12 (0.89, 1.43) | |
| Mean aortic gradient | ||
| <40 mm Hg | 1.24 (0.73, 2.13) | |
| 40 to 70 mm Hg | Referent | |
| >70 mm Hg | 0.84 (0.43, 1.66) | |
The unadjusted column represents results from individual logistic regressions, whereas the adjusted column represents results from one multivariable logistic regression. FAC indicates fractional area change; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MPI, myocardial performance index; MR, mitral regurgitation; PASP, pulmonary artery systolic pressure; RA, right atrium; RV, right ventricle; STS‐PROMM, Society of Thoracic Surgeons predicted risk of mortality or major morbidity; TR, tricuspid regurgitant.
A Cox model was then used to predict long‐term all‐cause mortality at the median follow‐up of 2 years (maximum of 4.2 years), and 5 echocardiographic parameters were independently predictive after adjusting for STS‐PROM (Table 5): LV mass index (standardized hazard ratio [HR], 1.38; 95% CI, 1.03, 1.91), RA area index (HR, 1.28; 95% CI, 0.94, 1.70), mean aortic gradient <40 mm Hg (HR, 2.23; 95% CI, 1.04, 4.76), mitral regurgitation grade (HR, 1.45; 95% CI, 0.97, 2.16), and LVEDVi (HR, 0.54; 95% CI, 0.35, 0.82). Addition of these echocardiographic parameters led to an increase in Harrell's c‐statistic from 0.82 for STS‐PROM alone to 0.85 for STS‐PROM plus echocardiographic parameters.
Table 5.
Optimal Echocardiographic Predictors of 2‐Year Mortality
| Unadjusted | Adjusted | |
|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| STS‐PROM | 1.005 (1.004, 1.007) | 1.005 (1.004, 1.007) |
| LVEF | ||
| <35% | 2.18 (0.92, 5.20) | |
| 35% to 55% | 2.08 (1.03, 4.21) | |
| 55% to 75% | Referent | |
| >75% | 2.26 (0.85, 6.07) | |
| LV end‐diastolic volume index | 0.66 (0.54, 0.98) | 0.54 (0.35, 0.82) |
| E/e’ | 1.51 (1.18, 1.92) | |
| LV mass index | 1.00 (0.72, 1.38) | 1.38 (1.03, 1.91) |
| MR grade | 1.72 (1.18, 2.52) | 1.45 (0.97. 2.16) |
| LA volume index | 1.19 (1.00, 1.69) | |
| RV FAC | 0.93 (0.69, 1.25) | |
| RV end‐diastolic area index | 1.16 (0.87, 1.55) | |
| RV MPI | 1.37 (1.11, 1.68) | |
| PASP | 1.48 (1.14, 1.91) | |
| TR grade | 1.87 (1.31, 2.66) | |
| RA area index | 1.70 (1.32, 2.19) | 1.28 (0.94, 1.70) |
| Mean aortic gradient | ||
| <40 mm Hg | 1.64 (0.85, 3.14) | 2.23 (1.04, 4.76) |
| 40 to 70 mm Hg | Referent | |
| >70 mm Hg | 0.13 (0.48, 2.66) | |
The unadjusted column represents results from individual logistic regressions, whereas the adjusted column represents results from one multivariable logistic regression. FAC indicates fractional area change; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MPI, myocardial performance index; MR, mitral regurgitation; PASP, pulmonary artery systolic pressure; RA, right atrium; RV, right ventricle; STS‐PROM, Society of Thoracic Surgeons predicted risk of mortality; TR, tricuspid regurgitant.
In sensitivity analyses, regression models were rerun with bootstrapping (1000 repetitions); these models identified the same echocardiographic optimal predictors and yielded similar effect sizes for each predictor. The intervariable correlation between the echocardiographic parameters included in the adjusted multivariable models was low (R, 0.02–0.31).
Discussion
Our 2‐center study has shown that the preoperative echocardiogram contributes incremental value to identify high‐risk candidates for SAVR. In particular, small LV cavity size, LV diastolic dysfunction (high E/e’ ratio), and RV dysfunction (high RV MPI) were identified as powerful predictors of in‐hospital mortality and major morbidity after SAVR. Enlarged RA cavity size, small LV cavity size, LV hypertrophy, MR, and low mean aortic gradient <40 mm Hg were predictive of long‐term mortality over a median follow‐up period of 2 years. None of these echocardiographic parameters are currently considered in risk scores for patient selection in AVR.
The magnitude of the incremental value provided by the echocardiogram is important, comparable to other well‐accepted cardiovascular prognostic tests, such as the coronary artery calcium scan in primary prevention (increase in c‐statistic, +0.04; IDI, 1.5%).17, 18 In the current study, as in our previous study of patients undergoing isolated CABG,7 measures of LV diastolic dysfunction and RV dysfunction emerged as the main incremental predictors of postoperative outcomes. Moreover, measures of LV remodeling and low mean aortic gradient emerged as strong predictors of postoperative outcomes in the setting of SAVR, reflecting the deleterious effect of this phenotypic response to chronic pressure overload.
Consistent with our findings, Boldt et al. found that patients with AS often have elevated RV filling pressures indicative of subclinical RV dysfunction,19 and Haddad et al. found that RV MPI assessed by intraoperative transesophageal echocardiography was associated with a higher incidence of circulatory failure after aortic and mitral valve surgery.20 Maslow et al. found that RV FAC was associated with a greater requirement for inotropic or mechanical support and longer length of stay after CABG.21 The RV MPI and FAC are recommended by the Guidelines for the Echocardiographic Assessment of the Right Heart from the American Society for Echocardiography12 and have the advantage of (1) being readily measurable from most routine echocardiography acquisition protocols and (2) being validated by prognostic data. Right atrial (RA) dilation was also shown to be predictive, which is not surprising given that RA size is reflective of the chronicity of underlying RV dysfunction.22
Elevated LV mass is an established predictor of adverse cardiovascular events,23 which has garnered interest in the past 5 years as a risk factor in the setting of SAVR. LV mass reflects the severity and chronicity of the pressure overload caused by AS, as well as adaptive or maladaptive efforts to remodel in response. Our findings are consistent with work from the Cleveland Clinic showing that LV mass was predictive of late mortality,24 whereas concentric geometry and small LV cavity size were predictive of early mortality and prolonged intubation.25 The negative impact of a small LV cavity size is supported by anecdotal accounts of surgeons and cardiologists who recall such patients to be “difficult to manage” and prone to perioperative complications. One advantage of our study is that we used Simpson's biplane method in all patients to measure LV volumes with greater precision than a single linear dimension.10
The constellation of low mean aortic gradient, small LV cavity size, and concentric hypertrophy were identified as predictors of adverse events; these features are associated with the phenotype of paradoxical low‐gradient severe AS, which has received considerable attention.26 This phenotype is often misleading for clinicians because establishing the diagnosis can be challenging and the prognosis falsely reassuring given a heart that is not dilated or hypokinetic (to the contrary, the LV often appears hyperdynamic). However, studies have convincingly shown, and our data reaffirm, that paradoxical low‐gradient AS constitutes a particularly high‐risk group with operative risk of 6% to 33% and medically treated survival <50% at 3 years.27, 28, 29, 30 Patients with paradoxical low‐gradient AS are particularly vulnerable to the deleterious effects of patient‐prosthesis mismatch and, accordingly, may be good candidates for TAVR.
The evidence for (unrepaired) MR as a risk factor for mortality after SAVR, as shown in our Cox multivariable model, is consistent with an analysis from the Johns Hopkins cardiac surgery database showing that moderate MR was an independent predictor of long‐term mortality after SAVR, especially when the etiology was organic and the degree of MR was less likely to improve.31 An analysis from the PARTNER trial similarly showed that significant MR was associated with an HR of 1.77 for 2‐year mortality post‐SAVR.32 Despite the impact of MR on mortality, the optimal management of such patients with moderate MR undergoing SAVR is not clear, given that the operative risk of double‐valve surgery continues to be markedly greater than single‐valve surgery.33, 34
This study had a number of limitations. First, approximately one half of patients were excluded because they did not have a digitally available echocardiogram performed at the study hospitals within the 3‐month time frame. The likelihood of selection bias is low given that included and excluded patients had nearly identical age, sex, and predicted and observed mortality. Second, our findings cannot be directly extrapolated to patients undergoing TAVR given that these patients were not included in our cohort. Third, although this is one of the largest series of SAVR with echocardiographic data, statistical power to assess short‐term mortality remains limited given the rarity of this occurrence. Fourth, although most of our echocardiographic predictors are routinely measured during a standard complete exam (mean aortic gradient, LV mass, MR grade, RA area, and E/e’), some may not be and thus could lengthen the exam interpretation time by a few minutes (LVEDV, RV MPI). Fifth, the results remain to be validated in an external cohort of AVR patients, although it is reassuring to note that the echocardiographic predictors identified in this study are consistent with those identified in a separate cohort of CABG patients.7
In conclusion, the preoperative echocardiogram is readily available and contributes incremental—yet underutilized—prognostic information than can be added to existing risk scores. The growing body of evidence from our studies in CABG and AVR, as well as studies from other groups, suggests that findings of LV diastolic dysfunction and RV function should be incorporated in cardiac surgery risk prediction models. Furthermore, findings of elevated LV mass, small LV cavity size, and low mean aortic gradient reflect the clinical entity of paradoxical low‐gradient AS that confers a high risk of adverse events. Incorporating such parameters will lead to refined estimates of risk, which, in turn, will assist clinicians in guiding patients toward the most appropriate therapeutic pathway.
Sources of Funding
This work was supported by a fellowship award from the Fonds de recherche du Québec en Santé (FRQ‐S), and Dr Afilalo is currently supported by a Clinical Research Scholar award from the FRQ‐S.
Disclosures
Dr Lawrence Rudski owns shares in General Electric.
(J Am Heart Assoc. 2015;4:e002129 doi: 10.1161/JAHA.115.002129)
References
- 1. Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta‐analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. [DOI] [PubMed] [Google Scholar]
- 2. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 3. O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task F . The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg. 2009;88:S23–S42. [DOI] [PubMed] [Google Scholar]
- 4. Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task F . The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3–valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S43–S62. [DOI] [PubMed] [Google Scholar]
- 5. Clark MA, Duhay FG, Thompson AK, Keyes MJ, Svensson LG, Bonow RO, Stockwell BT, Cohen DJ. Clinical and economic outcomes after surgical aortic valve replacement in Medicare patients. Risk Manag Healthc Policy. 2012;5:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; Investigators PT . Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 7. Afilalo J, Flynn AW, Shimony A, Rudski LG, Agnihotri AK, Morin JF, Castrillo C, Shahian DM, Picard MH. Incremental value of the preoperative echocardiogram to predict mortality and major morbidity in coronary artery bypass surgery. Circulation. 2013;127:356–364. [DOI] [PubMed] [Google Scholar]
- 8. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; Initiative S . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 9. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M; American Society of E, European Association of E . Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23; quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing G, American Society of Echocardiography's G, Standards C, European Association of E . Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 11. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 12. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786‐688. [DOI] [PubMed] [Google Scholar]
- 13. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ; American Society of E . Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 14. Shahian DM, Edwards FH, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, O'Brien SM, Shewan CM, Dokholyan RS, Peterson ED; Society of Thoracic Surgeons Quality Measurement Task F . Quality measurement in adult cardiac surgery: part 1—conceptual framework and measure selection. Ann Thorac Surg. 2007;83:S3–S12. [DOI] [PubMed] [Google Scholar]
- 15. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512–5528. [DOI] [PubMed] [Google Scholar]
- 16. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 17. Mohlenkamp S, Lehmann N, Moebus S, Schmermund A, Dragano N, Stang A, Siegrist J, Mann K, Jockel KH, Erbel R; Heinz Nixdorf Recall Study I . Quantification of coronary atherosclerosis and inflammation to predict coronary events and all‐cause mortality. J Am Coll Cardiol. 2011;57:1455–1464. [DOI] [PubMed] [Google Scholar]
- 18. Dupuis JY. Predicting outcomes in cardiac surgery: risk stratification matters? Curr Opin Cardiol. 2008;23:560–567. [DOI] [PubMed] [Google Scholar]
- 19. Boldt J, Zickmann B, Ballesteros M, Dapper F, Hempelmann G. Right ventricular function in patients with aortic stenosis undergoing aortic valve replacement. J Cardiothorac Vasc Anesth. 1992;6:287–291. [DOI] [PubMed] [Google Scholar]
- 20. Haddad F, Denault AY, Couture P, Cartier R, Pellerin M, Levesque S, Lambert J, Tardif JC. Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high‐risk valvular surgery. J Am Soc Echocardiogr. 2007;20:1065–1072. [DOI] [PubMed] [Google Scholar]
- 21. Maslow AD, Regan MM, Panzica P, Heindel S, Mashikian J, Comunale ME. Precardiopulmonary bypass right ventricular function is associated with poor outcome after coronary artery bypass grafting in patients with severe left ventricular systolic dysfunction. Anesth Analg. 2002;95:1507–1518, table of contents. [DOI] [PubMed] [Google Scholar]
- 22. Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation. 2005;112:I212–I218. [DOI] [PubMed] [Google Scholar]
- 23. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 24. Beach JM, Mihaljevic T, Svensson LG, Rajeswaran J, Marwick T, Griffin B, Johnston DR, Sabik JF, Blackstone EH. Coronary artery disease and outcomes of aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol. 2013;61:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duncan AI, Lowe BS, Garcia MJ, Xu M, Gillinov AM, Mihaljevic T, Koch CG. Influence of concentric left ventricular remodeling on early mortality after aortic valve replacement. Ann Thorac Surg. 2008;85:2030–2039. [DOI] [PubMed] [Google Scholar]
- 26. Pibarot P, Dumesnil JG. Low‐flow, low‐gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. [DOI] [PubMed] [Google Scholar]
- 27. Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler‐Klein J, Beanlands RS, Mathieu P, Magne J, Pibarot P. Predictors of outcomes in low‐flow, low‐gradient aortic stenosis: results of the multicenter TOPAS Study. Circulation. 2008;118:S234–S242. [DOI] [PubMed] [Google Scholar]
- 28. Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, Hodge DO, Tajik AJ. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940–1946. [DOI] [PubMed] [Google Scholar]
- 29. Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau T, Petit‐Eisenmann H, Gori M, Jobic Y, Bauer F, Chauvel C, Leguerrier A, Tribouilloy C. Aortic valve replacement for low‐flow/low‐gradient aortic stenosis operative risk stratification and long‐term outcome: a European multicenter study. J Am Coll Cardiol. 2008;51:1466–1472. [DOI] [PubMed] [Google Scholar]
- 30. Clavel M‐A, Berthelot‐Richer M, Le Ven F, Capoulade R, Dahou A, Dumesnil JG, Mathieu P, Pibarot P. Impact of classic and paradoxical low flow on survival after aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol. 2015;65:645–653. [DOI] [PubMed] [Google Scholar]
- 31. Barreiro CJ, Patel ND, Fitton TP, Williams JA, Bonde PN, Chan V, Alejo DE, Gott VL, Baumgartner WA. Aortic valve replacement and concomitant mitral valve regurgitation in the elderly: impact on survival and functional outcome. Circulation. 2005;112:I443–I447. [DOI] [PubMed] [Google Scholar]
- 32. Barbanti M, Webb JG, Hahn RT, Feldman T, Boone RH, Smith CR, Kodali S, Zajarias A, Thompson CR, Green P, Babaliaros V, Makkar RR, Szeto WY, Douglas PS, McAndrew T, Hueter I, Miller DC, Leon MB; Placement of Aortic Transcatheter Valve Trial I . Impact of preoperative moderate/severe mitral regurgitation on 2‐year outcome after transcatheter and surgical aortic valve replacement: insight from the placement of aortic transcatheter valve (PARTNER) trial cohort A. Circulation. 2013;128:2776–2784. [DOI] [PubMed] [Google Scholar]
- 33. Alghamdi AA, Elmistekawy EM, Singh SK, Latter DA. Is concomitant surgery for moderate functional mitral regurgitation indicated during aortic valve replacement for aortic stenosis? A systematic review and evidence‐based recommendations. J Card Surg. 2010;25:182–187. [DOI] [PubMed] [Google Scholar]
- 34. Turina J, Stark T, Seifert B, Turina M. Predictors of the long‐term outcome after combined aortic and mitral valve surgery. Circulation. 1999;100:II48–II53. [DOI] [PubMed] [Google Scholar]


