Abstract
Background
US blood pressure reduction policies are largely restricted to hypertensive populations and associated benefits are often estimated based on unrealistic interventions.
Methods and Results
We used multivariable linear regression to estimate incidence rate differences contrasting the impact of 2 pragmatic hypothetical interventions to reduce coronary heart disease, stroke, and heart failure (HF) incidence: (1) a population‐wide intervention that reduced systolic blood pressure by 1 mm Hg and (2) targeted interventions that reduced the prevalence of unaware, untreated, or uncontrolled blood pressure above goal (per Eighth Joint National Committee treatment thresholds) by 10%. In the Atherosclerosis Risk in Communities Study (n=15 744; 45 to 64 years at baseline, 1987–1989), incident coronary heart disease and stroke were adjudicated by physician panels. Incident HF was defined as the first hospitalization with discharge diagnosis code of “428.” A 10% proportional reduction in unaware, untreated, or uncontrolled blood pressure above goal resulted in ≈4.61, 3.55, and 11.01 fewer HF events per 100 000 person‐years in African Americans, and 3.77, 1.63, and 4.44 fewer HF events per 100 000 person‐years, respectively, in whites. In contrast, a 1 mm Hg population‐wide systolic blood pressure reduction was associated with 20.3 and 13.3 fewer HF events per 100 000 person‐years in African Americans and whites, respectively. Estimated event reductions for coronary heart disease and stroke were smaller than for HF, but followed a similar pattern for both population‐wide and targeted interventions.
Conclusions
Modest population‐wide shifts in systolic blood pressure could have a substantial impact on cardiovascular disease incidence and should be developed in parallel with interventions targeting populations with blood pressure above goal.
Keywords: blood pressure, coronary heart disease, epidemiology, heart failure, stroke
Introduction
Decades of research have characterized the associations between elevated blood pressure and a spectrum of cardiovascular diseases (CVD) including cerebral vascular disease, heart failure (HF), and coronary heart disease (CHD).1, 2, 3, 4, 5 Among blood pressure–related deaths, published estimates suggest that approximately one third of excess CHD and all‐cause mortality can be attributed to elevated systolic blood pressure (SBP) at levels designated as nonhypertensive,6, 7 indicating that benefits achieved from decreases in blood pressure are not limited to populations with hypertension.8, 9, 10, 11, 12 Experimental and observational studies have also demonstrated the efficacy of lifestyle‐based blood pressure interventions, such as increased physical activity and the Dietary Approaches to Stop Hypertension (DASH) diet, for CVD prevention.13, 14, 15, 16, 17 This body of evidence supports the development of population‐wide interventions to reduce blood pressure alongside interventions targeted to populations with blood pressure above goal for CVD reduction.18
Several authors have estimated the theoretical effects of shifting the distribution of blood pressure, either by lowering the mean population blood pressure or by decreasing the proportion of the population classified as hypertensive.8, 11, 19, 20, 21, 22, 23 Framingham Heart Study investigators reported that a 2‐mm Hg population‐wide diastolic blood pressure (DBP) reduction was associated with an estimated 17% decrease in the prevalence of hypertension, and a 6% reduction in the risk of CHD.19 Other published studies examined CHD and stroke events, but were conducted in predominantly white or European populations and considered blood pressure treatment guidelines no longer in use.8, 19, 20, 23 The degree to which modest population‐wide blood pressure shifts may affect the incidence of HF also remains unknown, as do the effects of blood pressure shifts in African American populations,19 who shoulder higher burdens of elevated blood pressure as well as CVD than do white populations.1 Further, the majority of published studies estimated cardiovascular benefits from interventions that completely eliminated hypertension or uncontrolled hypertension from the population, despite the implausibility of such goals.24, 25, 26 Therefore, in a biracial, population‐based setting, we assessed the impact of 2 types of pragmatic, hypothetical interventions on reducing the incidence of CHD, stroke, and HF after full implementation: a population‐wide intervention that reduced SBP by 1 or 2 mm Hg and targeted interventions that achieved a 10% reduction in the proportion of the population with unaware, untreated, or uncontrolled blood pressure above goal.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective, population‐based investigation of the etiology and natural history of CVD and its risk factors.27 From 1987 to 1989, ARIC investigators sampled 15 792 predominately white and African American participants between the ages of 45 and 64 from 4 geographic regions in the United States: Washington County, Maryland; suburban Minneapolis, Minnesota; Forsyth County, North Carolina; and Jackson, Mississippi. The latter 2 communities contributed the majority of African Americans to the cohort. Physical examinations and standardized questionnaires were administered by trained study personnel at baseline and during 4 follow‐up examinations. Cohort follow‐up for identification and classification of health outcomes is ongoing. The ARIC study obtained institutional review board approval from all participating institutions, and informed consent was obtained at each study visit.
The following sequential exclusions were applied: participants who reported races other than African American or white (n=48); participants missing information to classify prevalent CHD, stroke, or HF or with prevalent CHD, stroke, or HF from each respective analysis (CHD [n=1106], stroke [n=315], or HF [n=1035]). Prevalent CHD was defined at baseline by self‐reported history of a physician‐diagnosed myocardial infarction, myocardial infarction identified by electrocardiography, or prior coronary revascularization. Prevalent stroke was defined as a self‐reported history of physician‐diagnosed stroke.28 Prevalent HF was defined by current use of medication prescribed for HF or manifest HF defined by the Gothenburg criteria stage 3.29 After these exclusions, a total of 14 638, 15 429, and 14 709 participants were available for the evaluation of incident CHD, stroke, and HF, respectively. Follow‐up time was calculated from study enrollment to the first CHD, stroke, or HF event, loss to follow‐up, death, or December 31, 2011.
Exposure and Covariate Assessment
Seated blood pressure measurements were taken after a 5‐minute rest using a random‐zero sphygmomanometer; the mean of the second and third readings from the baseline examination was used for analysis. We used the 2014 Guidelines for the Management of High Blood Pressure from the Eighth Joint National Committee (JNC 8) to identify participants with blood pressure above goal: for participants aged ≥60 years, SBP ≥150 mm Hg or a DBP ≥90 mm Hg; for participants aged <60, SBP ≥140 mm Hg or a DBP ≥90 mm Hg.18 All study participants with blood pressure levels below goal were classified as unexposed, irrespective of medication use or history of hypertension, as they were ineligible for interventions targeted to populations with blood pressure above goal (Figure 1). Antihypertensive medication use, race, age, and gender were assessed at study baseline.
Figure 1.
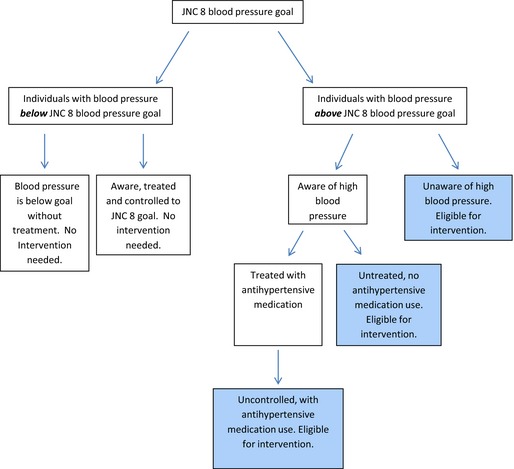
Diagram defining categories of blood pressure above and below the Eighth Joint National Committee (JNC 8) treatment goal. Groups targeted by hypothetical interventions achieving 10% proportional reductions in unaware, untreated, and uncontrolled blood pressure above the JNC 8 treatment goal are shaded blue. JNC 8 indicates, 2014 Evidence‐based Guidelines for the Management of High Blood Pressure in Adults report from the Panel Appointed to the Eighth JNC.
Contemporary race‐specific weighted proportions of unaware, untreated, and uncontrolled blood pressure above goal in whites (n=1494, 11.9% blood pressure above goal: 40% unaware; 14% untreated; 46% uncontrolled) and African Americans (n=1004, 26.4% blood pressure above goal: 20% unaware; 22% untreated; 58% uncontrolled) 45 to 64 years of age were estimated from the National Health and Nutrition Examination Survey (NHANES, 2009–12). Individuals with hypertension who were aware and treated to JNC 8 treatment goals were not included in the intervention group (Figure 1).
Outcome Ascertainment and Definitions
ARIC Study participants were interviewed annually by phone and all hospitalizations and deaths during the preceding year were identified, abstracted, and adjudicated according to study criteria. Local hospital discharge records and vital records were also surveyed to detect hospitalizations and deaths of cohort participants. An incident CHD event was defined as a validated definite or probable hospitalized myocardial infarction, a definite CHD death, an unrecognized myocardial infarction defined by electrocardiographic assessment at study visits, or coronary revascularization.30 An incident stroke event was defined as a first definite or probable hospitalized stroke occurring in a participant free of a baseline history of physician‐diagnosed stroke.28 Incident HF was identified as the first occurrence of either a hospitalization with discharge diagnosis code of 428.X in any position or an I50 or 428 listed on a death certificate.31
Statistical Analysis
To estimate the number of incident CHD, stroke, and HF events per 100 000 person‐years (PY) potentially prevented after a population‐wide 1‐ or 2‐mm Hg SBP reduction, we used a least‐squares linear regression approach32 to estimate race‐specific incidence rate differences (IRD) adjusted for age, gender, and antihypertensive medication use. These models provided estimates of the IRDs for CHD, stroke, or HF associated with a 1 mm Hg decrement in SBP at study baseline potentially achievable after lifestyle interventions were fully implemented16, 17, 33, 34, 35; estimates for a 2‐mm Hg reduction were obtained by multiplying the SBP regression coefficient by 2.
To evaluate interventions targeted to populations with blood pressure above goal (Figure 1) after full implementation, we first estimated race‐, gender‐, and age‐ (in 5‐year increments) specific IRDs using the least‐squares regression approach32 for the association between blood pressure above goal and incident CHD, stroke, or HF. Reduction on the incidence rate after a 10% reduction in unaware, untreated, or uncontrolled blood pressure above goal at study baseline was then estimated in the ARIC study using the following equation: IRDijk×(proportionl−proportionm) where i, j, and k index race, gender, and 5‐year age categories, and proportion is the race‐specific proportion of blood pressure above goal estimated in NHANES36 pre‐ (l subscript) and post (m subscript)‐ intervention that shifted 10% of the proportion of the population with unaware, untreated, or uncontrolled blood pressure above goal to unexposed (ie, below goal blood pressure). Results were presented per 100 000 PY and represented a special case of the population attributable risk that considers partial, rather than complete, elimination of the risk factor. Here, we considered partial elimination of blood pressure above goal, achieved after fully implementing interventions that decreased the proportion of the population with unaware, untreated, or uncontrolled blood pressure by 10%.19 Age‐ and gender‐specific results were then collapsed by race using a case‐load‐weighted summation method,37, 38 and 95% confidence intervals (CIs) were obtained using bootstrapping.39
As a sensitivity analysis, we also estimated the impact of a 10% reduction in the total population with blood pressure above goal. Hypothetical interventions that achieved a 5% and 20% reduction in the proportion of individuals with blood pressure above goal as well as the proportions of individuals with unaware, untreated, and uncontrolled blood pressure above goal were also examined. Contemporary race‐specific population projections for the number of events prevented by the population‐wide and the targeted interventions were calculated by multiplying the race‐specific IRD estimates by the race‐specific total population aged 45 to 64 years without a history of CHD, stroke, or HF, calculated by applying weighted prevalence proportions estimated in NHANES (2009–2012) (African Americans: CHD [n=40, weighted prevalence proportion 4.0%], stroke [n=62, weighted prevalence proportion 6.1%], HF [n=38, weighted prevalence proportion 3.8%]; whites: CHD [n=70, weighted prevalence proportion 3.3%] stroke [n=55, weighted prevalence proportion 2.2%], HF [n=42, weighted prevalence proportion 3.8]) to the 2010 US census population. All statistical analyses were performed with SAS 9.3 software (SAS Institute, Cary, NC) and Stata12 (StataCorp, College Station, TX).
Results
At study baseline, a maximum of 15 744 (27% African American) ARIC cohort members were available for analysis (Table 1). African Americans were more likely to be female and on average to have higher estimated SBP and diastolic blood pressure than white participants. African American individuals were over twice as likely to have blood pressure above goal and to report the use of antihypertensive medications. Although African Americans were slightly younger at baseline, they contributed on average 1.2 years less person time through 2011 than did whites.
Table 1.
Baseline Characteristics of the ARIC Study Cohort (N=15 744) by Race, 1987–1989
| Baseline Characteristics | African Americans (n=4266) | Whites (n=11 478) |
|---|---|---|
| Prevalent CHD, N (%) | 171 (4.1) | 594 (5.3) |
| Prevalent stroke, N (%) | 53 (1.3) | 52 (0.5) |
| Prevalent HF, N (%) | 296 (7.1) | 455 (4.0) |
| Mean follow‐up in y, (SD) | 19.1 (6.5) | 20.3 (5.6) |
| Mean age in y, (SD) | 53.6 (5.8) | 54.4 (5.7) |
| Female, N (%) | 2635 (61.8) | 6050 (52.7) |
| Reported antihypertensive use, N (%) | 1728 (51.5) | 2271 (22.7) |
| Mean systolic blood pressure, mm Hg (SD) | 128.9 (21.6) | 118.5 (17.0) |
| Mean diastolic blood pressure, mm Hg (SD) | 79.7 (12.3) | 71.5 (10.1) |
| Blood pressure categoriesa | ||
| Blood pressure below JNC 8 goal, N (%) | 3031 (71.2) | 10 285 (89.7) |
| Blood pressure above JNC 8 goal, N (%) | 1229 (28.8) | 1186 (10.3) |
| Above JNC 8 goal and unaware | 332 (27.0) | 419 (35.3) |
| Above JNC 8 goal, aware but untreated | 272 (22.1) | 258 (21.8) |
| Above JNC 8 goal, treated, but uncontrolled | 608 (49.5) | 487 (41.1) |
ARIC indicates Atherosclerosis Risk in Communities Study; CHD, coronary heart disease; HF, heart failure; JNC, Joint National Committee.
Blood pressure above goal, blood pressure values that exceed thresholds for management of blood pressure defined by JNC 8; blood pressure below goal, blood pressure values below thresholds for management of blood pressure defined by JNC 8.
Over a mean 20 years of follow‐up, 1803 incident CHD events, 1147 incident stroke events, and 2537 incident HF events were identified (Tables 1 and 2). Age‐adjusted incidence rates for CHD, stroke, and HF were higher among African Americans than whites. The discrepancy by race was especially apparent for HF, where estimated incidence rates were 1193/100 000 PY among African Americans but 786/100 000 PY among whites.
Table 2.
Incident CHD, Stroke, and HF Events and Incidence Rates in the Cohort (N=15 744), by Race, 1987–2011 (ARIC Study)
| Incident Characteristics | African Americans (n=4266) | Whites (n=11 478) | ||
|---|---|---|---|---|
| No. Events | IRa | No. Events | IRa | |
| Incident CHD | 554 | 711 | 1249 | 563 |
| Incident Stroke | 458 | 585 | 689 | 301 |
| Incident HF | 857 | 1193 | 1680 | 786 |
ARIC indicates Atherosclerosis Risk in Communities Study; CHD, coronary heart disease; HF, heart failure; IR, incidence rate; N, events, number of events; n, maximum number of individuals before exclusions.
IR per 100 000 person‐years.
A population‐wide hypothetical intervention that achieved an overall 1 mm Hg decrement in SBP at study baseline after full implementation yielded a greater estimated number of preventable events per 100 000 PY for HF (Figure 2A, Table 3), than for CHD and stroke. Notably, the estimated benefits from modest population‐wide decrements in SBP were consistently greater for African Americans than for whites; the greatest difference by race was observed for incident HF, for which a 1 mm Hg population‐wide decrement in SBP prevented 7.0 additional events per 100 000 PY in African Americans when compared to whites. If applied nationwide, a hypothetical 1 mm Hg shift in SBP among African American and white US populations aged 45 to 64 years was estimated to prevent ≈9338 incident HF events, 6210 incident CHD events, and 3761 incident stroke events annually (Table 4). As expected, the hypothetical intervention achieving the larger SBP reduction of 2 mm Hg was associated with larger reductions in the incidence of CHD, stroke, and HF for both racial groups (Figure 2B, Tables 3 and 4).
Figure 2.
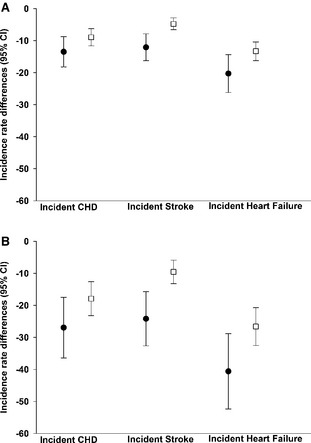
Incidence rate differences in CHD, stroke, and HF per 100 000 person‐years associated with a 1 mm Hg (A) and a 2 mm Hg (B) reduction in SBP in African Americans (black circles) and whites (white squares), the ARIC cohort study, 1987–2011. A, Population‐wide 1 mm Hg reduction in systolic blood pressure. B, Population‐wide 2 mm Hg reduction in systolic blood pressure. ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; HF, heart failure; SBP, systolic blood pressure.
Table 3.
Estimated Events Reduced for CHD, Stroke, and HF From 2 Population‐Wide Hypothetical Blood Pressure Reduction Interventions, by Race (N=15 744), 1987–1989, the ARIC Study
| Outcome | African Americans | Whites | ||||
|---|---|---|---|---|---|---|
| No. Events | Events Reduced by Population‐Wide Intervention (95% CI) | No. Events | Events Reduced by Population‐Wide Intervention (95% CI) | |||
| 1‐mm Hg Decreasea | 2‐mm Hg Decreasea | 1‐mm Hg Decreasea | 2‐mm Hg Decreasea | |||
| CHD | 554 | 13.5 (8.8–18.2) | 27.0 (17.5–36.4) | 1249 | 9.0 (6.3–11.6) | 17.9 (12.6–23.2) |
| Stroke | 458 | 12.1 (7.9–16.3) | 24.2 (15.8–32.6) | 689 | 4.8 (2.9–6.6) | 9.6 (5.9–13.3) |
| HF | 857 | 20.3 (14.4–26.2) | 40.6 (28.8–52.4) | 1680 | 13.3 (10.4–16.3) | 26.6 (20.7–32.5) |
ARIC indicates Atherosclerosis Risk in Communities Study; CHD, coronary heart disease; HF, heart failure.
Per 100 000 person‐years.
Table 4.
Estimated Events Reduced Annually for CHD, Stroke, and HF From 2 Population‐Wide Hypothetical Blood Pressure Reduction Interventions, by Race, 2010 US Census Population Aged 45 to 64
| Outcome | African Americans | Whites | ||||
|---|---|---|---|---|---|---|
| Population (N)a | Population‐Wide Intervention | Population (N)a | Population‐Wide Intervention | |||
| 1‐mm Hg Decrease | 2‐mm Hg Decrease | 1‐mm Hg Decrease | 2‐mm Hg Decrease | |||
| CHD | 8 701 219 | 1175 | 2349 | 55 946 031 | 5035 | 10 070 |
| Stroke | 8 501 707 | 1029 | 2057 | 56 575 526 | 2733 | 5465 |
| HF | 8 700 428 | 1766 | 3532 | 56 930 452 | 7572 | 15 144 |
CHD indicates coronary heart disease; HF, heart failure.
Total 2010 US census race specific populations aged 45 to 64 (African Americans n=9 042 518, whites n=57 864 260) were used to calculate the population with prevalent CHD, stroke or HF excluded, respectively, using weighted prevalence proportions for history of CHD, stroke, and HF by above‐goal blood pressure.
We then contrasted the hypothetical population‐wide SBP reduction with interventions targeted to populations with blood pressure above goal that achieved a 10% proportional reduction in unaware, untreated, or uncontrolled blood pressure. For example, before intervention, 26.4% of African Americans and 11.9% of whites 45 to 64 years of age were classified as having blood pressure above goal (NHANES 2009–2012; Table 5); we therefore evaluated a targeted intervention that achieved a 10% proportional decrease in unaware blood pressure above goal (ie, 20% to 18% among African Americans and 40% to 36% among whites, respectively) resulting in post‐intervention proportions of blood pressure above goal of 25.84% for African Americans and 11.41% for whites (Table 5). Similar to results from the population‐wide SBP interventions, 10% proportional reductions in unaware, untreated, or uncontrolled blood pressure above goal produced the largest reduction in events for HF, the magnitude of which varied by race (Table 6, Figure 3). Specifically, a 10% proportional reduction in unaware, untreated, or uncontrolled blood pressure above goal at study baseline resulted in ≈4.61 (95% CI: 2.50–7.36), 3.55 (95% CI: 1.79–5.73), and 11.01 (95% CI: 6.17–17.52) fewer HF events per 100 000 PY, respectively, in African Americans and 3.77 (95% CI: 1.88–6.51), 1.63 (95% CI: 0.65–3.04), and 4.44 (95% CI: 2.49–6.81) fewer HF events per 100 000 PY, respectively, in whites (Table 6, Figure 3). If 10% proportional reductions in unaware, untreated, or uncontrolled blood pressure above goal were achieved nationwide in African Americans and white populations aged 45 to 64, ≈2200, 1017, and 3164 fewer incident HF events, respectively, could be prevented annually; ≈1390, 650, and 2016 incident CHD and 962, 497, and 1505 stroke events would be prevented annually given 10% proportional reductions in unaware, untreated, or uncontrolled blood pressure above goal, respectively (Table 7).
Table 5.
Proportion of African American and White NHANES Participants Aged 45 to 64 Years With Blood Pressure Above JNC 8 Goal Before and After Targeted Interventions Improving Unaware, Untreated, and Uncontrolled Blood Pressure by 10%, 2009–2012
| Race | Proportion of Participants With Blood Pressure Above Goal | |||
|---|---|---|---|---|
| Before Intervention | After 10% Reduction in Proportion of Unaware Blood Pressure Above Goal | After 10% Reduction in Proportion of Untreated Blood Pressure Above Goal | After 10% Reduction in Proportion of Uncontrolled Blood Pressure Above Goal | |
| African Americans | 26.36 | 25.84 | 25.78 | 24.82 |
| Whites | 11.88 | 11.41 | 11.71 | 11.33 |
JNC indicates Joint National Committee; NHANES, National Health and Nutrition Examination Survey.
Table 6.
Estimated Events Reduced for CHD, Stroke, and HF From a Hypothetical Intervention That Achieves a 5%, 10%, or 20% Reduction of Unaware, Untreated, or Uncontrolled BP Above JNC 8 Goals, by Race (N=15 744), 1987–2011, ARIC Study
| Event Type | BP Category | Events Reduceda Per 100 000 PYs (95% CI) by Interventions Achieving Reductions in BP Above Goal | |||||
|---|---|---|---|---|---|---|---|
| 5% Proportional Reduction | 10% Proportional Reduction | 20% Proportional Reduction | |||||
| African American | White | African American | White | African American | White | ||
| Hypothetical intervention–reduce unaware BP (above JNC 8 treatment threshold) | |||||||
| CHD | BP above JNC 8 goal | 1.55 (0.66–2.58) | 1.05 (0.40–1.78) | 3.10 (1.31–5.15) | 2.09 (0.81–3.57) | 6.21 (2.63–10.31) | 4.19 (1.62–7.13) |
| Stroke | BP above JNC 8 goal | 1.48 (0.73–2.37) | 0.73 (0.32–1.27) | 2.95 (1.46–4.75) | 1.47 (0.64–2.55) | 5.95 (2.92–9.50) | 2.93 (1.27–5.09) |
| HF | BP above JNC 8 goal | 2.30 (1.25–3.68) | 1.89 (0.94–3.25) | 4.61 (2.50–7.36) | 3.77 (1.88–6.51) | 9.21 (5.00–14.72) | 7.54 (3.76–13.01) |
| Hypothetical intervention–reduce untreated BP (above JNC 8 treatment threshold) | |||||||
| CHD | BP above JNC 8 goal | 1.26 (0.55–2.13) | 0.41 (0.13–0.74) | 2.52 (1.10–4.25) | 0.81 (0.27–1.48) | 5.04 (2.19–8.51) | 1.63 (0.53–2.97) |
| Stroke | BP above JNC 8 goal | 1.27 (0.49–2.26) | 0.29 (0.10–0.56) | 2.53 (0.98–4.53) | 0.57 (0.20–1.12) | 5.07 (1.95–9.06) | 1.14 (0.40–2.24) |
| HF | BP above JNC 8 goal | 1.78 (0.89–2.87) | 0.81 (0.32–1.52) | 3.55 (1.79–5.73) | 1.63 (0.65–3.04) | 7.10 (3.57–11.47) | 3.26 (1.29–6.08) |
| Hypothetical intervention–reduce uncontrolled BP (above JNC 8 treatment threshold) | |||||||
| CHD | BP above JNC 8 goal | 3.61 (1.54–5.79) | 1.44 (0.55–2.57) | 7.22 (3.08–11.57) | 2.87 (1.11–5.15) | 14.44 (6.17–23.14) | 5.75 (2.22–10.30) |
| Stroke | BP above JNC 8 goal | 3.52 (1.72–5.65) | 0.98 (0.33–2.02) | 7.04 (3.44–11.31) | 1.95 (0.65–4.05) | 14.08 (6.87–22.62) | 3.90 (1.30–8.10) |
| HF | BP above JNC 8 goal | 5.51 (3.09–8.76) | 2.22 (1.25–3.40) | 11.01 (6.17–17.52) | 4.44 (2.49–6.81) | 22.02 (12.34–35.04) | 8.87 (4.99–13.62) |
ARIC indicates Atherosclerosis Risk in Communities Study; BP, blood pressure; CHD, coronary heart disease; HF, heart failure; IRD, incidence rate differences; JNC 8, the 2014 Guidelines for the Management of High Blood Pressure from the Eighth Joint National Committee.
Events reduced calculated as IRDijk×(proportionl−proportionm) where i, j, and k index race, gender, and 5‐year age categories, proportion is the race‐specific proportion of BP above goal36 pre‐ (l subscript) and post (m subscript)‐ intervention, per 100 000 person years.
Figure 3.
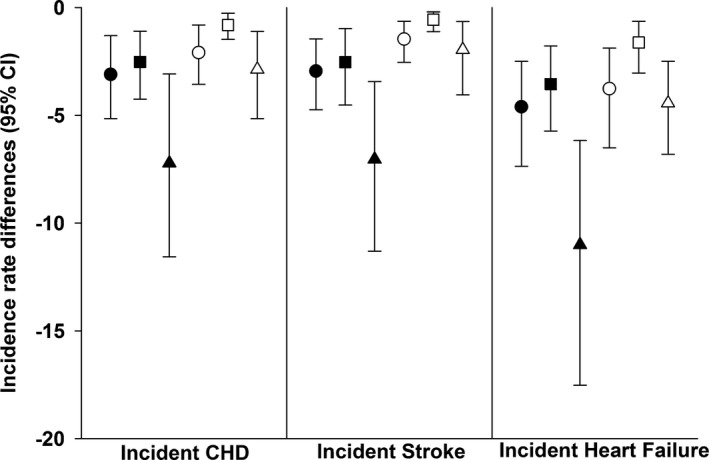
IRDs for CHD, stroke and HF per 100 000 person‐years associated with 10% proportional reductions in unaware (circle), untreated (squares), or uncontrolled (triangles) blood pressure above JNC 8 treatment goal in African American (black symbols) and white (white symbols) ARIC participants, 1987–2011. ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; HF, heart failure; IRDs, incidence rate differences; JNC, Joint National Committee.
Table 7.
Estimated Events Reduced for CHD, Stroke, and HF From Hypothetical Targeted BP Reduction Interventions, by Race, 2010 US Census Population Aged 45 to 64
| Event Type | BP Category | Events Reduced Annually by Interventions Achieving Reductions in BP Above Goal | |||
|---|---|---|---|---|---|
| Population (N) | 10% Proportional Reduction | ||||
| African Americana | Whitea | African American | White | ||
| Hypothetical intervention to reduce unaware BP above goal | |||||
| CHD | BP above JNC 8 goal | 8 701 219 | 55 946 031 | 211 | 1169 |
| Stroke | BP above JNC 8 goal | 8 501 707 | 56 575 526 | 213 | 749 |
| HF | BP above JNC 8 goal | 8 700 428 | 56 930 452 | 335 | 1865 |
| Hypothetical intervention to reduce untreated BP above goal | |||||
| CHD | BP above JNC 8 goal | 8 701 219 | 55 946 031 | 246 | 404 |
| Stroke | BP above JNC 8 goal | 8 501 707 | 56 575 526 | 238 | 259 |
| HF | BP above JNC 8 goal | 8 700 428 | 56 930 452 | 373 | 644 |
| Hypothetical intervention to reduce uncontrolled BP above goal | |||||
| CHD | BP above JNC 8 goal | 8 701 219 | 55 946 031 | 654 | 1362 |
| Stroke | BP above JNC 8 goal | 8 501 707 | 56 575 526 | 632 | 873 |
| HF | BP above JNC 8 goal | 8 700 428 | 56 930 452 | 991 | 2173 |
| Hypothetical intervention to reduce total population with BP above goal | |||||
| CHD | BP above JNC 8 goal | 8 701 219 | 55 946 031 | 1121 | 2934 |
| Stroke | BP above JNC 8 goal | 8 501 707 | 56 575 526 | 1083 | 1880 |
| HF | BP above JNC 8 goal | 8 700 428 | 56 930 452 | 1699 | 4682 |
BP indicates blood pressure; CHD, coronary heart disease; HF, heart failure; JNC, Joint National Committee.
Total 2010 US census race‐specific populations aged 45 to 64 (African Americans n=9 042 518, whites n=57 864 260) were used to calculate the population with prevalent CHD, stroke, or HF excluded, respectively, using weighted prevalence proportions for history of CHD, stroke, and HF by above‐goal BP.
Sensitivity analyses examining 5% to 20% proportional reductions in the total population with blood pressure above goal target a larger segment of the population with blood pressure above goal and demonstrated considerably larger reductions in CHD, stroke, and HF events than interventions targeting unaware, untreated, or uncontrolled blood pressure above JNC 8 goals (Tables 7 and 8). Heterogeneity by race for interventions achieving reductions in the total population with blood pressure above goal was also observed, with event reductions for African Americans approximately twice as large as event reductions for whites.
Table 8.
Estimated Events Reduced for CHD, Stroke, and HF From a Hypothetical Intervention That Achieves a 5%, 10%, or 20% Reduction in BP Above JNC 8 Goals, by Race (N=15 744), 1987–2011, ARIC Study
| Event Type | BP Category | Events Reduceda Per 100 000 PYs (95% CI) by Interventions Achieving Reductions in BP Above Goal | |||||
|---|---|---|---|---|---|---|---|
| 5% Proportional Reduction | 10% Proportional Reduction | 20% Proportional Reduction | |||||
| African American | White | African American | White | African American | White | ||
| CHD | BP above JNC 8 goal | 6.42 (2.77–9.83) | 2.89 (1.13–4.93) | 12.84 (5.53–19.67) | 5.78 (2.26–9.86) | 25.68 (11.06–39.33) | 11.56 (4.52–19.73) |
| Stroke | BP above JNC 8 goal | 6.26 (3.09–9.75) | 1.99 (0.80–3.77) | 12.53 (6.19–19.49) | 3.99 (1.60–7.55) | 25.05 (12.37–38.98) | 7.98 (3.19–15.09) |
| HF | BP above JNC 8 goal | 9.58 (5.64–14.36) | 4.92 (2.63–7.77) | 19.17 (11.29–28.72) | 9.84 (5.27–15.55) | 38.33 (22.58–57.44) | 19.67 (10.54–31.09) |
ARIC indicates Atherosclerosis Risk in Communities Study; BP, blood pressure; CHD, coronary heart disease; HF, heart failure; IRD, incidence rate differences; JNC 8, the 2014 Guidelines for the Management of High Blood Pressure from the Eighth Joint National Committee; PY, Person years.
Events reduced calculated Person years as IRDijk×(proportionl−proportionm) where i, j, and k index race, gender, and 5‐year age categories, proportion is the race‐specific proportion of BP above goal36 pre‐ (l subscript) and post (m subscript)‐ intervention, per 100 000.
Discussion
Predicted benefits from blood pressure reductions estimated in a biracial, population‐based cohort showed that a modest population‐wide 1–mm Hg decrement in SBP could prevent substantial numbers of cardiovascular events. Interventions targeted to the population with blood pressure above goal that achieved a 10% proportional reduction in unaware, untreated, or uncontrolled blood pressure also achieved meaningful cardiovascular event reductions, but were of smaller magnitude. Of the 3 CVD outcomes investigated, hypothetical interventions that decreased SBP or blood pressure above goal had the greatest estimated impact on reducing HF incidence. With both intervention types, the estimated benefits of lowering blood pressure on CVD events were greater for African Americans compared to whites.
Decades of research have demonstrated that the medical management of blood pressure among hypertensive populations is effective in reducing the risk of CVD and addresses the medical responsibility to lower the risk of clinical events among those at highest risk.7, 40, 41, 42 Accordingly, clinical and public health efforts to improve hypertension awareness, treatment, and control have translated into cardioprotective benefits, as shown between 1998 and 2008 when the mean SBP for US adults with hypertension declined by ≈10 mm Hg.40 However, declines in SBP were largely limited to hypertensive populations, as the average SBP for the entire US population changed little over the same time span.40
The large cardioprotective benefits associated with the medical management of blood pressure led earlier studies to evaluate the hypothetical impact of 100% elimination of hypertension on the incidence of CVD through metrics like the population‐attributable fraction.24, 26 For reference, our results suggested that if 100% of aware and treated blood pressure above goal was controlled among African American and whites aged 45 to 64 years of age, ≈20 154 CHD events could be prevented annually in the United States, consistent with recently published estimates using the JNC 8 guidelines.24 Despite considerable clinical and public health efforts to improve hypertension management, no historical precedent is available for achieving 100% control; instead, nationally representative estimates suggested that ≈43.5% of adults with treatment‐eligible hypertension are not treated to goal.43 As an alternative, we estimated event reductions achieved by modest, but plausible decreases in the proportion of the population classified as having unaware, untreated, and uncontrolled blood pressure above goal. As expected, full implementation of interventions that achieved 10% changes in unaware, untreated, and uncontrolled blood pressure above goal were estimated to prevent considerably fewer CVD events than fully implemented interventions assuming 100% elimination of blood pressure above goal. This discrepancy reflects the proportion of the population for whom we assume a successful intervention. For example, in a population of 100 000 (prevalence of blood pressure above goal 20%; composed of 40% unaware, 14% aware but untreated, and 46% aware treated but uncontrolled), a successful intervention that reduced uncontrolled blood pressure above goal by 10% would target ≈920 people compared to the substantially larger population of 20 000 people targeted by an intervention assuming 100% elimination of blood pressure above goal.
Targeted interventions and population‐wide interventions are complementary, and it is admittedly difficult to compare the 2 since the clinical and public health prevention and control strategies, their associated costs, and target populations differ considerably. Targeted efforts to improve the management of hypertension have resulted in relatively low levels of unaware, untreated, and uncontrolled hypertension, particularly after the realignment introduced by the 2014 JNC 8 guideline for the management of high blood pressure.40, 43 Interventions targeted to populations with blood pressure above goal will therefore become less effective in lowering CVD incidence as blood pressure awareness, treatment, and control continue to improve, limiting the target population.40 Conversely, population‐wide lifestyle approaches to blood pressure reduction, as encouraged by the 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk, have been successful in reducing CVD across a wide range of blood pressures.15, 16, 44 For example, prior population‐wide intervention studies of salt reduction demonstrated decreases in morbidity, mortality, and healthcare costs with as little as 1 g less of salt per day (equivalent to 1/6 of a teaspoon)15 and are feasible, as evidenced by recent dietary salt reduction programs in the United Kingdom focused on manufactured food that decreased mean sodium content in the food supply by 7%.13 Other potential lifestyle interventions to reduce blood pressure include increasing physical activity,45, 46 reducing weight,47 and improving overall diet quality.46 Further studies are needed to inform safety48, 49 and selection of the most cost‐effective population‐wide interventions in the United States to decrease SBP.
Our results highlight the potential of population‐wide SBP reduction approaches to complement interventions targeted to clinically defined populations with blood pressure above goal. Interventions targeting the US white populations with uncontrolled blood pressure would need to achieve approximately a 30% proportional reduction in uncontrolled blood pressure in order to achieve event reduction comparable to those estimated for population‐wide interventions that achieve a 1‐mm Hg shift in SBP. African American populations, among whom blood pressure elevation occurs at earlier ages and show elevated rates of CVD in the United States,1, 40 likely would reap the greatest benefits of population‐wide and targeted above goal blood pressure interventions, particularly for uncontrolled blood pressure, suggesting that both types of interventions have potential for reducing these persistent health disparities.
The strengths of this study include the use of a large, biracial cohort with high retention and quality assurance protocols over an average of 20 years of follow‐up. There also are several limitations that deserve consideration. First, the ARIC cohort may not be fully generalizable to the US population, particularly for African Americans participants, who were primarily recruited from Jackson, Mississippi and Forsyth County, North Carolina; other US minority groups were not represented in this study. The ARIC study was also restricted to participants aged 45 to 64 years at study baseline. Additionally, several studies suggest that the national incidence of CVD events has changed since the 1980s, with the incidence of CHD and stroke declining and the incidence of HF increasing,1, 50, 51 which may over‐ or underestimate the number of events reduced by population‐wide and targeted blood pressure interventions. Secondly, we assumed the same incidence rate reduction when calculating the number of events that could be prevented from interventions that targeted unaware, untreated, and uncontrolled blood pressure above goal. We also estimated separate intervention effects, for unaware, untreated, and uncontrolled blood pressure above goal, although in practice, these interventions would likely be promoted in combination and associated with target‐specific IRDs. Third, if future guidelines, following the results of the Systolic Blood Pressure Intervention Trial (SPRINT), reduce the thresholds for treatment or goal for blood pressure control, interventions that reduce untreated or uncontrolled blood pressure could result in substantially larger reductions in events.52 Despite these limitations, few studies have evaluated pragmatic blood pressure interventions and even fewer have included African Americans, who shoulder historic blood pressure and CVD disparities, positioning this study to contribute increased understanding of the population management of elevated blood pressure.
In conclusion, population‐wide reductions in SBP of modest magnitude are predicted to have a substantial impact on CVD prevention and should be developed in parallel with interventions targeted to populations with blood pressure above goal. As advocated by many primary prevention statements,33, 53, 54 the management of blood pressure in adults should not be limited to those classified as hypertensive, but rather extend to the majority of the populations below hypertension treatment thresholds. Omitting such individuals leaves a large segment of the population at increased risk of cardiovascular events and constrains the ability of clinicians and public health practitioners to reduce the societal burden of blood pressure–related CHD, stroke, and HF.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Avery was partially supported by R00HL098458.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc. 2015;4:e002276 doi: 10.1161/JAHA.115.002276)
References
- 1. Roger VL, Go AS, Lloyd‐Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie‐Rosett J. Heart Disease and Stroke Statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kannel WB, Wolf PA, Verter J, McNamara PM. Epidemiologic assessment of the role of blood pressure in stroke: the Framingham study. JAMA. 1996;276:1269–1278. [PubMed] [Google Scholar]
- 3. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. [DOI] [PubMed] [Google Scholar]
- 4. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end‐stage renal disease in men. N Engl J Med. 1996;334:13–18. [DOI] [PubMed] [Google Scholar]
- 5. Five‐year findings of the hypertension detection and follow‐up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow‐up Program Cooperative Group. JAMA. 1979;242:2562–2571. [PubMed] [Google Scholar]
- 6. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- 7. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ; Committee tNHBPEPC . Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 8. Emberson J, Whincup P, Morris R, Walker M, Ebrahim S. Evaluating the impact of population and high‐risk strategies for the primary prevention of cardiovascular disease. Eur Heart J. 2004;25:484–491. [DOI] [PubMed] [Google Scholar]
- 9. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. [DOI] [PubMed] [Google Scholar]
- 10. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 11. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 12. Mason PJ, Manson JE, Sesso HD, Albert CM, Chown MJ, Cook NR, Greenland P, Ridker PM, Glynn RJ. Blood pressure and risk of secondary cardiovascular events in women: the Women's Antioxidant Cardiovascular Study (WACS). Circulation. 2004;109:1623–1629. [DOI] [PubMed] [Google Scholar]
- 13. Eyles H, Webster J, Jebb S, Capelin C, Neal B, Ni Mhurchu C. Impact of the UK voluntary sodium reduction targets on the sodium content of processed foods from 2006 to 2011: analysis of household consumer panel data. Prev Med. 2013;57:555–560. [DOI] [PubMed] [Google Scholar]
- 14. Arroll B, Beaglehole R. Does physical activity lower blood pressure: a critical review of the clinical trials. J Clin Epidemiol. 1992;45:439–447. [DOI] [PubMed] [Google Scholar]
- 15. Bibbins‐Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, Williams B, Ford GA. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–233. [DOI] [PubMed] [Google Scholar]
- 17. He FJ, Pombo‐Rodrigues S, MacGregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open. 2014;4:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr. , Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr., Narva AS, Ortiz E. 2014 Evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 19. Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. [PubMed] [Google Scholar]
- 20. Erlinger TP, Vollmer WM, Svetkey LP, Appel LJ. The potential impact of nonpharmacologic population‐wide blood pressure reduction on coronary heart disease events: pronounced benefits in African‐Americans and hypertensives. Prev Med. 2003;37:327–333. [DOI] [PubMed] [Google Scholar]
- 21. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin P‐H, Karanja N, Simons‐Morton D, McCullough M, Swain J, Steele P, Evans MA, Miller ER, Harsha DW. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 22. Laaser U, Breckenkamp J, Ullrich A, Hoffmann B. Can a decline in the population means of cardiovascular risk factors reduce the number of people at risk? J Epidemiol Community Health. 2001;55:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooney M‐T, Dudina A, Whincup P, Capewell S, Menotti A, Jousilahti P, Njølstad I, Oganov R, Thomsen T, Tverdal A, Wedel H, Wilhelmsen L, Graham I; Investigators S . Re‐evaluating the Rose approach: comparative benefits of the population and high‐risk preventive strategies. Eur J Cardiovasc Prev Rehabil. 2009;16:541–549. [DOI] [PubMed] [Google Scholar]
- 24. Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, Guzman D, Williams L, Bibbins‐Domingo K, Coxson PG, Goldman L. Cost‐effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med. 2015;372:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willey JZ, Moon YP, Kahn E, Rodriguez CJ, Rundek T, Cheung K, Sacco RL, Elkind MS. Population attributable risks of hypertension and diabetes for cardiovascular disease and stroke in the northern Manhattan study. J Am Heart Assoc. 2014;3:e001106 doi: 10.1161/JAHA.114.001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez VA, Franklin SS, Tang S, Wong ND. Coronary heart disease events preventable by control of blood pressure and lipids in US adults with hypertension. J Clin Hypertens. 2007;9:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szklo M, Barnes R, Folsom A, Heiss G, Hutchinson R, Patsch W, Sharrett AR, Williams OD. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 28. Rosamond WD, Folsom AR, Chambless LE, Wang C‐H, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 29. Eriksson H, Caidaul K, Larsson B, Ohlson L‐O, Welin L, Wilhelmsen L, Svärdsudd K. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical‐epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. [DOI] [PubMed] [Google Scholar]
- 30. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA, The AI. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 31. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 32. Xu Y, Cheung YB, Lam KF, Tan SH, Milligan P. A simple approach to the estimation of incidence rate difference. Am J Epidemiol. 2010;172:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee I‐M, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith JSC, Svetkey LP, Wadden TA, Yanovski SZ. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;63. [DOI] [PubMed] [Google Scholar]
- 34. Jafar TH, Islam M, Hatcher J, Hashmi S, Bux R, Khan A, Poulter N, Badruddin S, Chaturvedi N. Community based lifestyle intervention for blood pressure reduction in children and young adults in developing country: cluster randomised controlled trial. BMJ. 2010;340:c2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou B, Wang HL, Wang WL, Wu XM, Fu LY, Shi JP. Long‐term effects of salt substitution on blood pressure in a rural North Chinese population. J Hum Hypertens. 2013;27:427–433. [DOI] [PubMed] [Google Scholar]
- 36. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012 NCHS Data Brief, no 133. Hyattsville, MD: National Center for Health Statistics; 2013. Available at: http://www.cdc.gov/nchs/data/databriefs/db133.htm. Accessed October 22, 2015. [PubMed] [Google Scholar]
- 37. Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Deswal A, Folsom AR, Heiss G. The potentially modifiable burden of incident heart failure due to obesity: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2010;172:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morgenstern H, Bursic E. A method for using epidemiologic data to estimate the potential impact of an intervention on the health status of a target population. J Community Health. 1982;7:292–309. [DOI] [PubMed] [Google Scholar]
- 39. Greenland S. Interval estimation by simulation as an alternative to and extension of confidence intervals. Int J Epidemiol. 2004;33:1389–1397. [DOI] [PubMed] [Google Scholar]
- 40. Egan BM, Zhao Y, Axon R. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 41. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the systolic hypertension in the elderly program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 42. Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’ Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 43. Navar‐Boggan A, Pencina MJ, Williams K, Sniderman AD, Peterson ED. Proportion of US adults potentially affected by the 2014 hypertension guideline. JAMA. 2014;311:1424–1429. [DOI] [PubMed] [Google Scholar]
- 44. He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 45. Laine J, Kuvaja‐Kollner V, Pietila E, Koivuneva M, Valtonen H, Kankaanpaa E. Cost‐effectiveness of population‐level physical activity interventions: a systematic review. Am J Health Promot. 2014;29:71–80. [DOI] [PubMed] [Google Scholar]
- 46. Mozaffarian D, Afshin A, Benowitz NL, Bittner V, Daniels SR, Franch HA, Jacobs DR, Kraus WE, Kris‐Etherton PM, Krummel DA, Popkin BM, Whitsel LP, Zakai NA. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126:1514–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kumanyika SK, Obarzanek E, Stettler N, Bell R, Field AE, Fortmann SP, Franklin BA, Gillman MW, Lewis CE, Poston WC, Stevens J, Hong Y. Population‐based prevention of obesity: the need for comprehensive promotion of healthful eating, physical activity, and energy balance: a scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science). Circulation. 2008;118:428–464. [DOI] [PubMed] [Google Scholar]
- 48. Messerli FH, Panjrath GS. The J‐curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54:1827–1834. [DOI] [PubMed] [Google Scholar]
- 49. O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez‐Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. [DOI] [PubMed] [Google Scholar]
- 50. Ford ES, Roger VL, Dunlay SM, Go AS, Rosamond WD. Challenges of ascertaining national trends in the incidence of coronary heart disease in the United States. J Am Heart Assoc. 2014;3:e001097 doi: 10.1161/JAHA.114.001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koton S, Schneider AC, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–268. [DOI] [PubMed] [Google Scholar]
- 52. National Institutes of Health the National Heart, Lung, and Blood Institute . Landmark NIH study shows intensive blood pressure management may save lives. 2015. Available at: http://www.nih.gov/news/health/sep2015/nhlbi-11.htm. Accessed October 22, 2015.
- 53. Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. [DOI] [PubMed] [Google Scholar]
- 54. Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. [DOI] [PubMed] [Google Scholar]


