Abstract
Background
There are few data relating novel measures of left ventricular (LV) mechanical function to cardiovascular disease (CVD) outcomes in the community. Whether distinct components of LV mechanical function provide information regarding risk for different CVD outcomes is unclear.
Methods and Results
We used speckle tracking echocardiography to quantify distinct components of LV mechanical function (measured as LV strain in multiple planes) in 2831 Framingham Offspring Study participants (mean age, 66 years; 57% women, 97% with LV fractional shortening >0.29). Participants were followed for 6.0±1.2 years for onset of 69 coronary heart disease (CHD), 71 heart failure (HF), and 199 mortality events. Adjusting for CVD risk factors, longitudinal LV strain appeared associated with incident CHD (hazards ratio [HR] per SD increment, 1.29; 95% confidence interval [CI], 1.00–1.67; P=0.05), whereas circumferential and radial strain were not (P>0.37 for both); however, the association of longitudinal strain with CHD was nonsignificant after Bonferroni correction. By contrast, circumferential strain was a significant predictor of incident HF (HR per SD increment, 1.79; 95% CI, 1.35–2.37; P<0.0001). Decrements in circumferential, radial, and longitudinal strain measures were related to all‐cause mortality (P<0.008 for all). Results remained similar in multivariable models adjusting additionally for the conventional echocardiographic measures of LV mass and fractional shortening.
Conclusions
In our large, community‐based sample, distinct components of LV mechanical function were associated with specific CVD outcomes. Additional studies are needed to replicate these findings and investigate the prognostic and therapeutic utility of these novel measures of LV mechanical function.
Keywords: cardiovascular disease, left ventricular strain, outcomes
Introduction
In the general population, asymptomatic left ventricular (LV) systolic dysfunction, conventionally defined by a low ejection fraction (EF), is associated with adverse outcomes, including heart failure (HF) and all‐cause mortality.1 Although EF is the predominant method for assessing LV systolic function, it has several well‐known limitations with respect to characterizing subclinical disease. As a single value, EF represents global pump function and may remain normal early in the course of the myocardial disease processes, even in the presence of regional impairment of LV function. Importantly, it is well known that EF can remain preserved even in the setting of overt clinical HF. Given the need for more‐sensitive measures of LV performance, advances in image analysis have led to noninvasive methods for assessing cardiac mechanical function in multiple planes.2, 3 Numerous studies have now demonstrated that speckle tracking analyses of LV strain (ie, myocardial tissue deformation) applied to routine echocardiography offer significant incremental prognostic information over EF in patients with known cardiovascular disease (CVD).4, 5, 6, 7, 8, 9 However, there are few studies of LV strain and outcomes in community‐dwelling individuals free of overt CVD.10, 11 Furthermore, the degree to which distinct components of LV deformation in various planes provide specific prognostic information regarding risk of individual CVD outcomes (eg, coronary disease vs. HF) remains unclear.12, 13
Echocardiographic speckle tracking analysis offers a detailed characterization of LV deformation in systole—including the extent to which the LV length shortens in the long axis (longitudinal strain), the extent to which the LV cavity circumference decreases in the short axis (circumferential strain), and the extent to which the LV walls thicken (transverse and radial strains).14, 15 The different LV strain components (eg, longitudinal vs. circumferential) reflect myofiber function in distinct orientations and at different layers of the ventricle (eg, subendocardium vs. mesomyocardium). Because marked alterations in a given LV strain measure can reflect changes in myocardial tissue function resulting from a certain type of myocardial stress, including chronic ischemia (which preferentially affects the subendocardium) and afterload stress (which preferentially affects the mesomyocardium),16, 17, 18, 19 a given LV strain measure may tend to be associated with a different CVD outcome (eg, ischemic vs. pump failure event) in the general population. To test this hypothesis, we comprehensively characterized LV mechanical strain components and prospectively evaluated the extent to which baseline measures of these components had varying associations with different CVD events as well as with all‐cause mortality in a large community‐based sample of middle‐aged to older men and women.
Methods
Study Sample
The study design and sampling of the Framingham Offspring Study and the Framingham Omni Study have been described.20, 21, 22 Offspring Study participants who attended examination cycle 8 (2005–2008) and Omni Study participants who attended examination cycle 3 (2007–2008) underwent a standardized medical history and examination (N=3319) and 3185 underwent routine echocardiography with digital image acquisition. A total of 3120 individuals had echocardiographic images deemed appropriate for speckle‐tracking analyses based on the following criteria: ≤1 segment of dropout for any of the predefined views (apical 2‐chamber, apical 4‐chamber, or parasternal short axis at the level of the mid‐ventricle) and absence of arrhythmia during image capture. Of this sample, 2861 had no history of previous CVD (coronary heart disease [CHD], HF, or cerebrovascular event) at the time of examination. After excluding 30 individuals who were missing data on key clinical covariates, the final sample comprised 2831 men and women. All study protocols were approved by the Institutional Review Board of Boston University Medical Campus, and all participants provided written informed consent.
Conventional Echocardiography
All echocardiographic M‐mode and 2‐dimensional (2D) images were acquired according to a standardized protocol using a Hewlett‐Packard 5500 machine (Philips Healthcare, Andover, MA). We applied the leading‐edge technique to M‐mode images for measures of end‐diastolic LV septal wall thickness (SWT), posterior wall thickness (PWT), LV end‐diastolic diameter (LVDD), and LV end‐systolic diameter (LVSD). The average of M‐mode measurements from ≥3 cardiac cycles was used to derive final measures. We calculated LV mass according to a previously validated formula23: 0.8 [1.04 (LVDD+SWT+PWT)3−(LVDD)3]+0.6. Fractional shortening (FS) was calculated as the difference between LVDD and LVSD, divided by LVDD.
Speckle‐Tracking Echocardiography
We used an offline speckle‐tracking software package (2D Cardiac Performance Analysis [CPA] v1.1; TomTec Imaging Systems, Unterschleißheim, Germany) to analyze LV deformation in each of the predefined 2D views (apical 2‐chamber [A2C], apical 4‐chamber [A4C], and mid‐ventricular parasternal short‐axis [SAX]) according to a standardized protocol.24 The TomTec 2D CPA v.1.1 analysis package allows for strain measurements to be made using an established speckle‐tracking algorithm that has been validated with sonomicrometry as well as cardiac magnetic resonance imaging (MRI) previously.2, 3, 25 The primary measurements of interest included the following LV peak systolic strains: global average longitudinal strain (average of longitudinal strain in the A2C and A4C views) and global average circumferential strain (in the SAX view). Secondary measurements of interest included global average transverse strain (average of transverse strain in the A2C and A4C views) and radial strain (in the SAX view). Herein, we use the term “transverse” strain to refer to radial strain measured in the apical (A2C and A4C) views and “radial” strain to refer to radial strain measured in the SAX view. For the present study, measurements in the 3 views were each performed by 3 separate technicians, where each technician was assigned to perform measurements for only 1 specific view; intraobserver reproducibility for each technician was assessed over the duration of the measurement period (12 months) with average coefficients of variation (CVs) <6% for global longitudinal and circumferential strain, and <9% for global transverse and radial strain. Before the start of measurements, interobserver reproducibility was also assessed, with average CVs of ≤4% for global longitudinal and circumferential strain, and <8% for global transverse and radial strain.24
Follow‐up and Outcomes
All Framingham participants are followed routinely for CVD events and death. Each suspected CVD event is adjudicated by a panel of 3 physicians who review data from examinations at the Framingham Heart Study, hospitalization records, and physician office visit records.26 Here, we specified 3 primary outcomes of interest: new‐onset CHD (comprising fatal or nonfatal myocardial infarction [MI], coronary insufficiency, and angina pectoris); HF (defined by published criteria27); and all‐cause mortality.
Statistical Analyses
We used means with SDs or percent frequencies to describe clinical and echocardiographic characteristics in the total sample. We assessed the cumulative incidence of each outcome by tertile of strain measure and used Fine‐Gray estimators to account for competing risks in analyses of CHD and HF.28 We used multivariable‐adjusted proportional hazards regression (Cox) models to quantify associations between each outcome and each LV mechanical function (strain) measure. We adjusted for (1) age, sex, and race/ethnicity and (2) additionally for body mass index, systolic and diastolic blood pressure, anti‐hypertensive treatment, total/HDL (high‐density lipoprotein) cholesterol ratio, diabetes, and smoking status. For each outcome, we used multiplicative interaction terms (between covariates and survival time) to confirm that the assumption of proportionality of hazards was satisfied. To account for multiple testing performed with 2 primary strain predictors and 3 primary outcomes, we used a Bonferroni‐corrected statistical significance threshold of P<0.008=(0.05/6).
In secondary analyses, we repeated analyses with additional adjustment for LV mass, LV mass‐to‐volume ratio, fractional shortening, and heart rate. We used Harrell's C statistic to quantify the incremental discriminatory ability to predict outcomes by adding strain measures to standard covariates.29 Additionally, for incident HF, we repeated multivariable analyses with adjustment for interim MI as a time‐varying covariate. For all models, we tested the linearity assumption of associations with strain using restricted cubic splines. Spline knots were selected based on tertile cutpoints in addition to the lower (5th) and upper (95th) percentile threshold values for each strain measure. We also tested for modification of strain effects by age and sex.
All analyses were performed using SAS software (version 9.3; SAS Institute Inc., Cary, NC). The authors have full access to, and take full responsibility for, the integrity of the data. All authors read and agreed to the manuscript as written.
Results
Clinical and echocardiographic characteristics of our study sample are shown in Table 1. The vast majority (97%) of individuals had “normal” LV systolic function (FS of ≥0.29). There were modest to moderate correlations between LV mass, FS, and individual LV strain components (Table 2); notable correlations were between longitudinal and transverse strains (r=−0.50), circumferential and radial strains (r=−0.48), and fractional shortening and circumferential strain (r=−0.46). During follow‐up (mean, 6.0±1.2) years, there were 69 incident CHD events, 71 HF events, and 199 deaths; there were 170 incident CVD events overall. Among deaths, 14 were attributable to CHD, 8 to cerebrovascular disease, and 13 to other CVD causes, with the remaining 164 deaths not attributable to a CVD cause.
Table 1.
Sample Characteristics
| Total Sample (N=2831) | |
|---|---|
| Clinical variables | |
| Age, y | 66±9 |
| Women, n (%) | 1613 (57) |
| Nonwhite race/ethnicity, n (%) | 259 (9) |
| Body mass index, kg/m2 | 28.3±5.5 |
| Systolic blood pressure, mm Hg | 128±17 |
| Diastolic blood pressure, mm Hg | 74±10 |
| Anti‐hypertensive treatment, n (%) | 1415 (50) |
| Hypertension, n (%) | 1679 (59) |
| Diabetes, n (%) | 365 (13) |
| Current smoker, n (%) | 236 (8) |
| Total/HDL cholesterol ratio | 3.48±1.06 |
| Conventional echocardiographic measuresa | |
| LV mass, g | 166±47 |
| LV end‐diastolic diameter, cm | 4.8±0.5 |
| LV end‐systolic diameter, cm | 3.0±0.4 |
| LV fractional shortening, % | 37.9±5.4 |
| Advanced echocardiographic measures | |
| LV longitudinal strain, % | −20.7±3.3 |
| LV transverse strain, % | 29.7±7.1 |
| LV circumferential strain, % | −32.1±5.8 |
| LV radial strain, % | 44.1±16.8 |
All values are shown as mean±SD or percent frequency. HDL indicates high‐density lipoprotein; LV, left ventricular.
Conventional echocardiographic measures were available for N=2549.
Table 2.
Age‐ and Sex‐Adjusted Pearson Correlations Between Conventional and Advanced Left Ventricular (LV) Measures
| LV Fractional Shortening | Longitudinal Strain | Transverse Strain | Circumferential Strain | Radial Strain | |
|---|---|---|---|---|---|
| LV mass | −0.03, P=0.14 | 0.11, P<0.0001 | −0.05, P=0.03 | 0.07, P=0.001 | −0.08, P=0.0002 |
| LV fractional shortening | −0.20, P<0.0001 | 0.18, P<0.0001 | −0.46, P<0.0001 | 0.18, P<0.0001 | |
| Longitudinal strain | −0.50, P<0.0001 | 0.31, P<0.0001 | −0.17, P<0.0001 | ||
| Transverse strain | −0.26, P<0.0001 | 0.13, P<0.0001 | |||
| Circumferential strain | −0.48, P<0.0001 |
Relations of LV Strain With Incident CHD
Based on the predefined Bonferroni‐adjusted significance threshold, none of the strain measures was significantly associated with CHD. Notably, there were no observed associations between transverse, circumferential (Figure 1), or radial strain with incidence of CHD (Table 3; P>0.30 for all). Nevertheless, we observed several potentially interesting findings. Individuals with worse (less negative) longitudinal strain demonstrated increased incidence of CHD (Figure 1). In Cox models adjusting for age, sex, race/ethnicity, and standard CVD risk factors (Table 3), worse (less negative) longitudinal strain also appeared associated with increased incidence of CHD (P=0.01 and 0.05, respectively), although these associations did not meet the Bonferroni threshold for statistical significance. Results were similar in models additionally adjusting for LV mass‐to‐volume ratio (data not shown). The association between longitudinal strain and incident CHD remained similar in magnitude in models additionally adjusting for both LV mass and fractional shortening (hazards ratio [HR] per SD increment, 1.29; 95% confidence interval [CI], 0.97–1.71; P=0.09).
Figure 1.

Cumulative incidence of coronary heart disease, heart failure, and all‐cause mortality are shown by tertiles of longitudinal strain (A through C) and circumferential strain (D through F). Incidence estimates for coronary heart disease and heart failure are adjusted for competing risk of mortality.
Table 3.
Associations of Cardiovascular Outcomes and Death With LV Strain Measuresa
| Independent Variables | Coronary Heart Disease | Heart Failure | Death | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 1 | ||||||
| Longitudinal strain (SD=3.3%) | 1.37 (1.06–1.76) | 0.01 | 1.45 (1.14–1.84) | 0.003 | 1.31 (1.14–1.52) | 0.0002 |
| Transverse strain (SD=7.1%) | 1.02 (0.80–1.29) | 0.89 | 0.73 (0.57–0.93) | 0.01 | 0.93 (0.81–1.07) | 0.32 |
| Circumferential strain (SD=5.8%) | 1.10 (0.85–1.42) | 0.48 | 1.70 (1.29–2.25) | 0.0002 | 1.30 (1.12–1.52) | 0.0007 |
| Radial strain (SD=16.8%) | 0.87 (0.67–1.13) | 0.30 | 0.64 (0.46–0.88) | 0.007 | 0.73 (0.61–0.87) | 0.0003 |
| Model 2 | ||||||
| Longitudinal strain | 1.29 (1.00–1.67) | 0.05 | 1.36 (1.07–1.73) | 0.01 | 1.34 (1.15–1.55) | 0.0002 |
| Transverse strain | 1.03 (0.82–1.29) | 0.81 | 0.76 (0.60–0.96) | 0.02 | 0.93 (0.80–1.06) | 0.28 |
| Circumferential strain | 1.12 (0.87–1.44) | 0.40 | 1.79 (1.35–2.37) | <0.0001 | 1.29 (1.11–1.51) | 0.0009 |
| Radial strain | 0.89 (0.68–1.15) | 0.37 | 0.69 (0.50–0.94) | 0.02 | 0.71 (0.60–0.85) | 0.0001 |
| Model 3 | ||||||
| Longitudinal strain | 1.29 (0.97–1.71) | 0.09 | 1.30 (0.99–1.70) | 0.06 | 1.35 (1.14–1.60) | 0.0005 |
| Transverse strain | 1.04 (0.81–1.33) | 0.77 | 0.80 (0.61–1.04) | 0.09 | 0.96 (0.82–1.12) | 0.57 |
| Circumferential strain | 1.11 (0.82–1.50) | 0.49 | 1.61 (1.15–2.26) | 0.006 | 1.21 (1.01–1.46) | 0.04 |
| Radial strain | 0.94 (0.72–1.24) | 0.68 | 0.86 (0.63–1.16) | 0.32 | 0.77 (0.64–0.92) | 0.005 |
Model 1 is adjusted for age, sex, and race/ethnicity (N=2748 for longitudinal and transverse strain, 2459 for circumferential and radial strain models). Model 2 is adjusted for age, sex, race/ethnicity, body mass index, systolic blood pressure, diastolic blood pressure, anti‐hypertensive treatment, total/HDL cholesterol, diabetes, and smoking status (N=2748 for longitudinal and transverse strain, 2459 for circumferential and radial strain models). Model 3 (secondary analysis) is adjusted for age, sex, race/ethnicity, body mass index, systolic blood pressure, diastolic blood pressure, anti‐hypertensive treatment, total/HDL cholesterol, diabetes, smoking status, LV mass, and LV fractional shortening (N=2485 for longitudinal and transverse strain, 2289 for circumferential and radial strain models). CI indicates confidence interval; CVD, cardiovascular disease; HDL, high‐density lipoprotein; HR, hazards ratio; LV, left ventricular.
Risk estimates are per 1 SD change in the strain value. There were 68 coronary heart disease events in 2554 persons at risk in Models 1 and 2 and 2311 persons at risk in Model 3, 70 heart failure events in 2671 persons at risk in Models 1 and 2 and 2410 persons at risk in Model 3, and 191 deaths among 2748 persons at risk (free of hard CVD at baseline) in Models 1 and 2 and 2485 persons at risk in Model 3 for longitudinal and transverse strain models. There were 60 coronary heart disease events in 2291 persons at risk in Models 1 and 2 and 2132 persons at risk in Model 3, 52 heart failure events in 2385 persons at risk in Models 1 and 2 and 2217 persons at risk in Model 3, and 163 deaths among 2459 persons at risk (free of hard CVD at baseline) in Models 1 and 2 and 2289 persons at risk in Model 3 for circumferential and radial strain models.
Relations of LV Strain With Incident HF
Individuals with worse strain in longitudinal and circumferential planes were more likely to develop incident HF over the follow‐up period (Figure 1). In age‐, sex‐, and race/ethnicity‐adjusted analyses (Table 3), decrements in longitudinal (HR, 1.45; P=0.003), circumferential (HR, 1.70; P=0.0002), and transverse (HR, 0.73; P=0.01) strains were associated with incidence of HF. Adjusting for standard risk factors, relations of incident HF remained significant for circumferential (HR, 1.79; P<0.0001) strain. These associations were similar in analyses additionally adjusting for interim MI during follow‐up modeled as a time‐varying covariate. In analyses adjusting for LV mass and fractional shortening, the association of circumferential strain with incident HF remained significant (P=0.006; Table 3).
Relations of LV Strain With All‐Cause Mortality
Presence of worse LV strain at baseline, particularly longitudinal strain, was associated with higher risk for all‐cause mortality (Figure 1). Worse longitudinal strain was related to increased risk for death in analyses adjusting for age, sex, race/ethnicity, and traditional risk factors (HR, 1.34; P=0.0002; Table 3), and additionally adjusting for LV mass and fractional shortening (HR, 1.35; P=0.0005). Decrements in circumferential and radial strain were also associated with mortality in models adjusting for age, sex, race/ethnicity, and standard risk factors (HR, 1.29; P=0.0009 and HR, 0.71; P=0.0001, respectively). Whereas the association of circumferential strain with death was attenuated in analyses adjusting for LV mass and FS (HR, 1.21; P=0.04), the association of radial strain with death remained significant (HR, 0.77; P=0.005).
Secondary Analyses
In secondary analyses, results were similar albeit attenuated in models additionally adjusting for heart rate (Table 4). In analyses of the discriminatory ability of distinct strain measures to predict outcomes, we observed statistically significant, albeit modest, increments in the C statistic for each of the associations reported above (Table 5). Because the associations of longitudinal strain (P=0.013) and circumferential strain (P=0.023) with incident HF were nonlinear (Figure 2), we repeated these multivariable models with these strain variables categorized by tertiles (Table 6); in analyses adjusting for standard CVD risk factors in addition to LV mass and fractional shortening, individuals in the better, compared to worst, tertiles of longitudinal and circumferential strain had lower risks for incident HF. In analyses of FS and incident events (Table 7), each 1 SD lower increment in FS was significantly associated all‐cause mortality, but not with incident HF or CHD; notwithstanding differences in the magnitude of associations, the results for incident HF were directionally concordant with results for some, but not all, of the distinct components of myocardial deformation represented by the individual strain variables (Table 3). In multivariable‐adjusted models where significant associations were observed, we observed no effect modification by age or sex on the associations of strain variables with the outcomes studied.
Table 4.
Multivariable‐Adjusted Associations of Outcomes With LV Strain Measuresa
| Independent Variables | Coronary Heart Disease | Heart Failure | Death | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 2: multivariable‐adjusted model | ||||||
| Longitudinal strain | 1.36 (1.03–1.79) | 0.03 | 1.29 (0.99–1.69) | 0.06 | 1.24 (1.05–1.46) | 0.01 |
| Transverse strain | 1.02 (0.81–1.29) | 0.87 | 0.79 (0.61–1.02) | 0.07 | 0.99 (0.85–1.14) | 0.85 |
| Circumferential strain | 1.14 (0.87–1.48) | 0.34 | 1.59 (1.18–2.14) | 0.002 | 1.21 (1.04–1.42) | 0.02 |
| Radial strain | 0.90 (0.68–1.17) | 0.43 | 0.82 (0.59–1.13) | 0.22 | 0.76 (0.64–0.91) | 0.002 |
| Model 3: secondary analyses | ||||||
| Longitudinal strain | 1.29 (0.96–1.74) | 0.09 | 1.14 (0.86–1.50) | 0.37 | 1.21 (1.02–1.44) | 0.03 |
| Transverse strain | 1.04 (0.81–1.34) | 0.75 | 0.84 (0.65–1.10) | 0.21 | 1.00 (0.85–1.17) | 0.97 |
| Circumferential strain | 1.11 (0.81–1.51) | 0.53 | 1.41 (1.00–2.00) | 0.05 | 1.11 (0.92–1.34) | 0.27 |
| Radial strain | 0.95 (0.72–1.26) | 0.72 | 0.98 (0.72–1.34) | 0.92 | 0.82 (0.68–0.98) | 0.03 |
Model 2 is adjusted for age, sex, race/ethnicity, body mass index, systolic blood pressure, diastolic blood pressure, anti‐hypertensive treatment, total/HDL cholesterol, diabetes, smoking status, and heart rate (N=2640). Model 3 is adjusted for age, sex, race/ethnicity, body mass index, systolic blood pressure, diastolic blood pressure, anti‐hypertensive treatment, total/HDL cholesterol, diabetes, smoking status, LV mass, LV fractional shortening, and heart rate (N=2485). CI indicates confidence interval; CVD, cardiovascular disease; HDL, high‐density lipoprotein; HR, hazards ratio; LV, left ventricular.
Risk estimates are per 1 SD change in the strain value. There were 64 coronary heart disease events in 2455 persons at risk in Model 2 and 2311 persons at risk in Model 3, 62 heart failure events in 2564 persons at risk in Model 2 and 2410 persons at risk in Model 3, and 175 deaths among 2640 persons at risk (free of hard CVD at baseline) in Model 2 and 2485 persons at risk in Model 3 for longitudinal and transverse strain models. There were 59 coronary heart disease events in 2250 persons at risk in Model 2 and 2132 persons at risk in Model 3, 49 heart failure events in 2343 persons at risk in Model 2 and 2217 persons at risk in Model 3, and 159 deaths among 2416 persons at risk (free of hard CVD at baseline) in Model 2 and 2289 persons at risk in Model 3 for circumferential and radial strain models.
Table 5.
Discriminatory Ability (C Statistic) of Strain Measures to Predict Outcomes
| Coronary Heart Disease | Heart Failure | Death | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C Statistic | P Value | C Statistic | P Value | C Statistic | P Value | ||||
| Model Without Strain Measure | Model With Strain Measure | Model Without Strain Measure | Model With Strain Measure | Model Without Strain Measure | Model With Strain Measure | ||||
| Model 2: multivariable‐adjusted model | |||||||||
| Longitudinal strain | 0.729 | 0.743 | 0.05 | 0.856 | 0.857 | 0.01 | 0.752 | 0.759 | 0.0002 |
| Circumferential strain | 0.734 | 0.734 | 0.40 | 0.864 | 0.875 | <0.0001 | 0.750 | 0.756 | 0.0009 |
| Model 3: secondary analyses | |||||||||
| Longitudinal strain | 0.758 | 0.767 | 0.09 | 0.886 | 0.884 | 0.06 | 0.759 | 0.766 | 0.0005 |
| Circumferential strain | 0.756 | 0.756 | 0.49 | 0.890 | 0.889 | 0.006 | 0.762 | 0.765 | 0.04 |
P values for incremental change in C statistics were determined using the Wald test.
Figure 2.
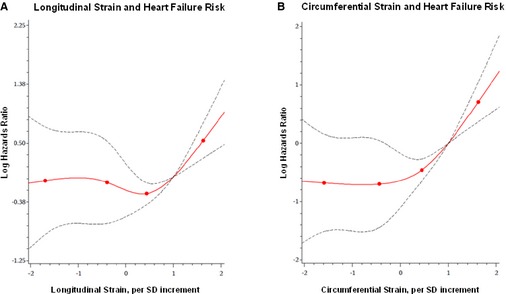
Multivariable‐adjusted splines display the association of longitudinal strain (A) and circumferential strain (B) with risk for heart failure. Knots shown denote 5th, 33rd, 67th, and 95th percentile values.
Table 6.
Associations of Longitudinal and Circumferential Strain Measures With Heart Failurea
| Predictors | No. Events/No. at Risk | Heart Failure | |
|---|---|---|---|
| HR (95% CI) | P Value | ||
| Longitudinal strain | |||
| 1st tertile | 15/807 | Referent | — |
| 2nd tertile | 13/820 | 0.73 (0.34, 1.55) | 0.41 |
| 3rd tertile | 31/783 | 1.29 (0.66, 2.53) | 0.46 |
| P trend | — | 0.34 | |
| Circumferential strain | |||
| 1st tertile | 12/768 | Referent | — |
| 2nd tertile | 11/736 | 0.97 (0.41, 2.29) | 0.94 |
| 3rd tertile | 23/713 | 2.24 (0.96, 5.21) | 0.06 |
| P trend | — | 0.05 | |
Analyses are adjusted for age, sex, race/ethnicity, body mass index, systolic blood pressure, diastolic blood pressure, anti‐hypertensive treatment, total/HDL cholesterol, diabetes, smoking status, LV mass, and LV fractional shortening (N=2410 for longitudinal strain, N=2217 for circumferential strain). CI indicates confidence interval; HDL, high‐density lipoprotein; HR, hazards ratio; LV, left ventricular.
Risk estimates are per 1 SD change in the strain value. There were 59 heart failure events for longitudinal strain model and 46 for circumferential strain.
Table 7.
Associations of Fractional Shortening With Cardiovascular Outcomes and Deatha
| Independent Variables | Coronary Heart Disease | Heart Failure | Death | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 1: age‐, sex‐, and race/ethnicity‐adjusted | ||||||
| Fractional shortening (SD=5.4%) | 0.93 (0.71–1.21) | 0.59 | 0.76 (0.59–0.99) | 0.04 | 0.79 (0.68–0.92) | 0.003 |
| Model 2: multivariable‐adjusted model | ||||||
| Fractional shortening | 0.91 (0.70–1.18) | 0.47 | 0.74 (0.57–0.96) | 0.02 | 0.80 (0.69–0.94) | 0.005 |
| Model 3: secondary analyses | ||||||
| Fractional shortening | 0.91 (0.70–1.18) | 0.46 | 0.75 (0.58–0.97) | 0.03 | 0.82 (0.70–0.95) | 0.009 |
Model 1 is adjusted for age, sex, and race/ethnicity (N=2546). Model 2 is adjusted for age, sex, race/ethnicity, body mass index, systolic blood pressure, diastolic blood pressure, anti‐hypertensive treatment, total/HDL cholesterol, diabetes, and smoking status (N=2546). Model 3 is adjusted for age, sex, race/ethnicity, body mass index, systolic blood pressure, diastolic blood pressure, anti‐hypertensive treatment, total/HDL cholesterol, diabetes, smoking status, and LV mass (N=2543). CI indicates confidence interval; CVD, cardiovascular disease; HDL, high‐density lipoprotein; HR, hazards ratio; LV, left ventricular.
Risk estimates are per 1 SD change in fractional shortening. There were 59 coronary heart disease, 59 heart failure events, and 163 deaths among 2546 persons at risk (free of hard CVD at baseline).
Discussion
In our large, community‐based sample, we performed comprehensive measurements of LV strain and investigated their relations to the incidence of distinct CVD events and death. Although the vast majority of individuals had conventionally defined normal LV function at baseline, measurable alterations in different LV strains were significantly and variably associated with different CVD outcomes. Specifically, worse longitudinal strain was the only strain‐based measure demonstrating any association with risk of CHD events, although this association did not meet the predefined threshold for statistical significance. On the other hand, circumferential strain was the measure most prominently associated with risk for HF. Notably, worse longitudinal strain was the strain‐based measure most prominently related to death from any cause, even after adjusting for standard risk factors and conventional measures of LV structure and function. Taken together, our data suggest that different LV strain measures may reflect distinctive components underlying cardiac dysfunction.
Previous studies of LV mechanical function and clinically important outcomes in the community have predominantly focused on a single primary measure of LV strain.7, 10, 11 Our study extends previous work by investigating LV strains in different planes and concurrently examining their association with distinct CVD outcomes and mortality. Alterations in different strains reflect the integrity and function of myofibers lying in a given orientation, based on location with respect to the inner‐ versus outermost layers of LV.30 Given their location‐dependent susceptibility to various cardiac stressors, myofibers are likely to be variably affected by accumulating risk for different CVD events. Noninvasive measures with the ability to reflect this variation may provide important detailed prognostic information regarding individuals at risk of CVD.
LV Strain and CHD
In our study, longitudinal strain was the only measure of LV deformation that demonstrated any consistent association with incident CHD. This finding is consistent with previous work demonstrating that alterations in longitudinal strain are associated with traditional risk factors for CHD (eg, hypertension) in the absence of overt disease31, 32, 33, 34; in turn, longitudinal strain is also considered among the most sensitive markers of active myocardial ischemia.35, 36 On echocardiography, longitudinal strain is measured along the endocardial surface of the LV cavity, in approximate alignment with the longitudinal orientation of myocardial fibers located along the subendocardial layers of the LV.37 Thus, echocardiographic longitudinal strain is considered the most sensitive measure of endocardial as well as subendocardial function, which is the myocardial layer of the ventricle considered most susceptible to stressors such as ischemia, mechanical stretch, and afterload resistance. Although previous studies have also demonstrated abnormalities in circumferential and radial strain in the setting of overt and severe ischemic heart disease,4, 38 longitudinal strain may represent a particularly sensitive marker of the cardiac dysfunction that occurs from early and chronic exposure to risk factors that specifically predispose to the future development of CHD. Indeed, we observed no significant relation of strain measured in other planes with incident CHD in our large ambulatory cohort, comprising individuals who were generally healthy without any prevalent CVD at baseline.
LV Strain and HF
In our large, community‐based cohort, circumferential strain was the measure of LV deformation that was the most consistently associated with incident HF, even in analyses adjusting for conventional parameters of LV structure and function in addition to traditional risk factors. Interestingly, Cho et al. similarly observed in a study of HF patients that decrements in circumferential, rather than longitudinal, strain predicted recurrent HF events or death.12 Of all quantifiable LV strain measures, circumferential strain is felt to best represent function of the mesomyocardial fibers that are oriented circumferentially and located between the subendo‐ and epicardial layers of the ventricle.37 Impairments in myofiber contraction in all orientations are likely precursors of global pump dysfunction. However, abnormalities of the circumferentially oriented mesomyocardial fibers, which contribute more to ejection fraction than the longitudinally oriented subendocardial fibers,39 may represent a more specific marker of impending global LV dysfunction with symptoms of congestion. It is well known that the 2 most important clinical contributors to incident HF are hypertension and previous MI, although a large proportion of individuals with either or both of these clinical traits do not go on to develop HF. Thus, abnormal circumferential strain could represent a marker of the more severe subclinical LV dysfunction that is more likely to lead to HF, with or without these risk factors present. Indeed, we observed that circumferential strain was associated with HF even after adjusting for previous and interim MI. Notably, we observed a nonlinear association of circumferential strain with HF with the possibility of a threshold effect that appears beyond 1.5 SDs away from the mean, in the range of what has been reported previously as a possible upper reference limit.40 Although assessed using cardiac MRI, a threshold value of LV circumferential strain has also been proposed previously by Choi et al. for predicting HF in the community.11 Taken together with previous work, the observation of a threshold effect is consistent with the hypothesis that circumferential strain remains preserved to compensate for early decrements in cardiac mechanical function—until the later stages of progression to HF when circumferential strain becomes overtly impaired even before detectable changes in EF.41
LV Strain and All‐Cause Mortality
In our study, decrements in longitudinal, circumferential, and radial strains were all associated with increased risk of death. Cross‐sectional and experimental studies suggest that longitudinal strain may serve as a marker of subendocardial cardiac dysfunction during the very early stages of disease progression, at which time circumferential and radial strains may remain preserved or even increase to compensate for relative decrements in the subendocardium, thus serving to maintain global pump function.16, 42 Our data also support this hypothesis, where individuals in the lowest (ie, best) tertile of circumferential strain at baseline appeared to fare just as poorly or even worse than those in the middle tertile with respect to risk for all‐cause mortality (Figure 1F). In aggregate, our findings indicate that overall CVD and mortality risk are highest for individuals with decrements in both longitudinal and circumferential strain. Notably, longitudinal strain was the most prominent predictor of all‐cause mortality, a finding previously reported in smaller samples.10 Though the majority of deaths in our analyses were not definitively attributable to a cardiovascular cause, the concomitant role of CVD in contributing to these deaths remains a possibility. In addition, decrements in longitudinal strain have been associated with a variety of disease states (eg, diabetes, chronic kidney disease, and subclinical hypothyroidism)43, 44, 45, 46; thus, longitudinal strain could represent an aggregate marker of subclinical cardiac stress in persons with comorbidities that predispose to noncardiovascular as well as cardiovascular causes of death.
Several limitations of the current study merit consideration. We observed associations between select LV strain measures and specific CVD outcomes that did not meet our prespecified threshold of statistical significance, possibly owing to inadequate power for analyses of a limited number of specific cardiovascular endpoints. Thus, additional prospective studies in larger cohorts with adequate event rates are needed to confirm, as well as extend, our results. Our echocardiographic measures of LV deformation may differ slightly from those made using alternate image analysis tools. Because our analyses were performed on previously acquired digital images, we used a non‐vendor‐specific software algorithm and package that can be applied to 2D images acquired from any echocardiography ultrasound machine.47, 48 This vendor‐independent approach to performing measures of LV deformation may augment the generalizability of our findings, given the fact that the same approach can be easily extended to other clinical research and practice settings. We did not include analyses of strain rate or regional strain, because the precision of these measures acquired from speckle tracking is reported to be lower than for global strain measures; accordingly, previous studies investigating the prognostic utility of speckle‐tracking echocardiography and outcomes have also focused on global strain measures.5, 8, 10 Because biplane Simpson's based measures of EF were unavailable in this study sample, our models included adjustment for LV fractional shortening, which is known to be limited as a conventional method for estimating global systolic function given its reliance on geometrical assumptions. Our study sample included middle‐aged to older men and women of predominantly European ancestry; thus, the extent to which our results may apply to other age or racial/ethnic groups remains unknown.
In summary, our investigation examining the relation of LV mechanical function with specific CVD outcomes and mortality represents the largest to date in a community‐based sample. In our study, advanced noninvasive measures of LV deformation were significantly associated with future risk of CVD and death, even though the vast majority of individuals had conventionally defined normal global LV function at baseline. Moreover, we observed that whereas higher longitudinal strain was related to incident CHD and particularly all‐cause mortality, higher circumferential strain emerged as the most prominent marker of risk for future HF. Further studies are warranted to replicate our findings and elucidate the underlying pathophysiological mechanisms by which distinct components of LV mechanical function contribute differentially to individual CVD outcomes—and the extent to which component measures of LV mechanical function may serve as disease‐specific markers for prognostication as well as targets for intervention.
Sources of Funding
This work was supported by the Ellison Foundation (Cheng), the National Heart, Lung and Blood Institute‘s Framingham Heart Study (Contract No.: N01‐HC‐25195), the NIGMS Interdisciplinary Training Grant for Biostatisticians T32 GM74905 (McCabe), and the following NHLBI grants: R00HL107642 (Cheng) and R01HL093328 (Vasan).
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002071 doi: 10.1161/JAHA.115.002071)
References
- 1. Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. [DOI] [PubMed] [Google Scholar]
- 2. Amundsen BH, Helle‐Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–793. [DOI] [PubMed] [Google Scholar]
- 3. Langeland S, D'Hooge J, Wouters PF, Leather HA, Claus P, Bijnens B, Sutherland GR. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005;112:2157–2162. [DOI] [PubMed] [Google Scholar]
- 4. Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Kober L, Pfeffer MA, Solomon SD. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56:1812–1822. [DOI] [PubMed] [Google Scholar]
- 5. Bertini M, Ng AC, Antoni ML, Nucifora G, Ewe SH, Auger D, Marsan NA, Schalij MJ, Bax JJ, Delgado V. Global longitudinal strain predicts long‐term survival in patients with chronic ischemic cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:383–391. [DOI] [PubMed] [Google Scholar]
- 6. Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, Monzy S, Roudaut R, Habib G, Lafitte S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr. 2010;23:1019–1024. [DOI] [PubMed] [Google Scholar]
- 7. Nahum J, Bensaid A, Dussault C, Macron L, Clemence D, Bouhemad B, Monin JL, Rande JL, Gueret P, Lim P. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging. 2010;3:249–256. [DOI] [PubMed] [Google Scholar]
- 8. Woo JS, Kim WS, Yu TK, Ha SJ, Kim SY, Bae JH, Kim KS. Prognostic value of serial global longitudinal strain measured by two‐dimensional speckle tracking echocardiography in patients with ST‐segment elevation myocardial infarction. Am J Cardiol. 2011;108:340–347. [DOI] [PubMed] [Google Scholar]
- 9. Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Moller JE, Velazquez EJ, Hassager C, Sogaard P, Kober L. Prediction of all‐cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013;61:2365–2373. [DOI] [PubMed] [Google Scholar]
- 10. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. [DOI] [PubMed] [Google Scholar]
- 11. Choi EY, Rosen BD, Fernandes VR, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida AL, Wu CO, Gomes AS, Bluemke DA, Lima JA. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34:2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2‐dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54:618–624. [DOI] [PubMed] [Google Scholar]
- 13. Kang Y, Xu X, Cheng L, Li L, Sun M, Chen H, Pan C, Shu X. Two‐dimensional speckle tracking echocardiography combined with high‐sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine‐based chemotherapy. Eur J Heart Fail. 2014;16:300–308. [DOI] [PubMed] [Google Scholar]
- 14. Mor‐Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. [DOI] [PubMed] [Google Scholar]
- 15. Mor‐Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. [DOI] [PubMed] [Google Scholar]
- 16. Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, Augeul L, Ovize M, Derumeaux G. Influence of afterload on left ventricular radial and longitudinal systolic functions: a two‐dimensional strain imaging study. Eur J Echocardiogr. 2009;10:914–921. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Khoury DS, Yue Y, Torre‐Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. [DOI] [PubMed] [Google Scholar]
- 18. Reant P, Labrousse L, Lafitte S, Bordachar P, Pillois X, Tariosse L, Bonoron‐Adele S, Padois P, Deville C, Roudaut R, Dos Santos P. Experimental validation of circumferential, longitudinal, and radial 2‐dimensional strain during dobutamine stress echocardiography in ischemic conditions. J Am Coll Cardiol. 2008;51:149–157. [DOI] [PubMed] [Google Scholar]
- 19. Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two‐dimensional strain imaging. J Am Soc Echocardiogr. 2008;21:1138–1144. [DOI] [PubMed] [Google Scholar]
- 20. Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham study. Am J Public Health Nations Health. 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 22. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 23. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 24. Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Reproducibility of speckle‐tracking‐based strain measures of left ventricular function in a community‐based study. J Am Soc Echocardiogr. 2013;26:1258–1266.e1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E, Abraham TP, Belohlavek M. Two‐dimensional strain–a Doppler‐independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr. 2005;18:1247–1253. [DOI] [PubMed] [Google Scholar]
- 26. Kannel WB, Wolf PA, Garrison RJ. The Framingham Study: an epidemiological investigation of cardiovascular disease. Section 34. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham heart study, 30‐year follow‐up. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. (NIH publication no. 87‐2703). [Google Scholar]
- 27. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 28. Lin G, So Y, Johnston G. Analyzing survival data with competing risks using SAS® software. Proceedings of the SAS® Global Forum 2012 Conference, Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 29. Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. [DOI] [PubMed] [Google Scholar]
- 30. Dorri F, Niederer PF, Lunkenheimer PP, Anderson RH. The architecture of the left ventricular myocytes relative to left ventricular systolic function. Eur J Cardiothorac Surg. 2010;37:384–392. [DOI] [PubMed] [Google Scholar]
- 31. Pavlopoulos H, Grapsa J, Stefanadi E, Philippou E, Dawson D, Nihoyannopoulos P. Is it only diastolic dysfunction? Segmental relaxation patterns and longitudinal systolic deformation in systemic hypertension. Eur J Echocardiogr. 2008;9:741–747. [DOI] [PubMed] [Google Scholar]
- 32. Kosmala W, O'Moore‐Sullivan TM, Plaksej R, Kuliczkowska‐Plaksej J, Przewlocka‐Kosmala M, Marwick TH. Subclinical impairment of left ventricular function in young obese women: contributions of polycystic ovary disease and insulin resistance. J Clin Endocrinol Metab. 2008;93:3748–3754. [DOI] [PubMed] [Google Scholar]
- 33. Kang SJ, Lim HS, Choi BJ, Choi SY, Hwang GS, Yoon MH, Tahk SJ, Shin JH. Longitudinal strain and torsion assessed by two‐dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase‐1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr. 2008;21:907–911. [DOI] [PubMed] [Google Scholar]
- 34. Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–1401. [DOI] [PubMed] [Google Scholar]
- 35. Montgomery DE, Puthumana JJ, Fox JM, Ogunyankin KO. Global longitudinal strain aids the detection of non‐obstructive coronary artery disease in the resting echocardiogram. Eur Heart J Cardiovasc Imaging. 2012;13:579–587. [DOI] [PubMed] [Google Scholar]
- 36. Munk K, Andersen NH, Terkelsen CJ, Bibby BM, Johnsen SP, Botker HE, Nielsen TT, Poulsen SH. Global left ventricular longitudinal systolic strain for early risk assessment in patients with acute myocardial infarction treated with primary percutaneous intervention. J Am Soc Echocardiogr. 2012;25:644–651. [DOI] [PubMed] [Google Scholar]
- 37. Bayer JD, Blake RC, Plank G, Trayanova NA. A novel rule‐based algorithm for assigning myocardial fiber orientation to computational heart models. Ann Biomed Eng. 2012;40:2243–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sarvari SI, Haugaa KH, Zahid W, Bendz B, Aakhus S, Aaberge L, Edvardsen T. Layer‐specific quantification of myocardial deformation by strain echocardiography may reveal significant cad in patients with non‐ST‐segment elevation acute coronary syndrome. JACC Cardiovasc Imaging. 2013;6:535–544. [DOI] [PubMed] [Google Scholar]
- 39. Ingels NB Jr. Myocardial fiber architecture and left ventricular function. Technol Health Care. 1997;5:45–52. [PubMed] [Google Scholar]
- 40. Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Age‐ and sex‐based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging. 2013;6:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1716–1717. [DOI] [PubMed] [Google Scholar]
- 42. Borg AN, Ray SG. A unifying framework for understanding heart failure? Response to “left ventricular torsion by two‐dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction”. J Am Soc Echocardiogr. 2009;22:318–320; author reply 321‐312. [DOI] [PubMed] [Google Scholar]
- 43. Zoroufian A, Razmi T, Taghavi‐Shavazi M, Lotfi‐Tokaldany M, Jalali A. Evaluation of subclinical left ventricular dysfunction in diabetic patients: longitudinal strain velocities and left ventricular dyssynchrony by two‐dimensional speckle tracking echocardiography study. Echocardiography. 2014;31:456–463. [DOI] [PubMed] [Google Scholar]
- 44. Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Leano R, Haluska BA, Stanton T. The association between left ventricular global longitudinal strain, renal impairment and all‐cause mortality. Nephrol Dial Transplant. 2014;29:1218–1225. [DOI] [PubMed] [Google Scholar]
- 45. Tadic M, Ilic S, Kostic N, Caparevic Z, Celic V. Subclinical hypothyroidism and left ventricular mechanics: a three‐dimensional speckle tracking study. J Clin Endocrinol Metab. 2014;99:307–314. [DOI] [PubMed] [Google Scholar]
- 46. Liu YW, Su CT, Huang YY, Yang CS, Huang JW, Yang MT, Chen JH, Tsai WC. Left ventricular systolic strain in chronic kidney disease and hemodialysis patients. Am J Nephrol. 2011;33:84–90. [DOI] [PubMed] [Google Scholar]
- 47. Nelson MR, Hurst RT, Raslan SF, Cha S, Wilansky S, Lester SJ. Echocardiographic measures of myocardial deformation by speckle‐tracking technologies: the need for standardization? J Am Soc Echocardiogr. 2012;25:1189–1194. [DOI] [PubMed] [Google Scholar]
- 48. Risum N, Ali S, Olsen NT, Jons C, Khouri MG, Lauridsen TK, Samad Z, Velazquez EJ, Sogaard P, Kisslo J. Variability of global left ventricular deformation analysis using vendor dependent and independent two‐dimensional speckle‐tracking software in adults. J Am Soc Echocardiogr. 2012;25:1195–1203. [DOI] [PubMed] [Google Scholar]


