Abstract
Background
We evaluated the relationship of aortic root dimension (ARD) with flow output and both peripheral and central blood pressure, using multivariable equations predicting ideal sex‐specific ARD at a given age and body height.
Methods and Results
We measured echocardiographic diastolic ARD at the sinuses of Valsalva in 3160 adults (aged 42±16 years, 61% women) from the fourth examination of the Strong Heart Study who were free of prevalent coronary heart disease, and we compared measured data with the theoretical predicted value to calculate a z score. Central blood pressure was estimated by applanation tonometry of the radial artery in 2319 participants. ARD z scores were divided into tertiles representing small, normal, and large ARD. Participants with large ARD exhibited greater prevalence of central obesity and higher levels of inflammatory markers and lipids (0.05<P<0.0001). Stroke volume, heart rate, and both cuff and central diastolic blood pressure were progressively greater from small to large ARD (all P<0.0001). Pulse pressure was higher in small ARD (P<0.0001). In multivariable analysis, ARD z score was related positively to stroke volume, either cuff or central diastolic blood pressure, and negatively to pulse pressure. Large ARD was also independently correlated to higher waist circumference and percentages of neutrophils and plasminogen activator inhibitor‐1 (all P<0.01).
Conclusions
Aortic root dilatation is associated with high diastolic blood pressure, high stroke volume, central fat distribution, and inflammatory status. In contrast, at a given diastolic blood pressure and stroke volume, aortic root dilatation is associated with lower pulse pressure and systolic blood pressure.
Keywords: aortic root dilatation, applanation tonometry, blood pressure, inflammation, neutrophil count, pulse pressure, stroke volume
Introduction
Aortic root (AR) dilatation has been associated with aortic regurgitation, hypertension, arteriosclerosis, and hypertensive end‐organ damage, including increased peripheral resistance, carotid intima–media thickness, and evidence of plaque.1, 2, 3, 4, 5 Although there is evidence of the relationship between AR dimension (ARD) and peripheral resistance,2 there are inconsistent findings regarding the relationship with components of blood pressure (BP). In clinical and epidemiologic settings, ARD is generally associated posi‐tively with diastolic BP but negatively with pulse pressure (PP) and systolic BP,6, 7 measured with cuff sphygmo‐manometry.
The effects of BP components and hemodynamics on the variance of ARD remain uncertain, given the difficulty of identifying a clear cause–effect relationship and the lack of data on the relationship between ARD and central BP. In addition, relationships between BP and ARD vary according to the level at which ARD is measured, namely, sinuses of Valsalva versus ascending aorta.8 Consequently, the nature of the association between hypertension and AR dilatation still needs to be clarified.1, 6, 8, 9, 10 This uncertainty makes these relationships similar to a “chicken and egg” question.
The physiological interaction between ARD and components of BP is regulated by key factors, including demographic, hemodynamic, and biological determinants. A number of previous reports strongly suggest that age, sex, and body size are the most relevant variables to be considered when estimating whether or not AR is dilated.6, 9, 11, 12, 13, 14 Analyses taking into account the effect of these potent determinants might contribute to clarification of the interplay among ARD, components of BP, and flow output, a potential component of ARD variance that has been neglected. To isolate the effect of demographics and anthropometrics on the variability of ARD, we previously analyzed ARD in 1207 multiethnic, nonobese, normotensive, nondiabetic participants who were free of cardiovascular (CV) disease or aortic valve disease11 to generate multivariable equations and nomograms to predict the ideal sex‐specific ARD at a given age and body size.
In the present project, we applied these equations to participants in the fourth examination of the Strong Heart Study to evaluate the relationship of differences between actual ARD and the theoretical predictions for age, sex, and body size with both peripheral and central BP components. We also considered the impact of flow output and other potentially important biological parameters.
Methods
Study Population
Initiated as a longitudinal population‐based survey of CV risk factors and CV disease in American Indians from 13 communities in Arizona, Oklahoma, and South and North Dakotas,14, 15, 16, 17 the Strong Heart Study was extended to members of large 3‐generation families (Strong Heart Family Study) during the fourth phase (between 2001 and 2003).15, 16
From the fourth examination Strong Heart Study cohort of 3642 participants, we excluded 27 participants with coronary heart disease, 14 participants with aortic stenosis or regurgitation, and 9 with more than mild mitral regurgitation. From the remaining 3592 participants who were free of CV disease, 3251 were aged >17 years, and 3160 (97%) of those had available echocardiographic measurement of ARD and were included in the analysis. Participants gave written informed consent under protocols approved by all participating communities and institutional review boards.
Laboratory Tests and Definitions
Fasting plasma glucose was measured by standard methods. Diabetes (fasting glucose ≥126 mg/dL or ongoing antidiabetic treatment) was diagnosed based on 1997 American Diabetes Association recommendations. Obesity was classified based on the 1998 National Institutes of Health guidelines (body mass index ≥30 kg/m2). Waist circumference was used as a measure of central fat distribution. Hypertension was defined by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure criteria (BP ≥140/90 mm Hg or use of antihypertensive treatment). C‐reactive protein, plasminogen activator inhibitor‐1, fibrinogen, and white cell count were measured by standard methods.
Fat‐free mass and adipose body mass were estimated by using an RJL bioelectric impedance meter (model B14101; RJL Equipment Co). Equations for estimating fat‐free mass in kilograms based on total body water, using bioelectric resistance, had been validated previously in the American Indian population.17 Adipose mass was estimated by subtracting fat‐free mass from body weight. BP was recorded according to the procedures reported in the study operation manuals (http://strongheart.ouhsc.edu/manual/PhaseIV/Volume3.pdf) and in pilot manuscripts,18, 19 with the participant sitting with the right arm on table and using a standard manometer. After 5 minutes of resting, the cuff was inflated to >30 mm Hg above the obliteration pressure, held constant for 5 seconds, and then slowly deflated (2 mm/s) while reading pressures for first and fifth Korotkoff phases. Before the second and third measurements, participants were requested to raise the arm for 5 seconds. After another 25 seconds with the arm on the table, the measurement was repeated for the second and third times. The average of the last 2 measurements was used for analysis. Cuff was determined based on the arm circumference, taken with participants sitting with the right arm hanging freely, with the right hand resting on the right knee, and with the tape measure placed horizontally at the midpoint between the acromion and olecranon. Results of arm circumference were rounded to the nearest centimeter.
Echocardiography
Echocardiograms were performed using phased‐array commercial echocardiographs with M‐mode, 2‐dimensional, and Doppler capabilities, as reported previously.18 Left ventricular dimensions and septal and posterior left ventricular wall thicknesses were measured based on American Society of Echocardiography recommendations.19, 20 Left ventricular volumes were estimated from linear dimensions by the z‐derived method21 and used to derive stroke volume. An estimate of arterial compliance was also generated by the ratio of stroke volume to PP.
Echocardiographic ARD was measured at the sinuses of Valsalva during diastole, by the leading edge‐to‐leading edge method.9 The raw measure of ARD was compared with the value predicted for age, sex, and body size, using an equation previously generated in a large multiethnic population of healthy persons.11 This approach has been validated recently in a population of patients with Marfan syndrome.22 Due to the high prevalence of obesity in the Strong Heart Study population, we used height as the measure of body size in the following equation (ARDpred indicates predicted ARD):
A sex‐specific z score was calculated by the individual difference between measured ARD and predicted ARD values, divided by the sex‐specific standard deviation of measured ARD in the present population (ARD‐z). Consequently, positive values of ARD‐z identified higher‐than‐predicted ARD, whereas negative values indicated ARD smaller than those predicted by age, sex, and body size. Cuff BP measured at the end of the echocardiogram by an aneroid sphygmomanometer was used for analysis in this study.
The reliability of our echocardiographic measurements was analyzed previously in a large population sample from a clinical trial.23
Carotid Ultrasonography and Applanation Tonometry
Carotid ultrasound was performed using a standardized protocol24 and commercial machines. The protocol has been reported extensively.25 Briefly, ECG‐gated 2‐dimensionally guided M‐mode tracings of the distal common carotid artery ≈1 cm proximal to the carotid bulb were obtained and read offline by an experienced cardiologist. Following calibration for depth and time, the end‐diastolic wall thickness (combined intima–media thickness of the far wall) and end‐diastolic and peak‐systolic internal diameters (by continuous tracing of the lumen–intima interface of the near and far walls) were measured on several cycles using electronic calipers and averaged. Carotid plaque was rare in this relatively young population and was not analyzed.
As reported previously,26 radial arterial pressure waveforms were obtained by applanation tonometry (SphygmoCor System) in 2319 adult participants from the Oklahoma and South and North Dakota field centers who were free of prevalent CV disease (73%) to generate estimates of central systolic and diastolic BP. Applanation tonometry has been validated to yield accurate estimates of intra‐arterial pressure.27 Brachial systolic and diastolic pressures for calibration were entered by averaging the last 2 of 3 sequential brachial pressure readings made at the end of the echocardiographic procedure (http://strongheart.ouhsc.edu/manual/PhaseIV/Volume5.pdf).
Statistical Analysis
Analyses were performed to examine the associations of variability of ARD‐z with 4 critical physiological components: (1) cuff diastolic BP, as the steady stress imposed on the Valsalva sinuses at the closure of aortic valve; (2) stroke volume, a measure of left ventricular performance28 that represents the flow volume distending the proximal aorta during ventricular contraction; (3) cuff PP, as the measure of the combined left ventricular ability to expel blood and the aortic capacitance (systolic BP was also used in alternative analyses); and (4) heart rate, as the marker of the frequency of the aortic distension per unit time. In the subpopulation in which applanation tonometry was available, estimates of central systolic diastolic and pulse BP were also evaluated in alternative analyses.
Data were analyzed using SPSS 22.0 (IBM Corp) and expressed as mean±1 SD or proportion in the chi‐square distribution. Indicator variables were included for the Arizona, South and North Dakota, and Oklahoma field centers. We analyzed main biological characteristics in tertiles of ARD‐z, labeled for convenience as small, normal, and large ARD, using ANOVA and simple main effect with Sidak correction for identification of differences. Tertile allocation was made automatically by the SPSS package. Multiple linear regression analysis was used to analyze the variance of ARD‐z in relation to the 4 main components, which we hypothesize contributed independently to modulation of ARD variance. In a second model, body composition, markers of inflammation, and lipid profile—identified as associated with ARD in the exploratory analyses—were added. Because the level of relatedness was high in this population, we also adjusted for a standard kinship coefficient based on the level of relatedness within a family, as reported previously.29 In the third model, antihypertensive therapy (classes of medications significantly different among the tertiles of ARD‐z) was also forced into the previous model. Variance inflation factor was computed to explore the level of multicollinearity. The variance inflation factor was maintained within the range considered to be acceptable (ie, <10).30 Although well below 10, the highest level of variance inflation factor was expectedly reached by the combination of fat‐free mass, fat mass, and waist circumference; these 3 parameters offer a high degree of biological collinenarity. The decision to maintain all 3 variables in the equation was made on the basis of the different biological meanings of these parameters, with the first 2 being expressions of general body composition and waist circumference being the marker of specific abdominal fat distribution. A 2‐tailed P≤0.05 was assumed to be statistically significant.
Results
The mean age of the study population was 42±16 years (range 18 to 93 years), and there was a predominance of women (61%). In men and women, respectively, arterial hypertension was present in 40% and 32%, obesity was present in 54% and 62% (both P<0.0001), and diabetes was present in 22% and 25% (P not significant).
Figure displays the normal distribution of ARD‐z. ARD‐z ranged between −3.75 and 4.04 SD from the mean predicted values.
Figure 1.
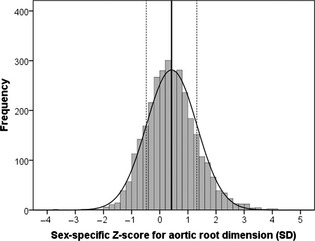
Distribution of z score of aortic root dimension. The straight continuous vertical line represents the mean, and the dotted lines represent 1 SD.
Table 1 presents the characteristics associated with tertiles of ARD‐z in the whole study population. Participants with large ARD were younger than those with small ARD and exhibited greater prevalence of central obesity, with greater amounts of body components (both fat and fat‐free mass) and higher levels of inflammatory markers and cholesterol (0.05<P<0.0001). Carotid intima–media complex was thicker and diastolic diameter was smaller in participants with small ARD than in those with normal or large ARD (both P<0.0001). Consequently, carotid relative wall thickness was progressively lower from small to large ARD (P<0.0001).
Table 1.
General Characteristics of Patients With Small, Normal, and Large ARD Based on Deviation From Predicted Values (z score)
| Small ARD (n=1007) | Normal ARD (n=1131) | Large ARD (n=1022) | Whole Cohort | |
|---|---|---|---|---|
| Age, y | 44.8±17.4a, b | 40.3±15.3 | 40.8±14.2 | 41.5±15.9 |
| Sex (% women) | 59.4 | 63.9 | 60.5 | 61.3 |
| Aortic root, cm | 3.08±0.22 | 3.31±0.27 | 3.63±0.32 | 3.34±0.35 |
| Aortic root/height, cm/m | 1.85±0.12a, b | 1.98±0.12 | 2.26±0.16 | 2.00±0.19 |
| Hypertension, % | 36.3 | 34.5 | 35.0 | 35.4 |
| Diabetes, % | 23.6 | 24.0 | 23.9 | 24.0 |
| Obesity, % | 47.8 a | 59.5 | 69.9 | 58.7 |
| Smokers—former smokers, % | 34 – 26 | 36 – 23 | 39 – 26 | 36 – 25 |
| BMI, kg/m2 | 30.4±6.9a, b | 32.8±7.5b | 34.6±8.0 | 32.6±7.68 |
| Waist girth, cm | 100.8±16.4a, b | 106.1±17.9b | 110.0±18.4 | 105.7±18.0 |
| Fat‐free mass, kg | 53.7±11.7a, b | 54.9±12.3b | 58.4±13.7 | 55.7±12.7 |
| Adipose mass, kg | 31.0±14.1a, b | 36.2±15.8b | 39.3±16.7 | 35.6±15.9 |
| C‐reactive protein, mg/L | 3.62 (1.64 to 8.33)a, b | 4.53 (2.07 to 9.2) | 4.41 (1.91 to 9.36) | 4.27 (1.86 to 8.87) |
| Fibrinogen, mg/dL | 386.2±86.6b | 394.6±88.2 | 394.9±94.4 | 392.22±90.03 |
| PAI‐1, mg/dL | 43.0 (25.0 to 65.0)a, b | 51.0 (30.0 to 80.0)b | 54.0 (32.0 to 82.0) | 49.0 (29.0 to 76.0) |
| White blood cell count, 109/L | 6.96±1.86a, b | 7.24±1.94 | 7.26±2.05 | 7.15±1.96 |
| Neutrophils, %a | 59.3±8.7b | 59.7±8.6b | 60.8±8.4 | 59.97±8.63 |
| Hematocrit, % | 42.6±4.8 | 42.1±4.8 | 42.4±4.8 | 42.35±4.82 |
| Fasting glucose, mg/dL | 114.3±51.7 | 115.3±53.2 | 116.0±53.2 | 115.27±52.80 |
| Cholesterol, mg/dL | 184.1±34.2 | 179.6±33.8b | 185.3±34.4 | 182.76±34.20 |
| Triglycerides, mg/dL | 160.4±91.0 | 159.5±87.6 | 167.5±94.0 | 162.28±90.70 |
| Carotid intima–media thickness, mm | 0.69±0.17a, b | 0.66±0.16 | 0.67±0.15 | 0.67±0.16 |
| Carotid diastolic diameter, mm | 5.72±0.67b | 5.79±0.63b | 6.00±0.66 | 5.84±0.66 |
| Carotid relative wall thickness | 0.24±0.06a, b | 0.23±0.05b | 0.22±0.05 | 0.23±0.05 |
ARD indicates aortic root dimension; BMI, body mass index; PAI‐1, plasminogen activator inhibitor‐1.
0.05<P<0.0001 vs normal ARD.
0.05<P<0.0001 vs large ARD.
Antihypertensive therapy was not different among the 3 groups, with similar proportions of patients taking diuretics, Ca++‐channel blockers, and beta blockers, but a significant difference was noted in anti–renin‐angiotensin system medications (anti‐RAS, namely, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers), which were taken more frequently in the subgroup with small ARD (21%) compared with the groups with normal ARD (17%) or large ARD (15%, P<0.001).
Stroke volume was progressively greater from small to large ARD (P<0.0001), paralleling trends in increasing heart rate (P<0.01) and cuff diastolic BP (Table 2) and decreasing PP (both P<0.0001), whereas systolic BP was similar among the 3 ARD groups. The applanation tonometry estimates of systolic, diastolic, and pulse central BP exhibited the same distributions as the cuff BP (Table 2). It should be noted that small ARD accommodates lower stroke volume but maintains the same systolic BP as large ARD. Estimated arterial compliance by the stroke volume/PP ratio was progressively lower as the ARD decreased (P<0.0001).
Table 2.
Physiological Correlates of ARD
| Small ARD (n=1007) | Normal ARD (n=1131) | Large ARD (n=1022) | Whole Cohort | |
|---|---|---|---|---|
| Stroke volume, mL/beat | 75.0±11.7a, b | 78.7±11.5b | 82.2±12.8 | 78.62±12.33 |
| Cuff systolic BP, mm Hg | 123.7±18.6 | 122.7±16.3 | 123.7±16.5 | 123.42±17.23 |
| Cuff diastolic BP, mm Hg | 75.2±10.7a, b | 76.9±11.1b | 79.1±10.9 | 77.08±11.02 |
| Cuff pulse pressure, mm Hg | 48.5±17.1a, b | 45.8±13.2b | 44.5±13.6 | 46.3±14.9 |
| Stroke volume/pulse pressure, mL/mm Hg | 1.71±0.60a, b | 1.84±0.55b | 1.99±0.61 | 1.85±0.60 |
| Heart rate, bpm | 66.4±10.9b | 67.2±10.5 | 67.9±10.8 | 67.22±10.75 |
| (n=756) | (n=763) | (n=800) | ||
| Central systolic BP, mm Hg | 113.7±18.4 | 112.6±17.4 | 113.4±16.8 | 113.18±17.51 |
| Central diastolic BP, mm Hg | 76.4±10.5a, b | 77.7±10.9b | 79.2±11.0 | 77.80±10.87 |
| Central pulse pressure, mm Hg | 37.3±14.9a, b | 34.9±12.7 | 34.1±12.7 | 35.38±13.51 |
| Stroke volume/pulse pressure, mL/mm Hg | 2.32±0.94a, b | 2.56±0.98b | 2.71±0.97 | 2.53±0.98 |
ARD indicates aortic root dimension; BP, blood pressure; bmp, beats per minute.
0.005<P<0.0001 vs normal ARD.
0.05<P<0.0001 vs large ARD.
Multivariable analysis was implemented on the basis of the exploratory statistics, thus ARD‐z variability was initially tested using the 4 hemodynamic components at the basis of our hypothesis (model 1) (Table 3). Eventually, all other potential confounders, including body fatness and markers of inflammation, were forced (model 2) (Table 3). ARD‐z was related positively to stroke volume, cuff diastolic BP, and heart rate and negatively to cuff PP (model 1, all P<0.0001). We also ran the same model using raw values of ARD normalized for height and adjusting for sex, age, and degree of relatedness. The relationship between ARD and hemodynamic components was almost identical to that reported in Table 3 (β‐coefficients were 0.21 for stroke volume, −0.08 for PP, 0.08 for diastolic BP, and 0.06 for heart rate, all P<0.0001).
Table 3.
Models of Multiple Regression Analysis Testing the Variability of ARD‐z Compared With Stroke Volume, Heart Rate, Diastolic Blood Pressure and Pulse Pressure
| Model 1 (Multiple R=0.35) | Model 2 (Multiple R=0.38) | |||||
|---|---|---|---|---|---|---|
| Standardized Coefficients β | P Value | Variance Inflation Factor | Standardized Coefficients β | P Value | Variance Inflation Factor | |
| Age, y | −0.07 | <0.0006 | 1.37 | −0.10 | <0.0001 | 1.69 |
| Female sex | 0.14 | <0.0001 | 1.20 | 0.10 | <0.009 | 4.01 |
| Stroke volume, mL/beat | 0.31 | <0.0001 | 1.22 | 0.25 | <0.0001 | 1.58 |
| Cuff pulse pressure, mm Hg | −0.11 | <0.0001 | 1.37 | −0.10 | <0.0001 | 1.38 |
| Cuff diastolic BP, mm Hg | 0.10 | <0.0001 | 1.16 | 0.09 | <0.0001 | 1.25 |
| Heart rate, bpm | 0.08 | <0.0001 | 1.07 | 0.04 | <0.02 | 1.17 |
| Degree of relatednessa | −0.05 | <0.0513 | 1.75 | −0.05 | <0.03 | 1.77 |
| Body fat, kg | — | — | — | 0.01 | <0.79 | 6.21 |
| Fat‐free mass, kg | — | — | — | −0.01 | <0.79 | 5.01 |
| Waist circumference, cm | — | — | — | 0.12 | <0.01 | 6.60 |
| White blood cell count, 109/L | — | — | — | −0.03 | <0.23 | 1.35 |
| Neutrophils, % | — | — | — | 0.07 | <0.001 | 1.24 |
| C‐reactive protein, mg/L | — | — | — | −0.01 | <0.58 | 1.46 |
| Plasma fibrinogen, mg/dL | — | — | — | 0.03 | <0.18 | 1.77 |
| PAI‐1, ng/mL | — | — | — | 0.05 | <0.01 | 1.24 |
| Triglycerides, mg/dL | — | — | — | 0.03 | <0.17 | 1.32 |
| Total cholesterol, mg/dL | — | — | — | −0.02 | <0.31 | 1.37 |
In Model 1, the 4 hemodynamic components were entered; in Model 2, parameters of body fatness and inflammation were forced. ARD‐z indicates sex‐specific z score for measured and predicted aortic root dimension values; BP, blood pressure; PAI‐1, plasminogen activator inhibitor‐1.
Relatedness was evaluated by standard kinship coefficients (0.25 for parent/offspring, 0.25 for full siblings, 0.125 for half siblings, and 0 for no consanguinity).29
The addition of the correlates identified in exploratory statistics (Table 1) did not modify the impact of the 4 primary covariates but added some information (model 2). In particular, large ARD was independently correlated to waist circumference (P<0.01), which blunted the effect of body composition components detected in univariate analysis. As expected, there was a moderately higher level of multicollinearity among waist circumference, fat‐free mass, and adipose mass but well below the level considered acceptable. In addition, ARD was positively correlated with the percentage of neutrophils of the white blood cell count and with the circulating level of plasminogen activator inhibitor‐1, whereas no relations were detected with lipid profile. Carotid intima–media thickness, added to model 2, did not exhibit any relationship with ARD (P=0.963). Finally, anti‐RAS medications were not independently related to ARD‐z (P=0.1), and forcing them into the model did not change the coefficients or the significance of covariates as shown in Table 3.
The same models as those in Table 3 were also run by changing cuff BP to estimates of central BP (Table 4) for participants in whom applanation tonometry was available. Using central instead of cuff BP did not change the profile of the explained variance of ARD displayed in Table 3, except for heart rate, which decreased its impact to a level that was no longer significant (P=0.4).
Table 4.
Models of Multiple Regression Analysis Testing the Variability of ARD‐z Compared With Stroke Volume, Heart Rate, Central Diastolic Blood Pressure and Central Pulse Pressure Estimated by Applanation Tonometry
| Model 1 (Multiple R=0.35) | Model 2 (Multiple R=0.38) | |||||
|---|---|---|---|---|---|---|
| Standardized Coefficients β | P Value | Variance Inflation Factor | Standardized Coefficients β | P Value | Variance Inflation Factor | |
| Age, y | −0.08 | <0.002 | 1.53 | −0.11 | <0.0002 | 1.87 |
| Female sex | 0.15 | <0.0001 | 1.18 | 0.11 | <0.009 | 3.98 |
| Stroke volume, mL/beat | 0.31 | <0.0001 | 1.23 | 0.25 | <0.0001 | 1.56 |
| Central pulse pressure, mm Hg | −0.09 | <0.001 | 1.64 | −0.09 | <0.0009 | 1.66 |
| Central diastolic BP, mm Hg | 0.10 | <0.0001 | 1.11 | 0.08 | <0.0004 | 1.19 |
| Heart rate, bpm | 0.05 | <0.02 | 1.09 | 0.02 | <0.40 | 1.18 |
| Degree of relatednessa | −0.03 | <0.30 | 1.65 | −0.03 | <0.21 | 1.68 |
| Body fat, kg | — | — | — | 0.01 | <0.85 | 5.59 |
| Fat‐free mass, kg | — | — | — | −0.02 | <0.69 | 5.10 |
| Waist circumference, cm | — | — | — | 0.13 | <0.02 | 6.28 |
| White blood cell count, 109/L | — | — | — | −0.02 | <0.36 | 1.34 |
| Neutrophils, % | — | — | — | 0.07 | <0.003 | 1.24 |
| C‐reactive protein, mg/L | — | — | — | −0.01 | <0.57 | 1.47 |
| Plasma fibrinogen, mg/dL | — | — | — | 0.03 | <0.2501 | 1.74 |
| PAI‐1, ng/mL | — | — | — | 0.05 | <0.0353 | 1.24 |
| Triglycerides, mg/dL | — | — | — | 0.03 | <0.1786 | 1.31 |
| Total cholesterol, mg/dL | — | — | — | −0.02 | <0.4864 | 1.32 |
In Model 1, the 4 hemodynamic components were entered; in Model 2, parameters of body fatness and inflammation were forced. ARD‐z indicates sex‐specific z score for measured and predicted aortic root dimension values; BP, blood pressure; PAI‐1, plasminogen activator inhibitor‐1.
Relatedness was evaluated by standard kinship coefficients (0.25 for parent/offspring, 0.25 for full siblings, 0.125 for half siblings, and 0 for no consanguinity).29
Other multivariable models were run using systolic BP (either cuff or central) instead of PP. The relationship of ARD to the covariates was confirmed by regression also using systolic BP (data not shown).
Discussion
In our analysis, we identified potential reasons for inconsistent findings in the literature about relationships between arterial hypertension and ARD. Whether ARD was related to BP values and how much other factors could interfere with this relationship were explored only marginally in previous large population‐based studies6 and never using central BP. We demonstrated that there are hemodynamic components strictly related to ARD that need to be considered as a whole to understand those relationships. We also demonstrated that other components of the variability of ARD did not interfere with the relationship of ARD to the hemodynamic components that we hypothesized to be primary physiological determinants of variability of ARD. An interesting parallelism has also been documented between ARD and carotid diastolic dimension.
Consequently, after controlling for the confounding influence of sex, age, and body size, ARD was influenced primarily by the distending pressure stretching the aortic wall when the aortic valve is closed and by the impact of the amount of blood expelled on each heart beat. The frequency of cardiac contraction also exhibited some influence, but the statistical effect was substantially diluted when other cofactors were considered, especially when BP was measured by applanation tonometry. In the scenario presented in our analysis, diastolic BP might be considered a determinant of ARD, and hypertensive persons with uncontrolled diastolic BP should be considered at risk of AR dilatation. In contrast, the comparison of the results of exploratory statistics with those of multivariable analysis indicated that PP decreased for larger ARD at a given diastolic BP, stroke volume, and heart rate, suggesting that small ARD might be a condition predisposing to systolic hypertension by reducing the capacitance of the proximal aorta.
Although the Framingham Heart Study already demonstrated directionally opposite relationships of ARD diastolic/mean BP (positive) and systolic/pulse BP (negative), stroke volume and other biologic correlates were not examined, and the BP used in the analysis was taken peripherally with sphygmomanometers. We extended those findings and documented, for the first time, that the scenario identified using cuff BP could be fully confirmed using central BP estimates and was not affected by other potential influences.
Our results and interpretations of our findings are consistent with another more recent longitudinal analysis from the Framingham Heart Study10 showing that longitudinal increase in AR size was related to a corresponding decrease in PP.
What is missing in all previous reports on ARD is the consideration of the stroke volume ejected into the aorta on each heart beat. Integration of the force imposing the steady tension on the AR, the stroke volume ejected that produces phasic aortic strain, and the frequency of these pulsatile deformations makes possible a better understanding of the relationship with systolic BP and PP.
The present study also identified a relationship between waist circumference and ARD that is independent of the 4 main hemodynamic correlates, a relationship that is also unaffected by influences of age, sex, and height. Central fat distribution is thus another important correlate of ARD and, in the multivariable analysis, obscures the relationship identified with body composition in the exploratory univariate analyses.
Central fat distribution has been reported to be associated with elevated levels of circulating inflammatory markers.31, 32 There are many conditions in which a high inflammatory state has been reported to be associated with AR dilatation,33, 34 thus the correlation between ARD and markers of inflammation, such as neutrophil percentage in the present study, was not a surprise.35 Our understanding of the role of the neutrophil in inflammation has changed fundamentally in recent years. Despite the traditional interpretation of neutrophils as pure markers of immune response to external factors, it is now recognized that activated neutrophils are similar to macrophages and produce a number of inflammatory cytokines, proteases, and chemoattractants36 that might play important roles in the process of arteriosclerosis and aortic dilatation.
The relationship of ARD with plasminogen activator inhibitor‐1 is also a new finding of our analysis. In addition to its activity on fibrinolysis, plasminogen activator inhibitor‐1 regulates circulating levels of a number of inflammatory cytokines, such as tumor necrosis factor‐α, interleukin‐6, and interferon‐γ,37 and might also be overexpressed as a consequence of the upregulation of proinflammatory cytokines (overproduced by visceral fat). Endothelium dysfunction, a consequence of the latent inflammatory status in visceral obesity, increases secretion of a number of endothelial products such as plasminogen activator inhibitor‐1,38 generating a vicious cycle. Experimental studies and human conditions strongly suggest that inflammation is a concurrent cause of aortic dilatation.39, 40
Our findings need to be interpreted taking into account the cross‐sectional nature of this study, which does not allow any cause–effect inference; however, it is interesting that despite the interference of a number of physiological and pathological confounders, a close correlation remains between ARD and the key hemodynamic parameters studied in this analysis. The equation tested in this analysis was derived from a multiethnic population but was applied to a specific ethnicity, a potential mismatch that should be taken into account when interpreting our results; however, the equation has already been validated in different populations.22
In addition, despite the good reliability tested in our echocardiography laboratory,23 the limitations inherent to ultrasound evaluation of CV structures (signal noise, acoustic artifacts) should always be considered when interpreting these data. In our analysis, the impact of these potential technical pitfalls is at least partially limited by the large number of observations, which dilutes the error.41
Finally, in evaluating the results obtained with central pressure, one needs to take into account that the calibration of the radial waveforms was done using brachial diastolic and systolic pressure, thus neglecting the possible amplification from brachial to radial site, with the possibility of a slight underestimation of calculated central pressure.42, 43
Perspectives
We used a novel approach to evaluate deviations of ARD from what would be appropriate for a given age, sex, and height, and that allowed us to identify a number of characteristics related to either small or large AR. This approach might be used in clinical practice to identify patients with AR dilatation and in longitudinal study to establish risk. Our findings also raise questions concerning the evolution of AR dilatation in hypertensive populations, based on hemodynamic characteristics of high blood pressure and the potential importance of the native AR in the progression toward isolated systolic hypertension. Further research needs to explore these aspects and possibly clarify whether findings at the level of the Valsalva sinuses may be extended to thoracic and abdominal aorta, as findings on carotid geometry might suggest.
Sources of Funding
This work was supported in part by grants RO1‐HL55673, U01‐HL54496, U01‐HL65521, and M10RR0047‐34 from the National Institutes of Health, Bethesda, Maryland.
Disclosures
None.
Acknowledgments
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. The authors wish to thank the Indian Health Service, the Strong Heart Study Participants, the Participating Tribal Communities and the Strong Heart Study Center Coordinators for their help in the realization of this project.
(J Am Heart Assoc. 2015;4:e002309 doi: 10.1161/JAHA.115.002309)
Views expressed in this paper are those of the authors and do not necessarily reflect those of the Indian Health Service or the Federal Government.
References
- 1. Palmieri V, Bella JN, Arnett DK, Roman MJ, Oberman A, Kitzman DW, Hopkins PN, Paranicas M, Rao DC, Devereux RB. Aortic root dilatation at sinuses of valsalva and aortic regurgitation in hypertensive and normotensive subjects: the Hypertension Genetic Epidemiology Network Study. Hypertension. 2001;37:1229–1235. [DOI] [PubMed] [Google Scholar]
- 2. Cuspidi C, Meani S, Fusi V, Valerio C, Sala C, Zanchetti A. Prevalence and correlates of aortic root dilatation in patients with essential hypertension: relationship with cardiac and extracardiac target organ damage. J Hypertens. 2006;24:573–580. [DOI] [PubMed] [Google Scholar]
- 3. Cipolli JA, Souza FA, Ferreira‐Sae MC, Pio‐Magalhaes JA, Figueiredo ES, Vidotti VG, Matos‐Souza JR, Franchini KG, Nadruz W Jr. Sex‐specific hemodynamic and non‐hemodynamic determinants of aortic root size in hypertensive subjects with left ventricular hypertrophy. Hypertens Res. 2009;32:956–961. [DOI] [PubMed] [Google Scholar]
- 4. Jiang JJ, Chen XF, Liu XM, Tang LJ, Lin XF, Pu ZX, Chen TL, Zhang Y, Wang YP, Wang JA. Aortic root dilatation is associated with carotid intima‐media thickness but not with carotid plaque in hypertensive men. Acta Cardiol. 2009;64:645–651. [DOI] [PubMed] [Google Scholar]
- 5. Chironi G, Orobinskaia L, Megnien JL, Sirieix ME, Clement‐Guinaudeau S, Bensalah M, Azarine A, Mousseaux E, Simon A. Early thoracic aorta enlargement in asymptomatic individuals at risk for cardiovascular disease: determinant factors and clinical implication. J Hypertens. 2010;28:2134–2138. [DOI] [PubMed] [Google Scholar]
- 6. Vasan RS, Larson MG, Levy D. Determinants of echocardiographic aortic root size. The Framingham Heart Study. Circulation. 1995;91:734–740. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell GF, Conlin PR, Dunlap ME, Lacourciere Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL Jr, Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51:105–111. [DOI] [PubMed] [Google Scholar]
- 8. Kim M, Roman MJ, Cavallini MC, Schwartz JE, Pickering TG, Devereux RB. Effect of hypertension on aortic root size and prevalence of aortic regurgitation. Hypertension. 1996;28:47–52. [DOI] [PubMed] [Google Scholar]
- 9. Roman MJ, Devereux RB, Kramer Fox R, O'Loughlin J. Two‐dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507–512. [DOI] [PubMed] [Google Scholar]
- 10. Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, Mitchell GF, Benjamin EJ, Vasan RS. Aortic root remodeling over the adult life course: longitudinal data from the Framingham Heart Study. Circulation. 2010;122:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devereux RB, de SG, Arnett DK, Best LG, Boerwinkle E, Howard BV, Kitzman D, Lee ET, Mosley TH Jr, Weder A, Roman MJ. Normal limits in relation to age, body size and gender of two‐dimensional echocardiographic aortic root dimensions in persons >/=15 years of age. Am J Cardiol. 2012;110:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheil MLK, Jenkins O, Sholler GF. Echocardiographic assessment of aortic root dimensions in normal children based on measurement of a new ratio of aortic size independent of growth. Am J Cardiol. 1995;75:711–715. [DOI] [PubMed] [Google Scholar]
- 13. Gautier M, Detaint D, Fermanian C, Aegerter P, Delorme G, Arnoult F, Milleron O, Raoux F, Stheneur C, Boileau C, Vahanian A, Jondeau G. Nomograms for aortic root diameters in children using two‐dimensional echocardiography. Am J Cardiol. 2010;105:888–894. [DOI] [PubMed] [Google Scholar]
- 14. Campens L, Demulier L, De GK, Vandekerckhove K, De WD, Roman MJ, Devereux RB, De PA, De BJ. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am J Cardiol. 2014;114:914–920. [DOI] [PubMed] [Google Scholar]
- 15. Drukteinis JS, Roman MJ, Fabsitz RR, Lee ET, Best LG, Russell M, Devereux RB. Cardiac and systemic hemodynamic characteristics of hypertension and prehypertension in adolescents and young adults: the Strong Heart Study. Circulation. 2007;115:221–227. [DOI] [PubMed] [Google Scholar]
- 16. Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, Devereux RB. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol. 2006;47:2267–2273. [DOI] [PubMed] [Google Scholar]
- 17. Stolarczyk LM, Heyward VH, Hicks VL, Baumgartner RN. Predictive accuracy of bioelectrical impedance in estimating body composition of Native American women. Am J Clin Nutr. 1994;59:964–970. [DOI] [PubMed] [Google Scholar]
- 18. Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the Strong Heart Study. Circulation. 2000;101:2271–2276. [DOI] [PubMed] [Google Scholar]
- 19. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 20. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux RB, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two‐ dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 21. de Simone G, Devereux RB, Ganau A, Hahn RT, Saba PS, Mureddu GF, Roman MJ, Howard BV. Estimation of left ventricular chamber and stroke volume by limited M‐mode echocardiography and validation by two‐dimensional and Doppler echocardiography. Am J Cardiol. 1996;78:801–807. [DOI] [PubMed] [Google Scholar]
- 22. van Kimmenade RRJ, Kempers M, de Boer MJ, Loeys BL, Timmermans J. A clinical appraisal of different Z‐score equations for aortic root assessment in the diagnostic evaluation of Marfan syndrome. Genet Med. 2013;15:528–532. [DOI] [PubMed] [Google Scholar]
- 23. Palmieri V, Bella JN, DeQuattro V, Roman MJ, Hahn RT, Dahlof B, Sharpe N, Lau CP, Chen WC, Paran E, de SG, Devereux RB. Relations of diastolic left ventricular filling to systolic chamber and myocardial contractility in hypertensive patients with left ventricular hypertrophy (The PRESERVE Study). Am J Cardiol. 1999;84:558–562. [DOI] [PubMed] [Google Scholar]
- 24. Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Relation of arterial structure and function to left ventricular geometric patterns in hypertensive adults. J Am Coll Cardiol. 1996;28:751–756. [DOI] [PubMed] [Google Scholar]
- 25. Roman MJ, Kizer JR, Best LG, Lee ET, Howard BV, Shara NM, Devereux RB. Vascular biomarkers in the prediction of clinical cardiovascular disease: the Strong Heart Study. Hypertension. 2012;59:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. [DOI] [PubMed] [Google Scholar]
- 27. Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. [DOI] [PubMed] [Google Scholar]
- 28. Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111:2306–2312. [DOI] [PubMed] [Google Scholar]
- 29. De Marco M, de Simone G, Roman MJ, Chinali M, Lee ET, Calhoun D, Howard BV, Devereux RB. Cardiac geometry and function in diabetic or prediabetic adolescents and young adults: the Strong Heart Study. Diabetes Care. 2011;34:2300–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–690. [Google Scholar]
- 31. Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 32. Despres JP. Abdominal obesity and cardiovascular disease: is inflammation the missing link? Can J Cardiol. 2012;28:642–652. [DOI] [PubMed] [Google Scholar]
- 33. Miller DV, Isotalo PA, Weyand CM, Edwards WD, Aubry MC, Tazelaar HD. Surgical pathology of noninfectious ascending aortitis: a study of 45 cases with emphasis on an isolated variant. Am J Surg Pathol. 2006;30:1150–1158. [DOI] [PubMed] [Google Scholar]
- 34. Gornik HL, Creager MA. Aortitis. Circulation. 2008;117:3039–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Herishanu Y, Rogowski O, Polliack A, Marilus R. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Haematol. 2006;76:516–520. [DOI] [PubMed] [Google Scholar]
- 36. Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford). 2010;49:1618–1631. [DOI] [PubMed] [Google Scholar]
- 37. Renckens R, Roelofs JJ, Bonta PI, Florquin S, de Vries CJ, Levi M, Carmeliet P, van't Veer C, van der Poll T. Plasminogen activator inhibitor type 1 is protective during severe Gram‐negative pneumonia. Blood. 2007;109:1593–1601. [DOI] [PubMed] [Google Scholar]
- 38. Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF. Among inflammation and coagulation markers, PAI‐1 is a true component of the metabolic syndrome. Int J Obes (Lond). 2006;30:1308–1314. [DOI] [PubMed] [Google Scholar]
- 39. Tambiah J, Franklin IJ, Trendell‐Smith N, Peston D, Powell JT. Provocation of experimental aortic inflammation and dilatation by inflammatory mediators and Chlamydia pneumoniae . Br J Surg. 2001;88:935–940. [DOI] [PubMed] [Google Scholar]
- 40. Cozijnsen L, Braam RL, Waalewijn RA, Schepens MA, Loeys BL, van Oosterhout MF, Barge‐Schaapveld DQ, Mulder BJ. What is new in dilatation of the ascending aorta? Review of current literature and practical advice for the cardiologist. Circulation. 2011;123:924–928. [DOI] [PubMed] [Google Scholar]
- 41. de Simone G, Muiesan ML, Ganau A, Longhini C, Verdecchia P, Palmieri V, Agabiti‐Rosei E, Mancia G. Reliability and limitations of echocardiographic measurement of left ventricular mass for risk stratification and follow‐up in single patients: the RES trial. Working Group on Heart and Hypertension of the Italian Society of Hypertension. Reliability of M‐mode Echocardiographic Studies. J Hypertens. 1999;17:1955–1963. [DOI] [PubMed] [Google Scholar]
- 42. Agnoletti D, Zhang Y, Salvi P, Borghi C, Topouchian J, Safar ME, Blacher J. Pulse pressure amplification, pressure waveform calibration and clinical applications. Atherosclerosis. 2012;224:108–112. [DOI] [PubMed] [Google Scholar]
- 43. Adji A, O'Rourke MF. Brachial artery tonometry and the Popeye phenomenon: explanation of anomalies in generating central from upper limb pressure waveforms. J Hypertens. 2012;30:1540–1551. [DOI] [PubMed] [Google Scholar]


