Abstract
Background
Primary stroke centers (PSCs) utilize more recombinant tissue plasminogen activator (rt‐PA) than non‐PSCs. The impact of PSCs on racial disparities in rt‐PA use is unknown.
Methods and Results
We used data from the Nationwide Inpatient Sample from 2004 to 2010, limited to states that publicly reported hospital identity and race. Hospitals certified as PSCs by The Joint Commission were identified. Adults with a diagnosis of ischemic stroke were analyzed. Rt‐PA use was defined by the International Classification of Diseases, 9th Revision procedure code 99.10. Discharges (304 152 patients) from 26 states met eligibility criteria, and of these 71.5% were white, 15.0% black, 7.9% Hispanic, and 5.6% other. Overall, 24.7% of white, 27.4% of black, 16.2% of Hispanic, and 29.8% of other patients presented to PSCs. A higher proportion received rt‐PA at PSCs than non‐PSCs in all race/ethnic groups (white 7.6% versus 2.6%, black 4.8% versus 2.0%, Hispanic 7.1% versus 2.4%, other 7.2% versus 2.5%, all P<0.001). In a multivariable model adjusting for year, age, sex, insurance, medical comorbidities, a diagnosis‐related group–based mortality risk indicator, ZIP code median income, and hospital characteristics, blacks were less likely to receive rt‐PA than whites at non‐PSCs (odds ratio=0.58, 95% CI 0.50 to 0.67) and PSCs (odds ratio=0.63, 95% CI 0.54 to 0.74) and Hispanics were less likely than whites to receive rt‐PA at PSCs (odds ratio=0.77, 95% CI: 0.63 to 0.95). In the fully adjusted model, interaction between race and presentation to a PSC for likelihood of receiving rt‐PA did not reach significance (P=0.98).
Conclusions
Racial disparities in intravenous rt‐PA use were not reduced by presentation to PSCs. Black patients were less likely to receive thrombolytic treatment than white patients at both non‐PSCs and PSCs. Hispanic patients were less likely to be seen at PSCs relative to white patients and were less likely to receive intravenous rt‐PA in the fully adjusted model.
Keywords: health disparities, health policy, stroke, stroke care, thrombolysis
Intravenous recombinant tissue plasminogen activator (rt‐PA) in acute ischemic stroke has been shown to improve outcomes in appropriately selected patients when administered within 4.5 hours.1, 2, 3 Despite multiple consensus guidelines, utilization of rt‐PA remains low.4, 5 Low treatment rates are partially explained by delayed hospital arrival, but provider‐level factors may also contribute.6 Utilization of rt‐PA varies further by age, socioeconomic status, hospital size and case volume, geography, and use of emergency medical services (EMS).7, 8, 9, 10, 11, 12
The past decade has witnessed broad adoption of stroke systems of care with EMS routing protocols focused on early detection of acute ischemic stroke, and prompt transport of patients to stroke centers.13, 14, 15 In December 2003, The Joint Commission established a national certification process for Primary Stroke Centers (PSCs), based on recommendations from the Brain Attack Coalition. Hospitals seeking certification were required to implement elements such as an acute stroke team, written care protocols, coordination with EMS, and creation of a stroke unit.13 Growing evidence indicates that PSCs utilize more rt‐PA than non‐PSCs and have lower mortality rates than noncertified hospitals.16, 17, 18, 19, 20
The role of race in rt‐PA utilization is uncertain. Some previous studies have shown that black patients receive IV rt‐PA 1/5 to 3/4 as often as white patients,8, 9, 21, 22, 23, 24 but other studies have reported no difference in rates of treatment.25, 26 Data for Hispanic patients are also conflicting. In one large nationwide population sample, Nasr et al found lower rates of rt‐PA use for Hispanics.24 In a separate study, Hispanic patients had equivalent odds of receiving therapy as white patients.27 Recognizing the limited and inconsistent evidence concerning stroke and racial disparities, the American Heart Association/American Stroke Association recommended further research on this important topic.28
Using a nationwide hospital administrative database, we sought to quantify access to PSCs by race and ethnicity and to compare rt‐PA use at PSCs and non‐PSCs. We hypothesized that disparities would be reduced at PSCs compared to hospitals without specialty stroke care.
Methods
Study Design
We performed a retrospective cohort study using data from the Nationwide Inpatient Sample (NIS) 2004–2010. The NIS, from the Agency for Healthcare Research and Quality's Healthcare Cost and Utilization Project, is the largest publicly available all‐payer inpatient care database.29 The analysis was limited to data from the 26 states that publicly identified both treating hospital and patient race/ethnicity, listed in Table 1. PSC data, including date of initial certification, were obtained via personal communication from The Joint Commission (Jean Range, Executive Director of Disease‐Specific Care Program, The Joint Commission, January 1, 2013).
Table 1.
States With Identifiable Hospitals and Race/Ethnicity in the Nationwide Inpatient Sample, 2004–2010
| Year | Total No. States | No. States w/Identifiable Hospitals and Race | States Reporting Hospital Identity and Race |
|---|---|---|---|
| 2004 | 37 | 18 | AZ, CA, CO, CT, FL, IA, MD, MA, MO, NH, NJ, NY, NC, RI, UT, VT, VA, WI |
| 2005 | 37 | 17 | AZ, CA, CO, CT, FL, IA, MD, MA, MO, NH, NJ, NY, NC, RI, UT, VT, WI |
| 2006 | 38 | 18 | AZ, CA, CO, CT, FL, IA, MD, MA, MO, NH, NJ, NY, NC, RI, UT, VT, VA, WI, |
| 2007 | 40 | 18 | AZ, CA, CO, CT, FL, IA, MD, MA, MO, NH, NJ, NY, NC, RI, UT, VT, VA, WI |
| 2008 | 42 | 23 | AZ, CA, CO, CT, FL, IA, KY, MD, MA, MO, NV, NH, NJ, NY, NC, OR, PA, RI, UT, VT, VA, WA, WI |
| 2009 | 44 | 23 | AZ, CA, CO, CT, FL, IL, IA, KY, MD, MA, MT, NV, NH, NJ, NY, OR, PA, RI, UT, VT, VA, WA, WI |
| 2010 | 45 | 23 | AZ, CA, CO, CT, FL, IL, IA, KY, MD, MA, MS, MT, NV, NJ, NY, NC, OR, PA, RI, UT, VT, VA, WI |
Patients age ≥18 years with a primary diagnosis of ischemic stroke defined by International Classification of Diseases, 9th Revision (ICD‐9) codes 433.x1, 434.x1, and 436 were identified. These codes demonstrate >85% positive predictive value for acute ischemic stroke.30 Patients transferred from other hospitals were excluded. Hospitalizations with complete data for all variables were included. Those missing information on death, sex, length of stay, or expected primary payer were excluded (Figure 1). Our primary end point, treatment with intravenous rt‐PA, was identified by International Classification of Diseases, 9th Revision procedure code 99.10.
Figure 1.
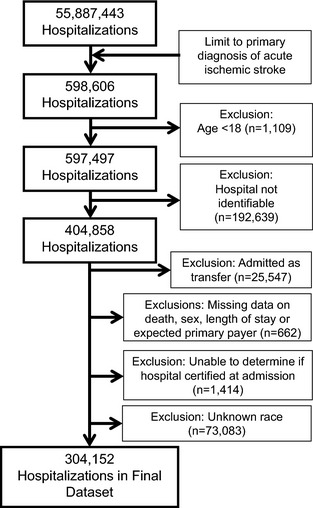
Flow diagram depicting the patients in the data set, exclusion criteria, and final number of hospitalizations included, 304 152.
This study used de‐identified data from an administrative claims database, so institutional review board approval and obtaining informed consent were not required.
Demographic Variables
The NIS classifies race/ethnicity uniformly as white, black, Hispanic, Asian/Pacific Islander, Native American, or other. Information from hospitals providing the race or ethnicity of patients are coded from state‐specific data into 1 of the 6 NIS classifications. Race and ethnicity are not reported separately and ethnicity takes precedence over race for coding. Other patient‐level variables included age, sex, year of discharge, expected primary payer (Medicaid, Medicare, private, or other), median household income in the patient's ZIP code, comorbid conditions (Table 2),31 and an all patient refined‐diagnosis related group (APR‐DRG) measure of the risk of inpatient mortality, which uses diagnoses and procedure codes to estimate the likelihood of dying during the hospitalization as minor, moderate, major, or extreme.32 The all patient refined‐diagnosis related group marker is not specific to stroke and does not include a measure of stroke severity. Hospital‐level variables included geographic region (Northeast, Midwest, South, or West), rural or urban location, status as a teaching hospital (yes/no), and annual ischemic stroke case volume (<100, 100 to 299, or ≥300).
Table 2.
Elixhauser Comorbidities
| AIDS |
| Alcohol abuse |
| (Deficiency) anemias |
| Rheumatoid arthritis |
| Blood loss anemia |
| Congestive heart failure |
| Chronic pulmonary disease |
| Coagulopathy |
| Depression |
| Diabetes (uncomplicated) |
| Diabetes (w/chronic complications) |
| Drug abuse |
| Hypertension |
| Hypothyroidism |
| Liver disease |
| Lymphoma |
| Fluid and electrolyte disorders |
| Metastatic cancer |
| Other neurological disorders |
| Obesity |
| Paralysis |
| Peripheral vascular disorders |
| Psychoses |
| Pulmonary circulation disorders |
| Renal failure |
| Solid tumor (without metastasis) |
| Peptic ulcer disease (no bleeding) |
| Valvular disease |
| Weight loss |
Statistical Analysis
Baseline characteristics were described for patients treated at PSCs and non‐PSCs using measures of central tendency (means, medians) for continuous variables and proportions for categorical variables. Differences between the groups were evaluated using Student t test, Wilcoxon rank‐sum, and χ2 tests, as appropriate. All data were stratified by race. Bonferroni correction was used to correct for multiple comparisons. A multivariable model was constructed to determine independent associations including year of discharge, age, sex, primary expected payer, median income by zip code, hospital region, teaching status, urban/rural location, and ischemic stroke admission volume, 29 Elixhauser comorbid conditions, and the all patient refined‐diagnosis related group measure of disease severity. This model was used first to determine rt‐PA use by each race individually at PSCs versus non‐PSCs and then separate models were constructed for each race, with white patients as the referent group. Because the introduction of diagnosis‐related group (DRG) 559 in 2006 may have encouraged more accurate coding for IV tPA use, we also performed a sensitivity analysis limiting our study population to 2006 and later. Our analytic models used NIS survey statistics and Taylor series estimation to account for the survey design and clustering within hospitals. The analysis was conducted using SAS‐callable‐SUDAAN version 11.0.1.
Results
Study Sample
Acute ischemic stroke was the primary diagnosis in 598 606 hospitalizations in the NIS between 2004 and 2010, and of these 304 152 from 26 states met all eligibility criteria, as diagrammed in Figure 1.
Demographic Characteristics
Of the 304 152 patients included in the analysis, 75 160 (24.7%) presented to a PSC and 228 992 (75.3%) presented to a non‐PSC. Overall, 71.5% of patients were white, 15.0% black, 7.9% Hispanic, and 5.6% were in other categories (Asian/Pacific Islander, Native American, or other). Patient and hospital characteristics are described in Table 3.
Table 3.
Patient and Hospital Characteristics
| Patients (%) | Overall | Presenting to a PSC | Presenting to a Non‐PSC |
|---|---|---|---|
| n=304 152 | n=75 160 (24.7%) | n=228 992 (75.3%) | |
| Race/ethnicitya | |||
| White | 217 399 (71.5) | 53 693 (71.4) | 163 706 (71.5) |
| Black | 45 635 (15.0) | 12 517 (16.7) | 33 118 (14.5) |
| Hispanic | 24 163 (7.9) | 3904 (5.2) | 20 259 (8.8) |
| Othersb | 16 955 (5.6) | 5046 (6.7) | 11 909 (5.2) |
| Femalea | 162 311 (53.4) | 39 138 (52.1) | 123 173 (53.8) |
| Age, ya | |||
| 18 to 44 | 11 931 (3.9) | 3346 (4.5) | 8585 (3.7) |
| 45 to 64 | 76 297 (25.1) | 20 170 (26.8) | 56 127 (24.5) |
| ≥65 | 215 924 (71.0) | 51 644 (68.7) | 164 280 (71.7) |
| Incomec,d | |||
| Lowest quartile | 71 340 (23.5) | 16 433 (21.9) | 54 907 (24.0) |
| Second quartile | 73 584 (24.2) | 16 688 (22.2) | 56 896 (24.8) |
| Third quartile | 73 348 (24.1) | 17 299 (23.0) | 56 049 (24.5) |
| Highest quartile | 78 935 (26.0) | 23 413 (31.2) | 55 522 (24.2) |
| Missing | 6945 (2.3) | 1327 (1.8) | 5618 (2.5) |
| Payment typea | |||
| Medicare | 206 772 (68.0) | 49 295 (65.5) | 157 513 (68.8) |
| Medicaid | 20 688 (6.8) | 4937 (6.6) | 15 751 (6.9) |
| Private, including HMO | 57 510 (18.9) | 15 735 (20.9) | 41 775 (18.2) |
| Self‐pay | 11 376 (3.7) | 3105 (4.1) | 8271 (3.6) |
| No charge | 1676 (0.6) | 468 (0.6) | 1208 (0.5) |
| Other | 6130 (2.0) | 1656 (2.2) | 4474 (2.0) |
| Hospital regiona | |||
| Northeast | 93 858 (30.9) | 15 978 (21.3) | 77 880 (34.0) |
| Midwest | 31 047 (10.2) | 8662 (11.5) | 22 385 (9.8) |
| South | 98 603 (32.4) | 34 692 (46.2) | 63 911 (27.9) |
| West | 80 644 (26.5) | 15 828 (21.1) | 64 816 (28.3) |
| Hospital locationa | |||
| Rural | 32 003 (10.5) | 2032 (2.7) | 29 971 (13.1) |
| Teaching hospitala | |||
| Yes | 125 088 (41.1) | 43 905 (58.4) | 81 183 (35.5) |
| Ischemic stroke volume, cases/yeara | |||
| <100 | 44 119 (14.5) | 977 (1.3) | 43 142 (18.8) |
| 100 to 299 | 141 594 (46.6) | 24 874 (33.1) | 116 720 (51.0) |
| ≥300 | 118 439 (38.9) | 49 309 (65.6) | 69 130 (30.2) |
HMO indicates health maintenance organization; PSC, Primary Stroke Center.
Chi‐square test between non‐PSC and PSC significant, P<0.001.
Others includes Asian/Pacific Islander, Native American, and other.
Chi‐square test between non‐PSC and PSC significant, P=0.005.
Median household income, by ZIP code.
Presentation to PSCs and Intravenous rt‐PA Use
Presentation to PSCs occurred for 24.7% of white patients, 27.4% of black patients, 16.2% of Hispanic patients, and 29.8% of patients of other races. A lower proportion of Hispanic patients were seen at PSCs. Figure 2 shows the proportion of patients presenting to PSCs over time by race/ethnicity. For presentation to PSCs, testing for differences between individual groups (white versus black, white versus Hispanic, white versus other, black versus Hispanic, black versus other, and Hispanic versus other) yielded P<0.001. In total, 3.6% of patients received intravenous rt‐PA. Utilization of rt‐PA occurred in 7.1% of patients at PSCs and 2.5% of patients at non‐PSCs. A higher proportion of patients received rt‐PA at PSCs in all racial/ethnic groups compared to treatment rates at non‐PSCs: white patients 7.6% versus 2.6% (P<0.001), black patients 4.8% versus 2.0% (P<0.001), Hispanic patients 7.1% versus 2.4% (P<0.001), and other racial groups 7.2% versus 2.5% (P<0.001), as depicted in Figure 3.
Figure 2.
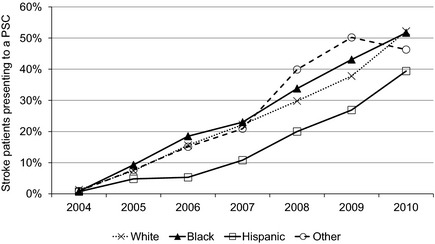
Proportion of stroke patients presenting to PSCs over time, by race/ethnicity. PSC indicates Primary Stroke Center.
Figure 3.
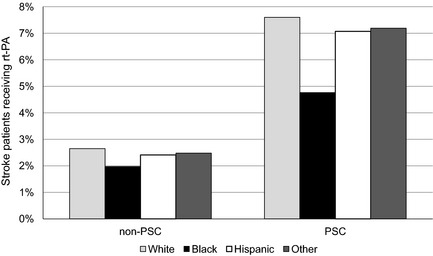
Proportion of patients receiving rt‐PA at non‐PSCs and PSCs, by race/ethnicity (white patients 2.6% vs 7.6%; black patients 2.0% vs 4.8%; Hispanic patients 2.4% vs 7.1%; other racial groups 2.5% vs 7.2%). PSC indicates Primary Stroke Center; rt‐PA, recombinant tissue plasminogen activator.
In the unadjusted analysis, the odds of receiving rt‐PA were greater at a PSC versus a non‐PSC for all groups (whites odds ratio (OR)=3.03, 95% CI: 2.65 to 3.45; blacks OR=2.49, 95% CI: 2.01 to 3.09; Hispanics OR=3.08, 95% CI: 2.40 to 3.94; other races OR=3.05, 95% CI: 2.35 to 3.97). Increased odds of rt‐PA use was further demonstrated in the fully adjusted analyses (whites OR=1.73, 95% CI: 1.50 to 2.00; blacks OR=1.47, 95% CI: 1.16 to 1.87; Hispanics OR=1.54, 95% CI: 1.16 to 2.05; other races OR=1.99, 95% CI: 1.49 to 2.66) as shown in Table 4. Testing for interaction between race and PSC status for the outcome of rt‐PA treatment, no significant effect was demonstrated either in the crude (P=0.62) or fully adjusted analysis (P=0.98). We found similar results for the sensitivity analysis limited to data from 2006 to 2010 (Table 5), the period after the introduction of DRG559 “Acute ischemic stroke with use of thrombolytic agent.”
Table 4.
Odds of Receiving rt‐PA at a PSC Versus Non‐PSC, by Race, in the NIS 2004–2010
| Race | Treatment Rate | Unadjusted | Model 1a | Model 2b | Model 3c | |
|---|---|---|---|---|---|---|
| PSC (%) | Non‐PSC (%) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| White | 7.60 | 2.65 | 3.03 (2.65 to 3.45) | 1.81 (1.60 to 2.05) | 1.73 (1.50 to 2.00) | 1.73 (1.50 to 2.00) |
| Black | 4.76 | 1.97 | 2.49 (2.01 to 3.09) | 1.63 (1.30 to 2.05) | 1.46 (1.15 to 1.85) | 1.47 (1.16 to 1.87) |
| Hispanic | 7.07 | 2.41 | 3.08 (2.40 to 3.94) | 1.77 (1.34 to 2.35) | 1.55 (1.17 to 2.05) | 1.54 (1.16 to 2.05) |
| Othersd | 7.19 | 2.48 | 3.05 (2.35 to 3.97) | 2.37 (1.82 to 3.10) | 2.00 (1.50 to 2.67) | 1.99 (1.49 to 2.66) |
APR‐DRG indicates all patient refined‐diagnosis related group; NIS, Nationwide Inpatient Sample; OR, odds ratio; PSC, Primary Stroke Center; rt‐PA, recombinant tissue plasminogen activator.
Adjusted for: year, age, sex, primary expected payer, median income quartiles by ZIP code, region, teaching hospital, urban hospital location, and volume of acute ischemic stroke annually at hospital.
Model 1+each of the 29 Agency for Healthcare Quality and Research (individual) comorbidities.
Model 2+APR‐DRG measure of disease severity to estimate the likelihood of dying during the hospitalization (minor, moderate, major, and extreme likelihoods of dying).
Others includes Asian/Pacific Islander, Native American, and other.
Table 5.
Sensitivity Analysis: Odds of Receiving rt‐PA at a PSC Versus Non‐PSC, by Race, in the NIS 2006–2010
| Race | Unadjusted | Model 1a | Model 2b | Model 3c |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| White | 2.52 (2.21 to 2.89) | 1.74 (1.54 to 1.96) | 1.65 (1.44 to 1.90) | 1.66 (1.44 to 1.90) |
| Black | 2.01 (1.61 to 2.51) | 1.66 (1.31 to 2.09) | 1.52 (1.18 to 1.95) | 1.52 (1.18 to 1.97) |
| Hispanic | 2.65 (2.07 to 3.40) | 1.80 (1.35 to 2.39) | 1.59 (1.19 to 2.13) | 1.59 (1.19 to 2.13) |
| Othersd | 2.75 (2.10 to 3.60) | 2.49 (1.89 to 3.28) | 2.13 (1.55 to 2.94) | 2.13 (1.55 to 2.94) |
APR‐DRG indicates all patient refined‐diagnosis related group; NIS, Nationwide Inpatient Sample; OR, odds ratio; PSC, Primary Stroke Center; rt‐PA, recombinant tissue plasminogen activator.
Adjusted for: year, age, sex, primary expected payer, median income quartiles by ZIP code, region, teaching hospital, urban hospital location, and volume of acute ischemic stroke annually at hospital.
Model 1+each of the 29 Agency for Healthcare Quality and Research (individual) comorbidities.
Model 2+APR‐DRG measure of disease severity to estimate the likelihood of dying during the hospitalization (minor, moderate, major, and extreme likelihoods of dying).
Others includes Asian/Pacific Islander, Native American, and other.
Comparing rt‐PA treatment rates between each of the nonwhite groups and whites showed that individuals in these racial groups were significantly less likely to be treated, after adjustment for covariates, at both PSCs and non‐PSCs (Table 6). Black patients were significantly less likely to be treated than whites at both PSCs and non‐PSCs in both the unadjusted and fully adjusted models. This trend for black patients was similar across all subgroups treated at PSCs, stratified by sex, age, insurance status, hospital region, and hospital stroke volume (Figure 4A). Hispanic patients and patients of other races were as likely to be treated as whites at PSCs in the unadjusted model but less likely to be treated in the fully adjusted model (Table 6). Rates of treatment at PSCs, stratified across subgroups, are shown for Hispanics versus whites and other races versus whites (Figure 4B and 4C).
Table 6.
Odds of Receiving rt‐PA Compared to Whites, Stratified by PSC Status, in the NIS 2004–2010
| Unadjusted OR (95% CI) | Fully Adjusted Modela OR (95% CI) | |||
|---|---|---|---|---|
| PSC | Non‐PSC | PSC | Non‐PSC | |
| Black vs white | 0.61 (0.52 to 0.71) | 0.74 (0.64 to 0.85) | 0.63 (0.54 to 0.74) | 0.58 (0.50 to 0.67) |
| Hispanic vs white | 0.92 (0.76 to 1.12) | 0.91 (0.76 to 1.09) | 0.77 (0.63 to 0.95) | 0.75 (0.63 to 0.88) |
| Othersb vs white | 0.94 (0.78 to 1.14) | 0.93 (0.79 to 1.11) | 0.75 (0.64 to 0.89) | 0.74 (0.62 to 0.88) |
APR‐DRG indicates all patient refined‐diagnosis related group; NIS, Nationwide Inpatient Sample; OR, odds ratio; PSC, Primary Stroke Center; rt‐PA, recombinant tissue plasminogen activator.
Multivariable model adjusted for: year, age, sex, primary expected payer, median income quartiles by ZIP code, region, teaching hospital, urban hospital location, volume of acute ischemic stroke annually at hospital, each of the 29 Agency for Healthcare Quality and Research (individual) comorbidities, and an APR‐DRG measure of disease severity to estimate the likelihood of dying during the hospitalization (minor, moderate, major, and extreme likelihoods of dying).
Others includes Asian/Pacific Islander, Native American, and other.
Figure 4.
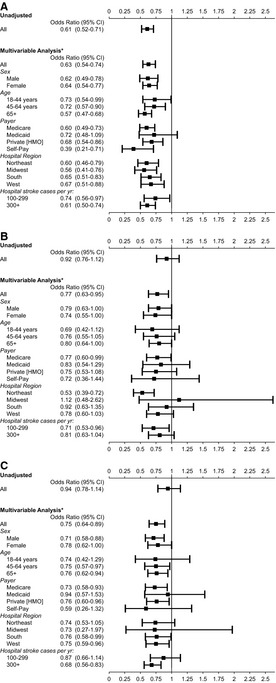
Odds of rt‐PA use at PSCs for (A) black patients, (B) Hispanic patients, and (C) Asian/Pacific Islander, Native American, and other patients, as compared to white patients. *Excluding the subgroup of interest, adjusted for: year, age, sex, primary expected payer, median income quartiles by ZIP code, region, teaching hospital, urban hospital location, volume of acute ischemic stroke annually at hospital, each of the 29 Agency for Healthcare Quality and Research (individual) comorbidities, and an APR‐DRG measure of disease severity to estimate the likelihood of dying during the hospitalization (minor, moderate, major, and extreme likelihoods of dying). APR‐DRG indicates all patient refined‐diagnosis related group; HMO, health maintenance organization; PSC, Primary Stroke Center; rt‐PA, recombinant tissue plasminogen activator.
Discussion
Racial and ethnic disparities in stroke are a pressing policy and public health issue, as minorities are projected to become a majority of the population in the United States by 2060.33, 34 In the present analysis, the proportion of patients receiving rt‐PA was similar in Hispanic patients and white patients. Hispanic patients presented less frequently to PSCs than white patients, consistent with a previous report.17 The disparity in presentation to PSCs persisted throughout the time period studied. Black patients presented to PSCs at rates that were similar to white patients, but they were less likely to receive rt‐PA at both PSCs and non‐PSCs in both the adjusted and unadjusted models. Patients in other racial groups (Hispanic or Asian/Pacific Islander, Native American, and other) received rt‐PA at a rate similar to white patients at both PSCs and non‐PSCs in the unadjusted model, but were less likely to receive rt‐PA after adjusting for covariates.
A greater burden of stroke incidence and mortality for black and Hispanic patients has been established in the literature, though reasons for this disparity are unclear.36, 37, 38, 39 Few studies have looked at presentation to PSCs by race and ethnicity.18, 40 In the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study, presentation to PSCs is similar among black and white patients, though subjects living in the Stroke Belt states were less likely to be seen at a PSC.11 PSC access in <60 minutes by ground ambulance is most available in major cities and access is limited in rural areas.
The present study demonstrates a difference in utilization of PSCs for Hispanic patients. Certain factors may play a role for this minority group, such as the growing rural Hispanic population or an inability to access the healthcare system for financial or legal reasons in areas where PSCs are readily available.41 Prior studies have suggested that geographic areas with a greater proportion of Hispanic individuals are actually more likely to have access to PSCs,42, 43 but realized access may be lower than geographic access. McDonald et al have shown that hospitals under governmental control were less likely to acquire PSC certification.43 This could disproportionately affect uninsured and underinsured minority patients.
Our study corroborates findings of increased rt‐PA utilization among all race/ethnicities at PSCs. This is consistent with the findings of Bhattacharya et al, who performed a chart review of 5 PSCs and 5 non‐PSCs in Michigan and found improved compliance with core measures for stroke care and increased use of rt‐PA for black patients at stroke centers.40 Our data demonstrate that whereas PSCs treat a higher proportion of black patients with rt‐PA than non‐PSCs, treatment rates in black patients continue to lag behind other racial and ethnic groups even at these specialty stroke centers. This finding is remarkably consistent across subgroups stratified by age, sex, insurance status, hospital region, and hospital stroke volume.
Trimble and Morgenstern described minority‐specific factors that contribute to inequalities in access and use of health services.44 On the patient level, mistrust or misunderstanding of the healthcare system, poor communication, low income, and lower education may delay or prevent care. There may be differences in stroke severity or other rt‐PA inclusion/exclusion criteria across racial groups. Black and Hispanic stroke patients present at younger ages,39, 45 and younger patients tend to suffer milder strokes, by National Institutes of Health Scale.46, 47 This could lead to withholding rt‐PA because of either diagnostic uncertainty or a low perceived likelihood of disability from the presenting symptoms, yet, in 1 multicenter study of young adults with stroke, black patients suffered worse 30‐day mortality and functional outcomes compared to white patients.48 Mexican‐American patients suffer worse functional outcomes after stroke, despite having a paradoxical lower mortality and longer survival period, emphasizing the importance of timely evaluation and treatment in the rapidly growing Hispanic population.49
Differences in time to presentation or utilization of EMS may also underlie differences in rt‐PA utilization.50, 51, 52, 53 Existing data on differences in prehospital delays and EMS use are conflicting. Studies in New York City, Minneapolis–St. Paul, and Houston failed to show differences in delay to emergency department arrival for black patients.54, 55, 56, 57 More recent studies demonstrated significant differences in delay to arrival within 3 hours22, 52, 58 that persist after adjusting for age, sex, stroke severity, and insurance status.26 Boehme et al found that black women, specifically, are more likely to arrive outside of the 3‐hour time window than white men, black men, or white women.59 In their analysis of >200 000 acute stroke cases in the Get With The Guidelines Stroke registry, Ekundayo et al found that minorities were less likely to use EMS,12 but in the Greater Cincinnati/Northern Kentucky Stroke Study there was no difference in EMS utilization between white and black patients.60 A better understanding of racial differences in time to presentation and use of EMS may help to increase our understanding of modifiable risk factors to inform future interventions, with the ultimate goal of eliminating disparities in stroke care.
Our analysis, which relies on administrative claims data, has limitations. The NIS coding for race/ethnicity is simplified from state‐specific values into 1 of 6 categories used in this analysis. Though reporting of race has increased over time, hospitals or Healthcare Cost and Utilization Project State Partners that do not supply these data were excluded, including a few states with large minority populations such as Georgia and Texas. Unknown race was common, likely due to the data being from hospital discharge information and not from reporting by the individuals themselves. Although the 26 states that provided data cover roughly 50% of the Hispanic and black population in the United States, the analysis does not constitute a nationally representative sample.61, 62
Our study defines PSCs by The Joint Commission certification, and does not recognize other national or state‐based certifications or identify hospitals that participate in national stroke care improvement programs, such as Get With The Guidelines. In our analysis, 25 of the 26 states had PSC hospitals, the exception being Massachusetts, which uses a state‐based certification program. Misclassification of these hospitals as nonstroke centers would likely bias our results toward the null and not change the observed disparities. A benefit of utilizing administrative data such as the NIS is the ability to compare hospitals with PSC certification to non‐PSC hospitals, even those that have elected not to share data with a quality improvement initiative or a stroke registry.
Because stroke diagnosis was identified by International Classification of Diseases, 9th Revision code, clinical information on stroke severity, such as the National Institutes of Health Scale or time of symptom onset, was unavailable for analysis. Lacking clinical data, we could not ascertain from our analysis the percentage of eligible patients who failed to receive rt‐PA, of any race. As discussed above, there may be differences in time to arrival, utilization of EMS, and stroke severity by race. All of these factors may be contributing to lower rt‐PA eligibility and thus lower treatment rates in black patients. Unfortunately, we are unable to test this hypothesis and existing literature is conflicting.8, 22, 54, 55, 56, 57 Prospective studies with detailed clinical data are needed to determine whether racial disparities persist after accounting for rt‐PA eligibility.
International Classification of Diseases, 9th Revision procedure code 99.10 was used to define rt‐PA. This underestimates rt‐PA use relative to pharmacy billing records, detecting 77% of rt‐PA cases in an analysis of the Get With The Guidelines data, and has the potential to bias the results if there are differences in coding across hospitals.63, 64 However, trends showing the increasing use of rt‐PA over time are consistent and financial pressures should encourage accurate coding at all hospitals. The NIS lacks data on Current Procedural Terminology codes 37201 and 37202, which might increase sensitivity for rt‐PA therapy use.65 Because our study excludes patients admitted as transfers, we are unable to investigate “drip and ship” cases, in which rt‐PA is administered in the emergency department of 1 hospital and then transferred to another hospital. It may be that non‐PSCs are more likely to transfer patients to another hospital after rt‐PA treatment. Excluding transfers avoids incorrectly attributing these patients to the receiving hospital. However, not accounting for drip and ship cases may underestimate rt‐PA utilization at non‐PSCs and lead us to overestimate the impact of certification.
In conclusion, our analysis provides an overview of utilization of specialty stroke care and rt‐PA administration among minority patients. While the increasing use of rt‐PA treatment over time is encouraging, efforts still need to be made to close the gaps in access to PSCs and rt‐PA use across racial and ethnic groups.
Sources of Funding
Dr Mullen received funding from National Heart, Lung, and Blood Institute (NHLBI) K12 HL083772 and R01 HS018362; Dr Kleindorfer received funding from National Institute of Neurological Disorders and Stroke (NINDS) U01NS041588; Dr Carr received funding from Agency for Healthcare Research and Quality (AHRQ) K08HS17960 and R01 HS018362; and Dr Albright received funding from AHRQ T32 HS013852‐10 and National Institute on Minority Health and Health Disparities National Institutes of Health (NIMHD NIH) P60 MD000502‐08S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, NIH, or the AHRQ.
Disclosures
Dr Carr spends a portion of his time in the Office of the Assistant Secretary for Preparedness and Response U.S. Department of Health and Human Services. The other authors have no conflicts to report.
Acknowledgments
We thank Jean Range, Executive Director, The Joint Commission, for providing PSC initial certification dates.
(J Am Heart Assoc. 2015;4:e001877 doi: 10.1161/JAHA.115.001877)
Data from this article were published in abstract form at the 2014 American Heart Association/American Stroke Association International Stroke Conference held in San Diego, California, February 12‐14.
The findings/conclusions of this report are those of the author and do not necessarily represent the views of the Department of Health and Human Services or its components.
References
- 1. Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC, Broderick JP, Levine SR, Frankel MP, Horowitz SH, Haley EC, Lewandowski CA, Kwiatkowski TP. Early stroke treatment associated with better outcome: the NINDS rt‐PA Stroke Study. Neurology. 2000;55:1649–1655. [DOI] [PubMed] [Google Scholar]
- 2. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP; Council AHAS . Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Physicians ACoE, Neurology AAo . Clinical policy: use of intravenous tPA for the management of acute ischemic stroke in the emergency department. Ann Emerg Med. 2013;61:225–243. [DOI] [PubMed] [Google Scholar]
- 4. Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, Bhatt DL, Grau‐Sepulveda MV, Peterson ED, Fonarow GC. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines‐Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6:543–549. [DOI] [PubMed] [Google Scholar]
- 5. Investigators CASPRC . Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–659. [DOI] [PubMed] [Google Scholar]
- 6. Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility Neurology. 2001;56:1015–1020. [DOI] [PubMed] [Google Scholar]
- 7. Willey JZ, Petersen N, Dhamoon MS, Stillman J, Boden‐Albala B, Elkind MS, Marshall RS. Safety of thrombolysis in patients over the age of 80. Neurologist. 2012;18:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnston SC, Fung LH, Gillum LA, Smith WS, Brass LM, Lichtman JH, Brown AN. Utilization of intravenous tissue‐type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity. Stroke. 2001;32:1061–1068. [DOI] [PubMed] [Google Scholar]
- 9. Kimball MM, Neal D, Waters MF, Hoh BL. Race and income disparity in ischemic stroke care: Nationwide Inpatient Sample database, 2002 to 2008. J Stroke Cerebrovasc Dis. 2012;23:17–24. [DOI] [PubMed] [Google Scholar]
- 10. Rost NS, Smith EE, Pervez MA, Mello P, Dreyer P, Schwamm LH. Predictors of increased intravenous tissue plasminogen activator use among hospitals participating in the Massachusetts Primary Stroke Service program. Circ Cardiovasc Qual Outcomes. 2012;5:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mullen MT, Judd S, Howard VJ, Kasner SE, Branas CC, Albright KC, Rhodes JD, Kleindorfer DO, Carr BG. Disparities in evaluation at certified primary stroke centers: reasons for geographic and racial differences in stroke. Stroke. 2013;44:1930–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekundayo OJ, Saver JL, Fonarow GC, Schwamm LH, Xian Y, Zhao X, Hernandez AF, Peterson ED, Cheng EM. Patterns of emergency medical services use and its association with timely stroke treatment: findings from Get With The Guidelines‐Stroke. Circ Cardiovasc Qual Outcomes. 2013;6:262–269. [DOI] [PubMed] [Google Scholar]
- 13. Alberts MJ, Latchaw RE, Jagoda A, Wechsler LR, Crocco T, George MG, Connolly ES, Mancini B, Prudhomme S, Gress D, Jensen ME, Bass R, Ruff R, Foell K, Armonda RA, Emr M, Warren M, Baranski J, Walker MD; Coalition BA . Revised and updated recommendations for the establishment of primary stroke centers: a summary statement from the Brain Attack Coalition. Stroke. 2011;42:2651–2665. [DOI] [PubMed] [Google Scholar]
- 14. Facts about primary stroke center certification. The Joint Commission website. 2015. Available at: http://www.jointcommission.org/facts_about_primary_stroke_center_certification/. Accessed January 30, 2015.
- 15. Song S, Saver J. Growth of regional acute stroke systems of care in the United States in the first decade of the 21st century. Stroke. 2012;43:1975–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prabhakaran S, McNulty M, O'Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke. 2012;43:875–877. [DOI] [PubMed] [Google Scholar]
- 17. Mullen MT, Kasner SE, Kallan MJ, Kleindorfer DO, Albright KC, Carr BG. Joint Commission primary stroke centers utilize more rt‐PA in the Nationwide Inpatient Sample. J Am Heart Assoc. 2013;2:e000071 doi: 10.1161/JAHA.112.000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xian Y, Holloway RG, Chan PS, Noyes K, Shah MN, Ting HH, Chappel AR, Peterson ED, Friedman B. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lichtman JH, Jones SB, Wang Y, Watanabe E, Leifheit‐Limson E, Goldstein LB. Outcomes after ischemic stroke for hospitals with and without Joint Commission‐certified primary stroke centers. Neurology. 2011;76:1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kleindorfer D, de los Rios La Rosa F, Khatri P, Kissela B, Mackey J, Adeoye O. Temporal trends in acute stroke management. Stroke. 2013;44:S129–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reed SD, Cramer SC, Blough DK, Meyer K, Jarvik JG. Treatment with tissue plasminogen activator and inpatient mortality rates for patients with ischemic stroke treated in community hospitals. Stroke. 2001;32:1832–1840. [DOI] [PubMed] [Google Scholar]
- 22. Hsia AW, Edwards DF, Morgenstern LB, Wing JJ, Brown NC, Coles R, Loftin S, Wein A, Koslosky SS, Fatima S, Sánchez BN, Fokar A, Gibbons MC, Shara N, Jayam‐Trouth A, Kidwell CS. Racial disparities in tissue plasminogen activator treatment rate for stroke: a population‐based study. Stroke. 2011;42:2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schumacher HC, Bateman BT, Boden‐Albala B, Berman MF, Mohr JP, Sacco RL, Pile‐Spellman J. Use of thrombolysis in acute ischemic stroke: analysis of the Nationwide Inpatient Sample 1999 to 2004. Ann Emerg Med. 2007;50:99–107. [DOI] [PubMed] [Google Scholar]
- 24. Nasr DM, Brinjikji W, Cloft HJ, Rabinstein AA. Racial and ethnic disparities in the use of intravenous recombinant tissue plasminogen activator and outcomes for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:154–160. [DOI] [PubMed] [Google Scholar]
- 25. Goldstein LB, Matchar DB, Hoff‐Lindquist J, Samsa GP, Horner RD. Veterans Administration Acute Stroke (VAST) study: lack of race/ethnic‐based differences in utilization of stroke‐related procedures or services. Stroke. 2003;34:999–1004. [DOI] [PubMed] [Google Scholar]
- 26. Siegler JE, Boehme AK, Albright KC, Martin‐Schild S. Ethnic disparities trump other risk factors in determining delay to emergency department arrival in acute ischemic stroke. Ethn Dis. 2013;23:29–34. [PMC free article] [PubMed] [Google Scholar]
- 27. Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, Zhao X, Peterson E, Fonarow GC. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. [DOI] [PubMed] [Google Scholar]
- 28. Cruz‐Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Howard G, Leira EC, Morgenstern LB, Ovbiagele B, Peterson E, Rosamond W, Trimble B, Valderrama AL; Council AHAS, Nursing CoC, Prevention CoEa, Research CoQoCaO . Racial‐ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. [DOI] [PubMed] [Google Scholar]
- 29. Overview of the National (Nationwide) Inpatient Sample (NIS) . Healthcare cost and utilization project website. 2015. Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 30, 2015.
- 30. Andrade SH, Tjia J, Cutrona S, Saczynski J, Goldberg R, Gurwitz J. Mini‐sentinel systematic evaluation of health outcome of interest definitions for studies using administrative data: cerebrovascular/transient ischemic attack report. 2011.
- 31. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 32. Averill RF, Goldfield NI, Muldoon J, Steinbeck BA, Grant TM. A closer look at all‐patient refined DRGs. J AHIMA. 2002;73:46–50. [PubMed] [Google Scholar]
- 33. U.S. Census Bureau projections show a slower growing, older, more diverse nation a half century from now. United States Census Bureau website. 2012. Available at: https://www.census.gov/newsroom/releases/archives/population/cb12-243.html. Accessed January 30, 2015.
- 34. Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG; Council AHAACCaS . Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. [DOI] [PubMed] [Google Scholar]
- 35. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; on behalf of the AHA SCaSS subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44:S126–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morgenstern LB, Smith MA, Sánchez BN, Brown DL, Zahuranec DB, Garcia N, Kerber KA, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Baek J, Lisabeth LD. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol. 2013;74:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, Kissela BM. Stroke incidence is decreasing in whites but not in blacks: a population‐based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36:374–386. [DOI] [PubMed] [Google Scholar]
- 40. Bhattacharya P, Mada F, Salowich‐Palm L, Hinton S, Millis S, Watson SR, Chaturvedi S, Rajamani K. Are racial disparities in stroke care still prevalent in certified stroke centers? J Stroke Cerebrovasc Dis. 2013;22:383–388. [DOI] [PubMed] [Google Scholar]
- 41. Berk ML, Schur CL. The effect of fear on access to care among undocumented Latino immigrants. J Immigr Health. 2001;3:151–156. [DOI] [PubMed] [Google Scholar]
- 42. Mullen MT, Wiebe DJ, Bowman A, Wolff CS, Albright KC, Roy J, Balcer LJ, Branas CC, Carr BG. Disparities in accessibility of certified primary stroke centers. Stroke. 2014;45:3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McDonald CM, Cen S, Ramirez L, Song S, Saver JL, Mack WJ, Sanossian N. Hospital and demographic characteristics associated with advanced primary stroke center designation. Stroke. 2014;45:3717–3719. [DOI] [PubMed] [Google Scholar]
- 44. Trimble B, Morgenstern LB. Stroke in minorities. Neurol Clin. 2008;26:1177–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fonarow GC, Reeves MJ, Zhao X, Olson DM, Smith EE, Saver JL, Schwamm LH; Investigators GWtG‐SSCa . Age‐related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121:879–891. [DOI] [PubMed] [Google Scholar]
- 46. Toni D, Ahmed N, Anzini A, Lorenzano S, Brozman M, Kaste M, Mikulik R, Putaala J, Wahlgren N; Investigators S . Intravenous thrombolysis in young stroke patients: results from the SITS‐ISTR. Neurology. 2012;78:880–887. [DOI] [PubMed] [Google Scholar]
- 47. Nedeltchev K, der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle HP, Schroth G, Remonda L, Sturzenegger M, Fischer U, Baumgartner RW. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005;76:191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsivgoulis G, Putaala J, Sharma VK, Balucani C, Martin‐Schild S, Giannopoulos S, Batala L, Krogias C, Palazzo P, Shahripour RB, Arvaniti C, Barlinn K, Strbian D, Haapaniemi E, Flamouridou M, Vadikolias K, Heliopoulos I, Voumvourakis K, Triantafyllou N, Azarpazhooh MR, Athanasiadis D, Kosmidou M, Katsanos AH, Vasdekis SN, Stefanis L, Stamboulis E, Piperidou C, Tatlisumak T, Alexandrov AV. Racial disparities in early mortality in 1,134 young patients with acute stroke. Neurol Sci. 2014;35:1041–1049. [DOI] [PubMed] [Google Scholar]
- 49. Lisabeth LD, Sánchez BN, Baek J, Skolarus LE, Smith MA, Garcia N, Brown DL, Morgenstern LB. Neurological, functional, and cognitive stroke outcomes in Mexican Americans. Stroke. 2014;45:1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moser DK, Kimble LP, Alberts MJ, Alonzo A, Croft JB, Dracup K, Evenson KR, Go AS, Hand MM, Kothari RU, Mensah GA, Morris DL, Pancioli AM, Riegel B, Zerwic JJ. Reducing delay in seeking treatment by patients with acute coronary syndrome and stroke: a scientific statement from the American Heart Association Council on Cardiovascular Nursing and Stroke Council. Circulation. 2006;114:168–182. [DOI] [PubMed] [Google Scholar]
- 51. Rossnagel K, Jungehülsing GJ, Nolte CH, Müller‐Nordhorn J, Roll S, Wegscheider K, Villringer A, Willich SN. Out‐of‐hospital delays in patients with acute stroke. Ann Emerg Med. 2004;44:476–483. [DOI] [PubMed] [Google Scholar]
- 52. Lacy CR, Suh DC, Bueno M, Kostis JB. Delay in presentation and evaluation for acute stroke: stroke Time Registry for Outcomes Knowledge and Epidemiology (S.T.R.O.K.E.). Stroke. 2001;32:63–69. [DOI] [PubMed] [Google Scholar]
- 53. Morris DL, Rosamond W, Madden K, Schultz C, Hamilton S. Prehospital and emergency department delays after acute stroke: the Genentech Stroke Presentation Survey. Stroke. 2000;31:2585–2590. [DOI] [PubMed] [Google Scholar]
- 54. Tuhrim S, Godbold JH, Goldman ME, Horowitz DR, Weinberger J. The Minorities Risk Factors and stroke Study (MRFASS). Design, methods and baseline characteristics. Neuroepidemiology. 1997;16:224–233. [DOI] [PubMed] [Google Scholar]
- 55. Smith MA, Doliszny KM, Shahar E, McGovern PG, Arnett DK, Luepker RV. Delayed hospital arrival for acute stroke: the Minnesota Stroke Survey. Ann Intern Med. 1998;129:190–196. [DOI] [PubMed] [Google Scholar]
- 56. Menon SC, Pandey DK, Morgenstern LB. Critical factors determining access to acute stroke care. Neurology. 1998;51:427–432. [DOI] [PubMed] [Google Scholar]
- 57. Goldstein LB, Edwards MG, Wood DP. Delay between stroke onset and emergency department evaluation. Neuroepidemiology. 2001;20:196–200. [DOI] [PubMed] [Google Scholar]
- 58. Kothari R, Jauch E, Broderick J, Brott T, Sauerbeck L, Khoury J, Liu T. Acute stroke: delays to presentation and emergency department evaluation. Ann Emerg Med. 1999;33:3–8. [DOI] [PubMed] [Google Scholar]
- 59. Boehme AK, Siegler JE, Mullen MT, Albright KC, Lyerly MJ, Monlezun DJ, Jones EM, Tanner R, Gonzales NR, Beasley TM, Grotta JC, Savitz SI, Martin‐Schild S. Racial and gender differences in stroke severity, outcomes, and treatment in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:e255–e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adeoye O, Lindsell C, Broderick J, Alwell K, Jauch E, Moomaw CJ, Flaherty ML, Pancioli A, Kissela B, Kleindorfer D. Emergency medical services use by stroke patients: a population‐based study. Am J Emerg Med. 2009;27:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ennis SR, Ríos‐Vargas M, Albert NG. The Hispanic Population: 2010. United States Census Bureau website. 2011. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. Accessed January 30, 2015.
- 62. Rastogi S, Johnson TD, Hoeffel EM, Drewery J, Malcolm P. The Black Population: 2010. United States Census Bureau website. 2011. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-06.pdf. Accessed January 30, 2015.
- 63. Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD‐9 codes substantially underestimate. Stroke. 2008;39:924–928. [DOI] [PubMed] [Google Scholar]
- 64. Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue‐type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qureshi AI, Harris‐Lane P, Siddiqi F, Kirmani JF. International Classification of Diseases and Current Procedural Terminology codes underestimated thrombolytic use for ischemic stroke. J Clin Epidemiol. 2006;59:856–858. [DOI] [PubMed] [Google Scholar]


