Abstract
Sporotrichosis is an infection of worldwide distribution caused by the dimorphic fungus, Sporothrix schenckii. Acquisition typically occurs via cutaneous inoculation with development of a localized cutaneous and/or lymphocutaneous infection. We present a rare case of osteoarticular sporotrichosis in a 39-year-old man and review the literature noting only 20 published cases since 1980. Recommendations on the diagnosis and management of this unusual infection are provided.
Keywords: Sporotrichosis, Sporothrix schenckii, Osteomyelitis, Osteoarticular disease, Treatment, Review
1. Introduction
Sporotrichosis is a fungal infection due to Sporothrix schenckii which is found worldwide in decaying vegetation, plants including rose bushes and sphagnum moss, and the soil [1]. After cutaneous inoculation, typically due to trauma associated with outdoor work, the fungus may cause cutaneous and/or lymphocutaneous infection. Rarely infection involves the joints and/or bones as a result of local inoculation or hematogenous spread. The infection is typically chronic and indolent in nature which may result in delayed diagnosis. We report a case of osteoarticular sporotrichosis, and provide a comprehensive review of the literature to describe the risk factors, diagnosis, and treatment of this unusual infection.
2. Case
A 39-year-old male presented on day 0 to our facility with left knee pain and swelling with progressive difficulty ambulating over the preceding 180 days. His history was significant for alcohol abuse and homelessness whereby he resided in a local park. There was no history of diabetes, immunosuppression, or intravenous drug use. Physical examination on presentation revealed a moderate left knee effusion with limited passive and active range of motion to 15 degrees, and a skin abrasion on the overlying skin. The remainder of the examination was unremarkable, and there was no other skin lesions or lymphadenopathy. Laboratory evaluation showed a white cell count of 8200 cells/mm3, erythrocyte sedimentation rate (ESR) of 53 mm/h, and a C-reactive protein (CRP) of 28.5 mg/L. An HIV test and a drug screen were negative. Kidney and liver testing was within normal limits, and a hemoglobin A1c was 5.9%.
The patient had presented previously to the Emergency Department (ED) at our facility on day −140 complaining of left knee pain and an ultrasound was done which found a mild to moderate joint effusion. An arthrocentesis was performed which showed a synovial white cell count of 7470 cells/mm3 with 87% neutrophils. Laboratory evaluation during this visit showed a white blood cell count of 8700 cells/mm3, erythrocyte sedimentation rate (ESR) of 50 mm/h, and a C-reactive protein (CRP) of 15.2 mg/L. The patient was diagnosed with a reactive joint effusion and discharged home from ED with pain medications and follow-up with an orthopedic surgeon. Sporothrix schenckii was identified from the arthrocentesis culture on day −126. When this result returned, an attempt was made to contact patient to return to ED for reassessment and initiation of antifungal therapy, however due to patient's homeless status and lack of contact information, he could not be reached.
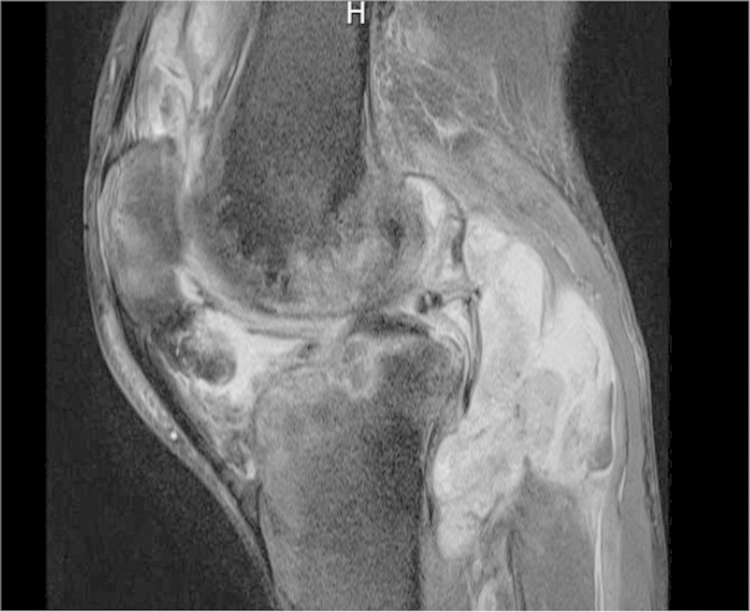
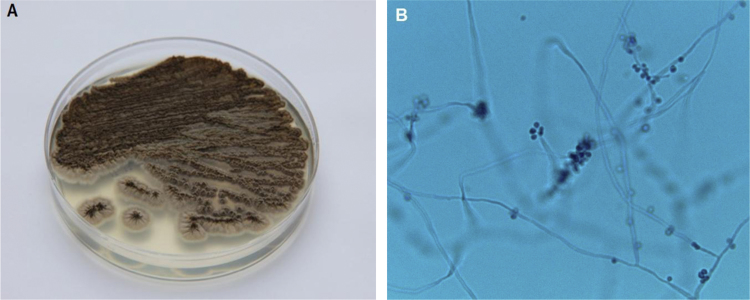
During the current visit (day 0), the patient again presented with ongoing left knee pain. MRI of the left knee on day +2 demonstrated large complex joint effusions and bone marrow edema within the femoral condyles and tibial plateaus consistent with osteomyelitis (Fig. 1). On day +3, the patient underwent surgical debridement and synovectomy. Bacterial and acid-fast bacilli (AFB) cultures collected during surgery were negative from all specimens. On day +14, growth on the fungal cultures (five specimens) showed Sporothrix schenckii (Fig. 2A and B). The identification of this fungus was based on growth of its mold form on a Sabouraud dextrose agar plate incubated at room temperature (30 °C) showing black-pigmented filamentous colonies, and microscopic slide examination showing lateral conidiophores with clusters of pyriform conidia appearing as flowers or bouquets. Identification was confirmed after transition to the yeast form after plating mycelia on rich culture media (e.g., brain heart infusion agar) at 37 °C. On day +3, the patient began treatment with oral itraconazole 200 mg twice daily based on the culture from the previous ED visit that had grown Sporothrix schenckii. His knee function progressively improved, and he is receiving a planned 12-month antifungal treatment course with good clinical response.
Fig. 1.
MRI demonstrating large complex joint effusions and bone marrow edema within the femoral condyles and tibial plateaus.
Fig. 2.
(A) Growth of filamentous colonies on Sabouraud dextrose agar plate incubated at 30 °C. Colonies at first often appear white to creamy, but then turn brown to black after a few days of incubation; the figure represents growth after 21 days of incubation. Colonies are typically wrinkled in appearance and over time form heaping, mountain-like colonies. (B) Microscopic image of hyphae which are septate and approximately 1–2 µm in diameter. Conidia are oval-shaped and classically occur in a flower or bouquet-like arrangement (arrow). Colonies incubated at 30 °C; lactophenol cotton blue stain, 500× magnification. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
Sporotrichosis is a disease of worldwide distribution most commonly found in tropical and temperate regions. The first case was described in 1898 by Schenck who demonstrated the pathogenicity of this fungus [2]. The disease was subsequently named S. schenckii, with recent data showing that different genetic lineages exist in varying geographical regions [1]. Classically, infection occurs after cutaneous injury from rose thorns, but exposures to decaying vegetation, sphagnum moss, and soil can also result in infection. Zoonotic acquisition is also possible after animal (e.g., cat, squirrel, and armadillo) scratches or bites. The incubation period is estimated as 3 weeks. The infection is often chronic in nature with a notable paucity of systemic symptoms, often resulting in delayed diagnosis.
Clinical manifestations most commonly include cutaneous (e.g., papulonodular lesions) and lymphocutaneous forms (adenopathy and lymphangitic spread) of the infection. Occasionally the infection involves the joints, most commonly the knee. Rare forms of sporotrichosis include osteomyelitis occurring after local or hematogenous spread. Local spread can occur when overlying skin lesions progress resulting in underlying erosive bone disease. Alternatively, sporotrichosis can involve the joint, and the adjacent bone may become secondarily infected. In both cases, bony disease typically occurs after a delay in the diagnosis and treatment of the primary infection. Hematogenous seeding of the bone can also occur especially in immunosuppressed hosts. In these cases, fungemia, pulmonary infection (which can occasionally occur after inhalation of the organism), and meningitis may also be noted.
We performed a review of the English literature (PubMed) from January 1, 1980 to December 31, 2015 using the following keywords: “Sporothrix schenckii” , “Sporothrix” , “sporotrichosis” , “osteomyelitis” , “osteoarticular” ," infectious arthritis" and found only 20 published cases of osseous sporotrichosis (Table 1) [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. Cases were included if the term “osteomyelitis” was included in the published case. For the published cases and our case, the median age of patients was 46 (range 8–77) years, with 71% occurred among males. Potential risk factors for infection included local trauma and immunosuppression including HIV-infection. Our case highlights that alcoholism is a potential risk factor for osseous sporotrichosis, similar to that seen in other cases of osteoarticular sporotrichosis [7], [8], [10], [12]. The most common bone involved was the tibia. The hand and wrist bones were also frequently involved, perhaps due to local trauma to the hands and subsequent spread from cutaneous lesions. Any bone can be involved with cases including the jaw as well as bones of the upper and lower extremities noted.
Table 1.
Summary of published cases and present case of osteomyelitis due to sporotrichosis, 1980–2015.
| First author, year, Ref. | No. of cases | Bone (s) involved | Other body sites | Age/sex | Risk factors | Treatment (surgical and pharmacologic) | Outcome |
|---|---|---|---|---|---|---|---|
| Goveia, 1981 [3] | 1 | Mandible, 5th finger, and metatarsal bone of foot | Lung, skin lesions including abscesses and nodules | 50/M | Cleaned teeth with broom straws, marijuana use, prednisone use for presumed sarcoidosis | AMB (2.8 g)+SSKI, debridement | Improved, but then recurrence with bilateral hand skin lesion requiring retreatment with AMB |
| Horsburgh, 1983 [4] | 1 | Wrist/carpal bones | None | 35/M | Landscaper | KTC, AMB, debridement | Clinical response |
| Kumar, 1984 [5] | 1 | Tibia | Diffuse skin lesions | 60/F | Gardener, low CD4 counts of unclear etiology | Curettage, SSKI and AMB | Cure |
| Chang, 1984 [6] | 1 | Metacarpal and finger bones | Diffuse skin lesions | 77/F | None reported | SSKI | Clinical response |
| Lesperance, 1988 [7] | 1 | Ulna and radius | Knee arthritis | 34/M | Alcoholism, construction worker | FLC, AMB (2 g), ITC, debridement | Clinical response |
| MacKenzie, 1988 [8] | 1 | Femur and tibia | None | 57/M | Cotton farmer, alcoholism, trauma | AMB×2 courses, debridement, impregnated KTC beads | Improved, but then recurrence after 1st AMB course. Unknown outcome post 2nd AMB course & KTC beads |
| Govender, 1989 [9] | 4 | Ulna | None | 8/M | None reported | SSKI, debridement | Cure |
| Tibia | None | 29/M | Local trauma | SSKI, debridement | Cure | ||
| Fibula | None | 50/M | None reported | SSKI, debridement | Cure | ||
| Ischium | None | 27/M | Farmer | SSKI, debridement | Cure | ||
| Winn, 1993 [10] | 1 | Femoral condyle | Diffuse skin nodules, wrist, positive blood culture | 51/M | Alcoholism, diabetes mellitus | AMB (450 mg), ITC, debridement | Clinical response |
| Patange, 1995 [11] | 1 | Patella, proximal tibia, and wrist | Diffuse skin lesions, positive blood cultures | 27/M | Outdoor exposures shoveling gravel | ITC | Cure |
| Zacharias, 1997 [12] | 1 | Knee | None | 46/M | Alcoholism | ITC, debridement | Cure |
| al-Tawfiq, 1998 [13] | 1 | Thumb and wrist | Diffuse skin lesions, positive blood culture | 47/M | HIV-infection | AMB (2.5 g), ITC | Cure |
| Appenzeller, 2006 [14] | 1 | Knee – not further specified | None | 35/M | Farmer | ITC, surgical resection of a cutaneous fistula | Cure |
| Mahajan, 2010 [15] | 1 | Middle finger | Diffuse skin lesions, knee arthritis | 25/F | None Reported | SSKI, incision and drainage of knee | Clinical response |
| Freitas, 2012 [16] | 2 | Ankle and knee | Diffuse skin and mucosal lesions | 46/F | HIV-infection | ITC, then AMB (1 g), then ITC | Cure |
| 47/M | HIV-infection | Cure | |||||
| Index finger | ITC and AMB (1 g) | ||||||
| Diffuse skin lesions and arm tenosynovitis | |||||||
| Eustace, 2013 [17] | 1 | Tibia and metatarsal | Diffuse skin lesions | 39/F | None reported | AMB (22 days), then ITC | Clinical response |
| de Carvalho Aguinaga, 2014 [18] | 1 | Thumb phalanx | Arm skin lesions | 48/F | Veterinarian, cat scratch, diabetes mellitus | ITC | Cure |
| Present Case, 2015 | 1 | Tibia and femur | None | 39/M | Alcoholism, Trauma | ITC, debridement | Clinical response |
AMB: Amphotericin B; FLC: Fluconazole; ITC: Itraconazole; KTC: Ketoconazole; SSKI: Saturated solution of Potassium Iodide.
Treatment of osteomyelitis due to sporotrichosis consisted of saturated solution of potassium iodide (SSKI) in a majority of cases before 1990 (Table 1). Of note, most cases treated with SSKI also underwent debridement which may have led to resolution of the infection as prior studies have shown failure of this medication in treating osseous forms of the disease [19]. Amphotericin B and itraconazole therapy were more commonly utilized in more recent cases. The outcome was favorable showing cure or good clinical response at the time of publication, with only two cases (one being the earliest case in our review) reporting recurrence of the infection requiring retreatment [3], [8].
Our case presented with progressive knee pain over a several month course and was diagnosed with culture-confirmed sporotrichosis involving the knee joint and the adjacent bony structures. Our review of the literature showed only 21 cases (including the present case) of osteomyelitis due to Sporothrix since 1980 [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. A prior review by Gladstone and Littman found only 22 published cases between the years of 1924–1970 [19], demonstrating the rarity of this condition. Similar to our review, earlier reports demonstrated that the infection is more common in males; often occurred among laborers working with the soil and/or plants; and that the tibia followed by the hand bones were the most common osseous structures involved [19].
The diagnosis of sporotrichosis is established by culture or histopathology findings; serologic testing is generally not useful. In cases of joint involvement, synovial fluid parameters often show relatively low white cell counts (<10,000 cells/mm3) with a neutrophil predominance [14]. The time for growth on cultures is approximately 8 days, but may require 2–4 weeks. Dimorphic fungi, such as Sporothrix, have the mold form present on cultures incubated at room temperatures (21–30 °C) with transition to the yeast form after plating mycelia on rich culture media (e.g., brain heart infusion agar) at 35–37 °C. Macroscopically Sporothrix appears as filamentous colonies that are white to creamy at first, then turning brown to black after several days (Fig. 2A); some strains have the ability to form dark colonies from the beginning of growth. Microscopically, Sporothrix schenckii assumes a filamentous form, composed of hyaline, septate hyphae 1–2 µm wide, with branching pyriform conidia in flower-like or bouquet arrangement (Fig. 2B). Histological findings can include granulomatous inflammation with budding yeasts that are round to oval, 3–5-μm in diameter, and appear as elongated, cigar-shaped buds on a narrow base. In our case, the diagnosis was established by positive cultures; tissue pathology was not obtained at the time of surgery.
Spontaneous resolution of this infection is rare, and thus treatment is required for most cases and is based on clinical presentation. The preferred agent in most cases is oral itraconazole [20]. For cutaneous and lymphocutaneous forms, the Infectious Diseases Society of America (IDSA) guidelines recommend itraconazole at 200 mg orally daily for at least 2–4 weeks after resolution of all lesions (typically for 3–6 months) [21]. A higher dose and longer duration is recommended for osteoarticular disease with itraconazole 200 mg orally twice daily for at least 12 months. Serum levels of itraconazole are obtained to ensure therapeutic levels are achieved (i.e., ≥1 μg/mL drawn 2 weeks after treatment initiation and measured as a trough). Of note, the itraconazole oral solution (taken on an empty stomach) is preferred due to higher bioavailability, but the capsule form (taken with food) can be also utilized.
Intravenous amphotericin B can be considered for initial therapy, especially in severe cases, but is typically not required and is associated with a higher risk of toxicity. Intraarticular injection of amphotericin has been utilized for articular cases [22], but is generally not recommended [21]. Other antifungal agents have displayed varying activity against S. schenckii. Voriconazole and echinocandins (e.g., micafungin and caspofungin) lack in vitro activity [23], [24], [25]. Fluconazole has some activity, but in generally is avoided because it is less effective compared with itraconazole [21]. Posaconazole may be a future option given its excellent in vitro activity [24], [25], but clinical data is currently limited to case reports [26], [27].
Additional treatment options for cutaneous and lymphocutaneous forms may include local hyperthermia (42–43 °C), SSKI, and terbinafine which have shown variable treatment responses. However, these therapies have no current role in more severe forms of sporotrichosis including osteoarticular disease. Finally, surgery may be used as an adjuvant to antifungal therapy, especially in cases with complex joint effusions and sequestrum, but is typically not needed in uncomplicated cases which are treated with antifungal agents. Cases involving joint destruction may subsequently require arthrodesis or arthroplasty [28].
In conclusion, we present an unusual case of osteoarticular sporotrichosis. Sporotrichosis should be considered in the differential diagnosis of infectious arthritis and/or osteomyelitis in the setting of a low synovial fluid white count, growth of mold on culture, and the presence of risk factors including skin trauma involving plants or vegetation. Diagnosis is established by fungal culture, and treatment is typically with a long course (12 months) of oral itraconazole.
Conflict of interest
None.
Acknowledgments
None.
References
- 1.Marimon R., Gené J., Cano J., Trilles L., Dos Santos Lazéra M., Guarro J. Molecular phylogeny of Sporothrix schenckii. J. Clin. Microbiol. 2006;44:3251–3256. doi: 10.1128/JCM.00081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schenck B.R. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Johns Hopkins Hosp. Bull. 1898;9:286–290. [Google Scholar]
- 3.Goveia G.L., Bellome J., Hiatt W.R. Disseminated sporotrichosis with mandibular involvement. J. Oral Surg. 1981;39:468–472. [PubMed] [Google Scholar]
- 4.Horsburgh C.R., Jr, Cannady P.B., Jr, Kirkpatrick C.H. Treatment of fungal infections in the bones and joints with ketoconazole. J. Infect. Dis. 1983;147:1064–1069. doi: 10.1093/infdis/147.6.1064. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R., van der Smissen E., Jorizzo J. Systemic sporotrichosis with osteomyelitis. J. Can. Assoc. Radiol. 1984;35:83–84. [PubMed] [Google Scholar]
- 6.Chang A.C., Destouet J.M., Murphy W.A. Musculoskeletal sporotrichosis. Skelet. Radiol. 1984;12:23–28. doi: 10.1007/BF00373171. [DOI] [PubMed] [Google Scholar]
- 7.Lesperance M., Baumgartner D., Kauffman C.A. Polyarticular arthritis due to Sporothrix schenckii. Mycoses. 1988;12:599–603. [PubMed] [Google Scholar]
- 8.MacKenzie T.R., Perry C., Pearson R., Gilula L.A. Imaging rounds #95. Atypical infection: osteoarticular sporotrichosis. Orthop. Rev. 1988;17:716–719. [PubMed] [Google Scholar]
- 9.Govender S., Rasool M.N., Ngcelwane M. Osseous sporotrichosis. J. Infect. 1989;19:273–276. doi: 10.1016/s0163-4453(89)90829-3. [DOI] [PubMed] [Google Scholar]
- 10.Winn R.E., Anderson J., Piper J., Aronson N.E., Pluss J. Systemic sporotrichosis treated with itraconazole. Clin. Infect. Dis. 1993;17:210–217. doi: 10.1093/clinids/17.2.210. [DOI] [PubMed] [Google Scholar]
- 11.Patange V., Cesani F., Phillpott J., Villanueva-Meyer J. Three-phase bone and Ga-67 scintigraphy in disseminated sporotrichosis. Clin. Nucl. Med. 1995;20:909–912. doi: 10.1097/00003072-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Zacharias J., Crosby L.A. Sporotrichal arthritis of the knee. Am. J. Knee Surg. 1997;10(3):171–174. [PubMed] [Google Scholar]
- 13.al-Tawfiq J.A., Wools K.K. Disseminated sporotrichosis and Sporothrix schenckii fungemia as the initial presentation of human immunodeficiency virus infection. Clin. Infect. Dis. 1998;26:1403–1406. doi: 10.1086/516356. [DOI] [PubMed] [Google Scholar]
- 14.Appenzeller S., Amaral T.N., Amstalden E.M., Bertolo M.B., Neto J.F., Samara A.M. Sporothrix schenckii infection presented as monoarthritis: report of two cases and review of the literature. Clin. Rheumatol. 2006;25:926–928. doi: 10.1007/s10067-005-0095-z. [DOI] [PubMed] [Google Scholar]
- 15.Mahajan V.K., Sharma N.L., Shanker V., Gupta P., Mardi K. Cutaneous sporotrichosis: unusual clinical presentations. Indian J. Dermatol. Venereol. Leprol. 2010;76:276–280. doi: 10.4103/0378-6323.62974. [DOI] [PubMed] [Google Scholar]
- 16.Freitas D.F., de Siqueira Hoagland B., do Valle A.C., Fraga B.B., de Barros M.B. Sporotrichosis in HIV-infected patients: report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med. Mycol. 2012;50:170–178. doi: 10.3109/13693786.2011.596288. [DOI] [PubMed] [Google Scholar]
- 17.Eustace K.E., Sampaio F.M., Lyra M.R., Quintella L., do Valle A.C. Cutaneous disseminated sporotrichosis complicated by osteomyelitis. Acta Derm. Venereol. 2013;93:192–193. doi: 10.2340/00015555-1403. [DOI] [PubMed] [Google Scholar]
- 18.de Carvalho Aguinaga F., Trope B.M., Fernandes N.C., Engel D.C., Ramos-e-Silva M. Sporotrichosis with bone involvement: an alert to an occupational disease. Case Rep. Dermatol. 2014;6:114–118. doi: 10.1159/000362184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladstone J.L., Littman M.L. Osseous sporotrichosis. Failure of treatment with potassium iodide and sulfadimethoxine and success with amphotericin B. Am. J. Med. 1971;51:121–133. doi: 10.1016/0002-9343(71)90329-9. [DOI] [PubMed] [Google Scholar]
- 20.Sharkey-Mathis P.K., Kauffman C.A., Graybill J.R., Stevens D.A., Hostetler J.S., Cloud G. Treatment of sporotrichosis with itraconazole. NIAID Mycoses Study Group. Am. J. Med. 1993;95:279–285. doi: 10.1016/0002-9343(93)90280-3. [DOI] [PubMed] [Google Scholar]
- 21.Kauffman C.A., Bustamante B., Chapman S.W., Pappas P.G., Infectious Diseases Society of America Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007;45:1255–1265. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 22.Downs N.J., Hinthorn D.R., Mhatre V.R., Liu C. Intra-articular amphotericin B treatment of Sporothrix schenckii arthritis. Arch. Intern. Med. 1989;149:954–955. [PubMed] [Google Scholar]
- 23.McGinnis M.R., Nordoff N., Li R.K., Pasarell L., Warnock D.W. Sporothrix schenckii sensitivity to voriconazole, itraconazole and amphotericin B. Med. Mycol. 2001;39:369–371. doi: 10.1080/mmy.39.4.369.371. [DOI] [PubMed] [Google Scholar]
- 24.Marimon R., Serena C., Gené J., Cano J., Guarro J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob. Agents Chemother. 2008;52:732–734. doi: 10.1128/AAC.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinel-Ingroff A. Comparison of In vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunce P.E., Yang L., Chun S., Zhang S.X., Trinkaus M.A., Matukas L.M. Disseminated sporotrichosis in a patient with hairy cell leukemia treated with amphotericin B and posaconazole. Med. Mycol. 2012;50:197–201. doi: 10.3109/13693786.2011.584074. [DOI] [PubMed] [Google Scholar]
- 27.Paixão A.G., Galhardo M.C., Almeida-Paes R., Nunes E.P., Gonçalves M.L., Chequer G.L. The difficult management of disseminated Sporothrix brasiliensis in a patient with advanced AIDS. AIDS Res. Ther. 2015;12:1–6. doi: 10.1186/s12981-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koëter S., Jackson R.W. Successful total knee arthroplasty in the presence of sporotrichal arthritis. Knee. 2006;13:236–237. doi: 10.1016/j.knee.2006.02.004. [DOI] [PubMed] [Google Scholar]