Abstract
OBJECTIVE: To study the relative diagnostic value of enterovirus-specific molecular biological and serological assays in patients with end-stage dilated cardiomyopathy, and to investigate the possible role of other cardiotropic viruses in dilated cardiomyopathy. DESIGN: Analysis of recipient myocardial tissue and serum from patients with dilated cardiomyopathy and controls undergoing cardiac transplantation for end-stage cardiac disease. SETTING: University virology department and transplantation unit. METHODS: Reverse transcriptase-polymerase chain reaction and nucleotide sequence analysis of myocardial RNA and DNA; enterovirus-specific in situ hybridization; enterovirus-specific immunoglobulin M detection. RESULTS: Enterovirus RNA was detected in myocardial tissue from only a small proportion of (five of 75) hearts. However, although enterovirus-specific immunoglobulin M responses were detected in 22 (28%) of 39 controls patients, a significantly higher prevalence was observed among patients with dilated cardiomyopathy (22 (56%) of 39 patients; P < 0.005). All enteroviruses detected in myocardium showed greatest nucleotide sequence homology with coxsackievirus type B3. Detection of enterovirus RNA in myocardium by the polymerase chain reaction and by in situ hybridisation gave comparable results. Other potentially cardiotropic virus genomes, including human cytomegalovirus, influenzaviruses, and coronaviruses were not detected in myocardium. CONCLUSION: This study found that enterovirus-specific immunoglobulin M responses provided the strongest evidence of enterovirus involvement in patients with end-stage dilated cardiomyopathy. However, the high background prevalence of these responses limits their diagnostic value. The finding that enteroviruses detected in myocardium were coxsackievirus type B3 accords with recent findings in patients with acute myocarditis, and indicates that this serotype is the major cardiotropic human enterovirus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander L. K., Small J. D., Edwards S., Baric R. S. An experimental model for dilated cardiomyopathy after rabbit coronavirus infection. J Infect Dis. 1992 Nov;166(5):978–985. doi: 10.1093/infdis/166.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles N. E., Rose M. L., Taylor P., Banner N. R., Morgan-Capner P., Cunningham L., Archard L. C., Yacoub M. H. End-stage dilated cardiomyopathy. Persistence of enterovirus RNA in myocardium at cardiac transplantation and lack of immune response. Circulation. 1989 Nov;80(5):1128–1136. doi: 10.1161/01.cir.80.5.1128. [DOI] [PubMed] [Google Scholar]
- Cambridge G., MacArthur C. G., Waterson A. P., Goodwin J. F., Oakley C. M. Antibodies to Coxsackie B viruses in congestive cardiomyopathy. Br Heart J. 1979 Jun;41(6):692–696. doi: 10.1136/hrt.41.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington J., Super M., Patel K., Grundy J. E., Griffiths P. D., Emery V. C. Use of the polymerase chain reaction to analyse sequence variation within a major neutralizing epitope of glycoprotein B (gp58) in clinical isolates of human cytomegalovirus. J Gen Virol. 1991 Aug;72(Pt 8):1985–1989. doi: 10.1099/0022-1317-72-8-1985. [DOI] [PubMed] [Google Scholar]
- Figulla H. R., Stille-Siegener M., Mall G., Heim A., Kreuzer H. Myocardial enterovirus infection with left ventricular dysfunction: a benign disease compared with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1995 Apr;25(5):1170–1175. doi: 10.1016/0735-1097(94)00517-t. [DOI] [PubMed] [Google Scholar]
- Keeling P. J., Lukaszyk A., Poloniecki J., Caforio A. L., Davies M. J., Booth J. C., McKenna W. J. A prospective case-control study of antibodies to coxsackie B virus in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994 Mar 1;23(3):593–598. doi: 10.1016/0735-1097(94)90742-0. [DOI] [PubMed] [Google Scholar]
- Khan M., Why H., Richardson P., Archard L. Nucleotide sequencing of PCR products shows the presence of Coxsackie-B3 virus in endomyocardial biopsies from patients with myocarditis or dilated cardiomyopathy. Biochem Soc Trans. 1994 May;22(2):176S–176S. doi: 10.1042/bst022176s. [DOI] [PubMed] [Google Scholar]
- Kämmerer U., Kunkel B., Korn K. Nested PCR for specific detection and rapid identification of human picornaviruses. J Clin Microbiol. 1994 Feb;32(2):285–291. doi: 10.1128/jcm.32.2.285-291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Lansdown A. B. Viral Infections and diseases of the heart. Prog Med Virol. 1978;24:70–113. [PubMed] [Google Scholar]
- Liljeqvist J. A., Bergström T., Holmström S., Samuelson A., Yousef G. E., Waagstein F., Jeansson S. Failure to demonstrate enterovirus aetiology in Swedish patients with dilated cardiomyopathy. J Med Virol. 1993 Jan;39(1):6–10. doi: 10.1002/jmv.1890390103. [DOI] [PubMed] [Google Scholar]
- Martin A. B., Webber S., Fricker F. J., Jaffe R., Demmler G., Kearney D., Zhang Y. H., Bodurtha J., Gelb B., Ni J. Acute myocarditis. Rapid diagnosis by PCR in children. Circulation. 1994 Jul;90(1):330–339. doi: 10.1161/01.cir.90.1.330. [DOI] [PubMed] [Google Scholar]
- Muir P. Enteroviruses and heart disease. Br J Biomed Sci. 1993 Sep;50(3):258–271. [PubMed] [Google Scholar]
- Muir P., Nicholson F., Banatvala J. E., Bingley P. J. Coxsackie B virus and postviral fatigue syndrome. BMJ. 1991 Mar 16;302(6777):658–659. doi: 10.1136/bmj.302.6777.658-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir P., Nicholson F., Jhetam M., Neogi S., Banatvala J. E. Rapid diagnosis of enterovirus infection by magnetic bead extraction and polymerase chain reaction detection of enterovirus RNA in clinical specimens. J Clin Microbiol. 1993 Jan;31(1):31–38. doi: 10.1128/jcm.31.1.31-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir P., Nicholson F., Tilzey A. J., Signy M., English T. A., Banatvala J. E. Chronic relapsing pericarditis and dilated cardiomyopathy: serological evidence of persistent enterovirus infection. Lancet. 1989 Apr 15;1(8642):804–807. doi: 10.1016/s0140-6736(89)92270-8. [DOI] [PubMed] [Google Scholar]
- Nicholson F., Ajetunmobi J. F., Li M., Shackleton E. A., Starkey W. G., Illavia S. J., Muir P., Banatvala J. E. Molecular detection and serotypic analysis of enterovirus RNA in archival specimens from patients with acute myocarditis. Br Heart J. 1995 Nov;74(5):522–527. doi: 10.1136/hrt.74.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
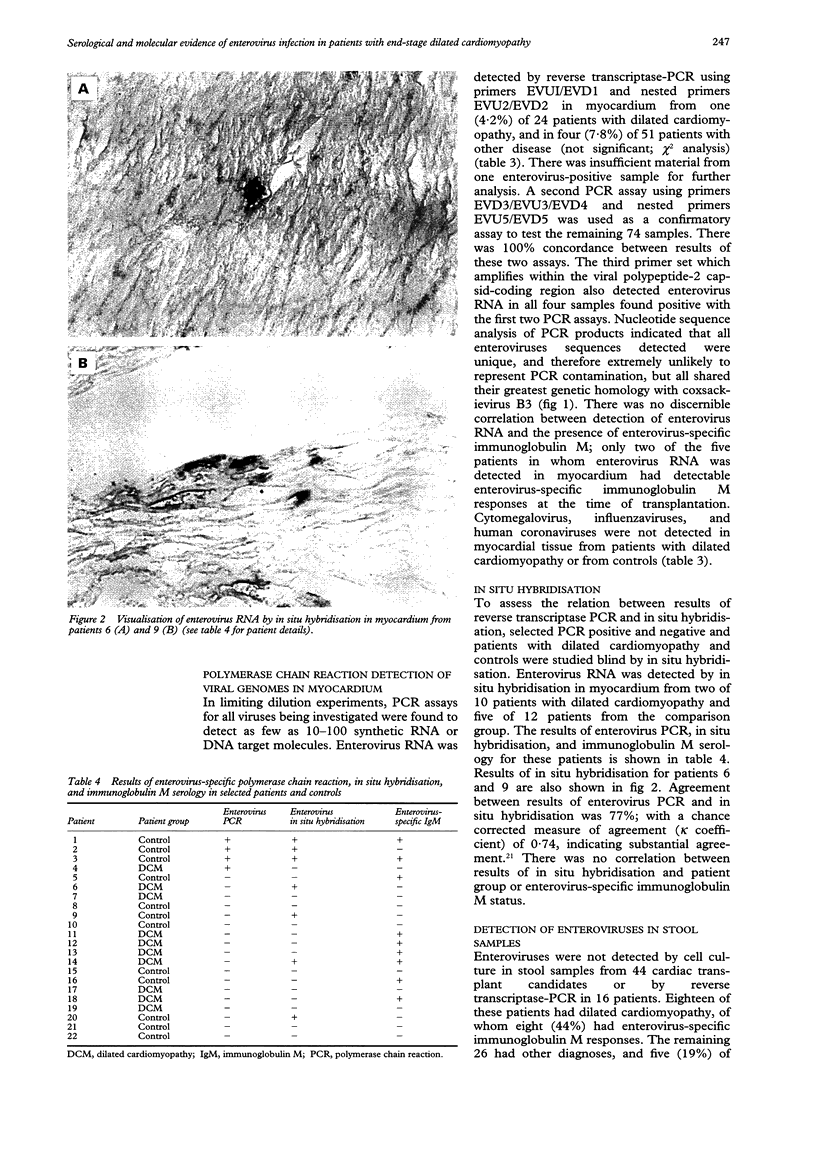
- Nicholson F., Meetoo G., Aiyar S., Banatvala J. E., Muir P. Detection of enterovirus RNA in clinical samples by nested polymerase chain reaction for rapid diagnosis of enterovirus infection. J Virol Methods. 1994 Jul;48(2-3):155–166. doi: 10.1016/0166-0934(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Riski H., Hovi T. Coronavirus infections of man associated with diseases other than the common cold. J Med Virol. 1980;6(3):259–265. doi: 10.1002/jmv.1890060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A., Sawyer M. H., Fast S., Lewinski C., Murphy N., Keyser E. F., Spadoro J., Kao S. Y., Loeffelholz M. Diagnosis of enteroviral meningitis by using PCR with a colorimetric microwell detection assay. J Clin Microbiol. 1994 Oct;32(10):2590–2592. doi: 10.1128/jcm.32.10.2590-2592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger A., Umlauft F., Weyrer K., Larcher C., Lyons J., Mühlberger V., Dietze O., Grünewald K. Detection of enteroviral ribonucleic acid in myocardial biopsies from patients with idiopathic dilated cardiomyopathy by polymerase chain reaction. Am Heart J. 1993 Aug;126(2):406–410. doi: 10.1016/0002-8703(93)91058-m. [DOI] [PubMed] [Google Scholar]
- Slifka M. K., Matloubian M., Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995 Mar;69(3):1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z., Chapman N. M., Hufnagel G., Tracy S., Romero J. R., Barry W. H., Zhao L., Currey K., Shapiro B. The cardiovirulent phenotype of coxsackievirus B3 is determined at a single site in the genomic 5' nontranslated region. J Virol. 1995 Aug;69(8):4607–4618. doi: 10.1128/jvi.69.8.4607-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Why H. J., Meany B. T., Richardson P. J., Olsen E. G., Bowles N. E., Cunningham L., Freeke C. A., Archard L. C. Clinical and prognostic significance of detection of enteroviral RNA in the myocardium of patients with myocarditis or dilated cardiomyopathy. Circulation. 1994 Jun;89(6):2582–2589. doi: 10.1161/01.cir.89.6.2582. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Yousef G. E., Cunningham L., Blake N. W., OuYang X., Bayston T. A., Kandolf R., Archard L. C. Attenuation of a reactivated cardiovirulent coxsackievirus B3: The 5'-nontranslated region does not contain major attenuation determinants. J Med Virol. 1993 Oct;41(2):129–137. doi: 10.1002/jmv.1890410208. [DOI] [PubMed] [Google Scholar]