Abstract
Mechanistic target of rapamycin complex 1 (mTORC1) negatively regulates autophagy at early stages by phosphorylating Unc51-like kinase 1 (ULK1). Our recent study expanded the roles of mTORC1 in autophagy by identifying ultraviolet radiation resistance-associated gene product (UVRAG) as a substrate of mTORC1. This finding has provided new insight into the roles of mTORC1 in cellular membrane processes and cancer.
Keywords: mTOR, mTORC1, autophagy, UVRAG, RUBICON, endosome, lysosome
There has been increasing interest in autophagy as an anticancer target, mainly because of its important role in cancer cell survival under conditions of metabolic stress, hypoxia, or anticancer treatment. One of the lessons from previous studies is that autophagy might function differently in cancer cells compared to normal cells. Understanding the differential regulation of autophagy in cancer cells might be central to the development of better strategies for cancer prevention or treatment. A key link between autophagy and cancer is through mechanistic target of rapamycin complex 1 (mTORC1), which mediates signaling of growth factor and nutrient availability to the autophagy machinery. mTORC1 regulates the balance between protein synthesis (anabolic process) and autophagy (catabolic process) in response to cellular metabolic status and environmental stress. Hyperactivation of mTORC1 is frequently found in many cancers, and mTORC1 inhibitors have shown promising outcomes in clinical trials for some types of cancer. The extent to which autophagy contributes to the anticancer effects of mTORC1 inhibitors remains unclear. This question might be key for better understanding of the roles of autophagy in cancer, given the important role of mTORC1 in cancer as well as its role in coordinating cell growth and autophagy.
mTORC1 negatively regulates autophagosome formation at early stages of autophagy by phosphorylating ULK1 and other early autophagy proteins.1-4 Our recent study revealed that mTORC1 also phosphorylates UV radiation resistance-associated gene product (UVRAG) and suppresses autophagosome maturation at late stages of autophagy (Fig. 1).5 UVRAG localizes in the endoplasmic reticulum (ER) and endosomes.6-8 UVRAG has a tumor suppressor function, and the UVRAG gene locus is frequently altered in breast, colorectal, and gastric cancers.9,10 One of the major functions of UVRAG is to regulate autophagosome maturation by binding to the homotypic fusion and vacuole protein sorting (HOPS) complex,7 a key component of the late endosome and lysosome fusion machinery. Another function of UVRAG is to stimulate the activity of vacuolar protein sorting 34 kDa (Vps34, also known as phosphatidylinositol 3-kinase class III), a critical component for autophagy. Our study showed that phosphorylation of UVRAG by mTORC1 facilitates its binding to RUN domain Beclin 1-interacting and cysteine-rich containing protein (RUBICON) under nutrient-enriched conditions. The phosphorylation-dependent binding of UVRAG to RUBICON has a dual effect on UVRAG functions: first, the enhanced binding of RUBICON suppresses the ability of UVRAG to stimulate the kinase activity of Vps34; and second, RUBICON binding interferes with the interaction between UVRAG and the HOPS complex, thereby inhibiting the lysosomal fusion machinery. This mTORC1-mediated regulation of UVRAG on the lysosomal fusion machinery is supported by the cellular localization of mTORC1 to late endosomes and lysosomes, where it is activated by amino acid-responsive signaling.
Figure 1.
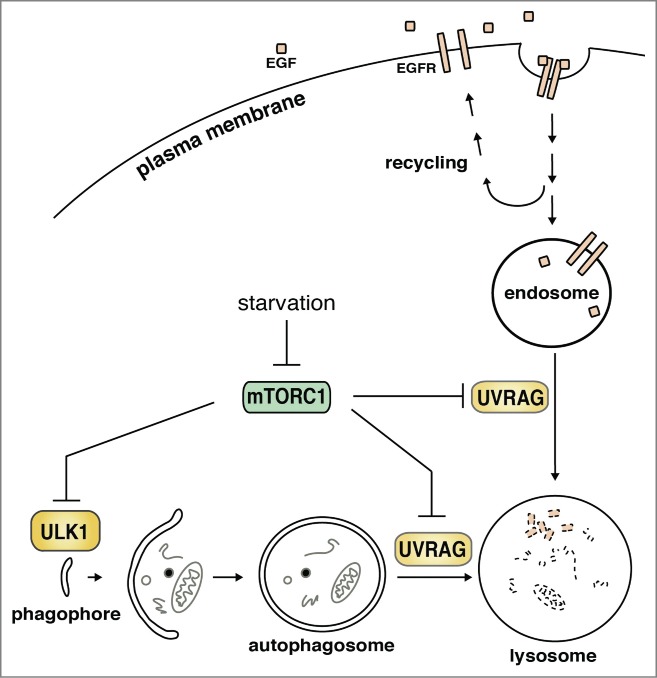
mTORC1 suppresses both early and late stages of autophagy. mTORC1 phosphorylates ULK1 and UVRAG to negatively regulate autophagy induction and autophagosome maturation, respectively. mTORC1-mediated phosphorylation of UVRAG also has a negative effect on endosomal maturation and the lysosomal degradation of EGFR.
Another aspect that is newly addressed by our study is the function of mTORC1 beyond its role in autophagy. Our study showed that mTORC1-mediated phosphorylation of UVRAG negatively regulates the lysosomal degradation of epidermal growth factor receptor (EGFR) by suppressing endosomal maturation. This finding, together with the lysosomal localization of mTORC1, indicates a broader range of mTORC1 functions at the membrane compartments harboring autophagosomes, late endosomes, and lysosomes. Although regulation of protein synthesis by mTORC1 might be its major contribution to cancer, it is possible that regulation of autophagy and/or endosomal maturation also plays an important role. This possibility was supported by our study using a xenograft approach, in which we showed that a dephosphorylation-mimicking mutation of the mTORC1 target site of UVRAG suppressed tumor growth.5 However, it remains unclear whether the effect of mTORC1 on cancer cell proliferation and tumor growth is due to autophagy or endosomal maturation.
The finding that mTORC1 regulates the late stage of autophagy gives rise to the question of why mTORC1 suppresses multiple steps instead of a key early step that governs downstream processes. A consequence of suppression at the stage of autophagosome maturation is prevention of recycling of autophagy components. However, inhibition of an early key component could lead to suppression of multiple downstream processes, including the recycling process. It is possible that the multi-step regulation of autophagy by mTORC1 allows for fine-tuning of different types of autophagy in response to various signals. Another possible interpretation is that mTORC1-mediated suppression of autophagosome maturation might be secondary to inhibition of endosomal maturation or the lysosomal fusion machinery. Since inhibition of the early steps of autophagy by mTORC1 could be effective enough to suppress the whole process of autophagy, inhibition of the lysosomal fusion machinery by mTORC1 might have a less significant effect on autophagy compared to its effect on endosomal maturation.
The role of the mTORC1-UVRAG pathway in regulation of lysosomal membrane processes might be important for many cancers, as supported by our study, thus adding to the multiple facets of mTORC1 in cancer. Whether cancer cells gain an advantage for growth or survival through suppression of autophagy is unclear, but might be possible considering the deletion of autophagy genes in many cancers. Our study suggests that the mTORC1-UVRAG pathway could be a potential target for an anticancer strategy. Before this possibility is tested, it would be necessary to clarify how significantly each of the membrane processes (autophagy and endosomal maturation) contributes to the effects of mTORC1 on cell proliferation and tumor growth. Further studies of mTORC1-regulated autophagic membrane dynamics might provide new insights into better strategies for cancer prevention and treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Kim lab members for comments.
Funding
This study was supported by grants from the National Institutes of Health (P30-DK050456, GM097057, AG039758), U.S. Department of Defense (W81XWH-13-1-0060), and American Diabetes Association (ADA 7-12-BS-093).
References
- 1.Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 2009; 284:12297-305; PMID:19258318; http://dx.doi.org/ 10.1074/jbc.M900573200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, et al.. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 2009; 20:1981-91; PMID:19211835; http://dx.doi.org/ 10.1091/mbc.E08-12-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 2009; 20:1992-2003; PMID:19225151; http://dx.doi.org/ 10.1091/mbc.E08-12-1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011; 13:132-41; PMID:21258367; http://dx.doi.org/ 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y, Jung CH, Seo M, Kim EK, Park J, Bae SS, Kim DH. mTORC1 Phosphorylates UVRAG to Negatively Regulate Autophagosome and Endosome Maturation. Mol Cell 2014; 57:207-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He S, Ni D, Ma B, Lee JH, Zhang T, Ghozalli I, Pirooz SD, Zhao Z, Bharatham N, Li B, et al.. PtdIns(3)P-bound UVRAG coordinates Golgi-ER retrograde and Atg9 transport by differential interactions with the ER tether and the beclin 1 complex. Nat Cell Biol 2013; 15:1206-19; PMID:24056303; http://dx.doi.org/ 10.1038/ncb2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, et al.. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol 2008; 10:776-87; PMID:18552835; http://dx.doi.org/ 10.1038/ncb1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 2008; 19:5360-72; PMID:18843052; http://dx.doi.org/ 10.1091/mbc.E08-01-0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knaevelsrud H, Ahlquist T, Merok MA, Nesbakken A, Stenmark H, Lothe RA, Simonsen A. UVRAG mutations associated with microsatellite unstable colon cancer do not affect autophagy. Autophagy 2010; 6:863-70; PMID:20724836; http://dx.doi.org/ 10.4161/auto.6.7.13033 [DOI] [PubMed] [Google Scholar]
- 10.Kim MS, Jeong EG, Ahn CH, Kim SS, Lee SH, Yoo NJ. Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability. Human Pathol 2008; 39:1059-63; PMID:18495205; http://dx.doi.org/ 10.1016/j.humpath.2007.11.013 [DOI] [PubMed] [Google Scholar]


