Abstract
RAF- and MEK-targeted therapies are approved for patients with BRAFV600E melanoma and under investigation in a several other tumor types, but resistance remains a major challenge. We uncovered yes-associated protein 1 (YAP1) as a mechanism of resistance to RAF-MEK inhibition in BRAF- and RAS-mutant cancers, providing a rationale for co-targeting YAP and RAF-MEK to enhance patient outcomes.
Keywords: BRAF, Hippo, MEK, resistance, RAS, targeted therapy, YAP
The identification of oncogenic driver mutations that promote tumor growth and the development of specific targeted therapies that act against these mutations has revolutionized the treatment of patients suffering from a wide spectrum of advanced-stage tumors.1,2 However, the clinical success of driver oncogene targeted therapy is limited by both primary and acquired drug resistance that frequently occurs in patients and is often lethal.3 The molecular events that mediate this resistance remain incompletely defined. Improving our understanding of the molecular basis of resistance is a central theme of molecular oncology because the knowledge gained not only provides fundamental biological insight into cancer cell signaling but also reveals novel therapeutic strategies to overcome resistance and increase patient survival.
Our recent work has focused on resistance to targeted therapies acting against aberrant RAS-RAF-MEK-ERK mitogen-activated protein kinase (MAPK) signaling in cancers.4,5 Resistance to RAF-MEK targeted therapy is a major clinical challenge. RAF-MEK inhibitors are initially, but only transiently, effective in some, but not all, patients with BRAF-mutant disease, and largely ineffective in patients with RAS-mutant disease because of resistance.6 We used a pooled small hairpin RNA screen in human BRAFV600E mutant lung cancer cells to identify genes that when silenced enhanced the response to the approved BRAF inhibitor vemurafenib.4 Through this genetic screen, we found that the Hippo pathway effector yes-associated protein 1 (YAP1) acts as a parallel survival input to promote resistance to RAF-MEK inhibitor therapy (Fig. 1). Combined YAP and RAF-MEK inhibition was synthetically lethal not only in several BRAF-mutant tumor types (melanoma, lung, colon, thyroid), but also in RAS-mutant tumors (lung, melanoma, pancreas). We found that YAP acted as a parallel survival factor by regulating expression of the antiapoptotic protein BCL-xL (BCL2L1) in concert with MAPK signaling, such that BCL-xL levels were suppressed to a degree sufficient to trigger apoptosis only upon combined YAP and RAF-MEK inhibition. We found that increased YAP expression in patients with BRAFV600E tumors was a biomarker of worse initial response and acquired resistance to RAF-MEK inhibition, establishing the clinical relevance of our findings. These data reveal YAP as a novel mechanism of resistance to RAF-MEK targeted therapy and unveil the synthetic lethality of YAP and RAF-MEK co-suppression as a promising strategy to enhance response and patient survival.
Figure 1.
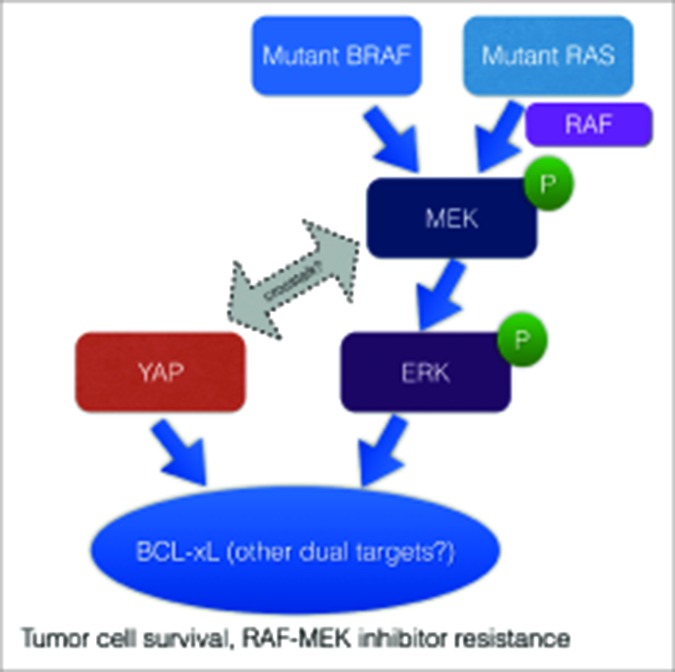
YAP promotes cancer cell survival and resistance to RAF-MEK-targeting agents. The figure shows a schematic of the role of YAP in BRAF- and RAS-mutant tumor cells and areas for future exploration, such as the basis of molecular crosstalk and co-regulation of target genes between YAP and the RAS-RAF-MEK-ERK (MAPK) pathway. MAPK, mitogen activated protein kinase; YAP, yes-associated protein.
Open Questions
Our study raised several new questions. First, the findings raise the possibility that YAP may enable survival and escape from other therapies targeting proteins that can activate MAPK pathway signaling. Such signaling components include mutant receptor tyrosine kinases (RTKs) such as epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK), which drive the growth of many lung adenocarcinomas and are targeted with approved EGFR and ALK kinase inhibitors, respectively.1 This represents an interesting area of ongoing research with potential implications for the treatment of many additional cancer patients.
Second, on a fundamental level the findings provide a rationale to investigate the molecular basis of the newly discovered crosstalk between YAP and MAPK pathway signaling. We found that the expression of many signaling components, including BCL-xL, is co-regulated by these 2 pathways in BRAF-mutant cancer cells. The factors underlying this co-regulation, and whether tissue- or genetic driver-specific regulatory events are critical aspects of this crosstalk, are currently unknown and a focus of ongoing efforts.
Third, pharmacologically blocking YAP remains a clinical challenge. Although YAP inhibitors have been reported,7 whether these agents are potent and selective enough to suppress the pathway with acceptable toxicity in patients is unclear. Therefore, the design and development of novel, more specific drugs that inhibit YAP may be warranted.
Implications
RAF and MEK inhibitors are effective in many, but not all, cancer patients. Furthermore, the efficacy of these targeted therapies is limited by the almost invariable development of acquired resistance in patients. Our study indicates that YAP can promote both primary and acquired resistance to RAF-MEK targeted therapies in cancer patients. The data provide a rational basis for the development and testing of a YAP inhibitor together with a RAF-MEK inhibitor to enhance the magnitude and duration of response to MAPK pathway blockade in patients. Furthermore, the findings augment an emerging body of literature on the role of YAP in cancer progression, with important implications for understanding the function of YAP in cell survival and growth.8,9 Dual basic and translational investigations promise to shed light on the emerging role of YAP in cancer biology and therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet 2013; 382:720-31; PMID:23972815; http://dx.doi.org/ 10.1016/S0140-6736(13)61715-8 [DOI] [PubMed] [Google Scholar]
- 2.Sawyers CL, van't Veer LJ. Reliable and effective diagnostics are keys to accelerating personalized cancer medicine and transforming cancer care: a policy statement from the american association for cancer research. Clin Cancer Res 2014; 20:4978-81; PMID:25204554; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-2295 [DOI] [PubMed] [Google Scholar]
- 3.Garraway LA, Janne PA. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discovery 2012; 2:214-26; PMID:22585993; http://dx.doi.org/ 10.1158/2159-8290.CD-12-0012 [DOI] [PubMed] [Google Scholar]
- 4.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al.. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nature genetics 2015; 47(3):250-6; PMID:25665005; http://dx.doi.org/ 10.1038/ng.3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin L, Asthana S, Chan E, Bandyopadhyay S, Martins MM, Olivas V, Yan JJ, Pham L, Wang MM, Bollag G, et al.. Mapping the molecular determinants of BRAF oncogene dependence in human lung cancer. Proc Natl Acad Sci U S A 2014; 111:E748-57; PMID:24550319; http://dx.doi.org/ 10.1073/pnas.1320956111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, et al.. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010; 467:596-9; PMID:20823850; http://dx.doi.org/ 10.1038/nature09454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Lin Z, Zhou Z, Shen HC, Yan SF, Mayweg AV, Xu Z, Qin N, Wong JC, Zhang Z, et al.. Structure-based design and synthesis of potent cyclic peptides inhibiting the YAP-TEAD protein-protein interaction. ACS Med Chem Lett 2014; 5:993-8; PMID:25221655; http://dx.doi.org/ 10.1021/ml500160m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 2014; 141:1614-26; PMID:24715453; http://dx.doi.org/ 10.1242/dev.102376 [DOI] [PubMed] [Google Scholar]
- 9.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 2015; 15:73-9; PMID:25592648; http://dx.doi.org/ 10.1038/nrc3876 [DOI] [PMC free article] [PubMed] [Google Scholar]


