Abstract
We recently published that the retinoid-responsive gene NR2F1 (nuclear receptor subfamily 2, group F, member 1) mediates postsurgical dormancy of local residual tumor cells and disseminated tumor cells. Importantly, the combination of azacytidine with retinoids induces dormancy of malignant tumor cells by reinstating the NR2F1-regulated gene program. These findings open the door to the development of strategies that may stop minimal residual disease from becoming life-threatening metastases.
Keywords: dormancy, histones, minimal residual disease, NR2F1, pluripotency, quiescence, retinoids
Disseminated tumor cells (DTCs) that leave the primary tumor and become lodged at secondary organs such as bone marrow (BM)1 can transit for years through a dormant phase before growing into a new metastasis. This notion opens a window of opportunity to therapeutically target these DTCs and prevent relapses and also indicates that dormant DTCs remain intact upon conventional neo- and adjuvant therapies. At present, the molecular mechanisms responsible for dormancy of residual tumor cells in humans remain unknown; however in the last decade new experimental models have provided insight into what such mechanisms might be.2,3 More importantly, our recent study3 is the first to track one of these mechanisms in human DTCs from patients thought to have clinically dormant disease.
Using a head and neck squamous carcinoma cell (HNSCC) patient-derived xenograft (PDX) model, we revealed that the retinoic acid responsive orphan nuclear receptor NR2F1 (nuclear receptor subfamily 2, group F, member 1) (also known as COUP-TFI and EAR3) is upregulated and induces dormancy of local and distant residual tumor cells after tumor surgery. NR2F1 is a key node in a transcription factor network that constitutes a tumor cell dormancy signature. When applied to gene expression profiles of estrogen receptor-positive (ER+) breast cancer patients, this signature predicted longer metastasis-free periods.4 Remarkably, the dormancy signature was also found in dormant DTCs in prostate cancer patients who had been asymptomatic for 7–18 y,3,5 highlighting its relevance to human disease. In the HNSCC model, NR2F1 was downregulated in primary tumors but became upregulated when malignant tumor cells entered dormancy and was silenced again upon dormancy interruption.3 The plasticity of NR2F1 expression suggested that changes in the epigenome of residual tumor cells could be controlled by external and internal signals and dictate the fate of DTCs. Interestingly, NR2F1 expression was induced in prostate tumors after androgen deprivation therapy.6 In addition, NR2F1 was shown to limit induced pluripotent stem cell (iPS) reprogramming, probably by modulating chromatin remodeling. Along these lines, we found that NR2F1 was key in maintaining a globally repressive chromatin in dormant tumor cells while simultaneously allowing for an active chromatin state in the promoters of specific dormancy genes, including its own promoter.3 This emphasizes the existence of an orchestrated epigenetic program that is modulated by NR2F1 and microenvironmental cues and leads to tumor cell dormancy. Defining the cues that trigger this dormancy program and finding ways to reinstate it may have valuable research and clinical implications.
Intrinsic and Extrinsic Signals Defining Dormancy of DTCs
Microenvironmental signals, originating from stroma-DTC interactions and/or as a consequence of therapies, may trigger epigenetic programs that promote dormancy gene expression in DTCs.2 Recently, transforming growth factor, β 2 (TGFβ2) was shown to induce dormancy of HNSCC cells and to be upregulated in dormant cells collected from mouse BM.7 In addition, TGFβ2 can be upregulated by retinoids,3 and bone morphogenetic protein 7 (BMP7), another member of the TGFβ family that is secreted by normal bone marrow cells, also induced dormancy of prostate cancer cells in mouse BM.8 Interestingly, BMP7, TGFβ2, and NR2F1 genes were all upregulated in BM DTCs collected from prostate cancer patients considered to be in clinical dormancy.3,5 This argues that these experimental models are helpful to elucidate relevant mechanisms of dormancy and that a commonality of pathways exists among different types of cancer. Interestingly, the sequential combination of DNA demethylating agent 5-azacytidine followed by the retinoic acid receptor (RAR) pan-agonist all trans retinoic acid (atRA), reprogrammed tumor cells into long-term dormancy by reinstating, at least in part, the NR2F1-induced dormancy program. The use of these drugs, which have already been approved by the U.S. Food and Drug Administration (FDA) and tested in clinical trials, may be the basis for future treatments to induce a chronically asymptomatic disease (see “Implications” below and Fig. 1).
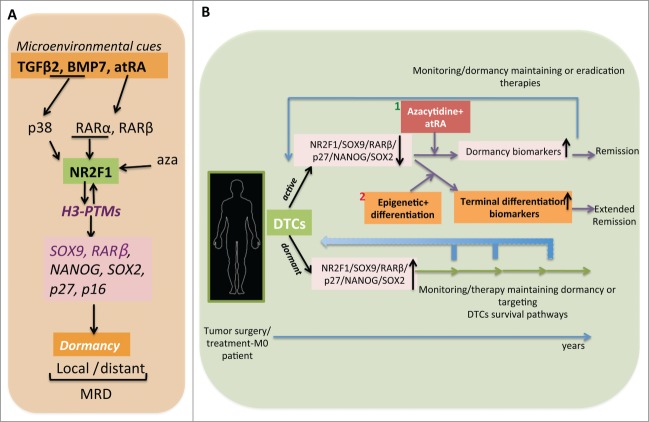
Figure 1.
Dormancy programs and their potential use in patient treatment. (A) Microenvironmental cues such as retinoids and stress signals enforce epigenetic changes in NR2F1 (nuclear receptor subfamily 2, group F, member 1) expression and activate an NR2F1-dependent dormancy program in local and distant residual tumor cells. This signature is defined by the re-expression of genes in the retinoic acid, pluripotency, and cell cycle inhibitor pathways. Moreover, NR2F1 maintains active histone markers on SOX9 (SRY [sex determining region Y]- box 9) and RARβ (retinoic acid receptor, β) promoters in dormant cells. However, at the same time NR2F1 is also responsible for a globally repressive chromatin state. Lastly, all trans retinoic acid (atRA) in combination with azacytidine (aza) enforces a long-sustained NR2F1-induced dormancy program. H3-PTMs, histone 3-post-translational modifications; MRD, minimal residual disease. (B) Possible therapeutic scenarios for patients with long-term MRD. Disseminated tumor cells (DTCs) collected from bone marrow (BM) of M0 stage patients (distant metastases are not detected) could be analyzed to determine their dormant or active status and select the appropriate therapeutic action. For example, if DTCs carry a dormancy signature (NR2F1high/NR2F1 targetshigh) either dormancy maintaining therapy could be applied or therapies that eliminate these cells could be used (lower part of the panel); if DTCs carry a signature indicative of reactivation (NR2F1low/NR2F1 targetslow) then the azacytidine+atRA therapy that we propose here may serve to convert these residual tumor cells back to dormancy. This combination might also benefit from the addition of inhibitors of the mitogen-activated protein kinase 1 (MAPK1) pathway (1). DTCs from BM aspirates could be tested for restoration of dormancy biomarkers and to examine the long-lasting treatment efficacy over years. Patients under this scenario would be considered in remission. Alternatively, the design of treatments that allow terminal differentiation of residual tumor cells would entirely prevent the reawakening of DTCs and induce an extended long-term remission phase (2).
The NR2F1-induced dormant phenotype observed was molecularly defined by the induction of a global repressive chromatin state and upregulation of the SOX9 (SRY [sex determining region Y]- box 9) and RARβ (retinoic acid receptor, β) genes, which in turn upregulated cyclin-dependent kinase (CDK) inhibitors fueling a strong G0/G1 arrest (Fig. 1A). Intriguingly, while limiting tumor initiating potential via quiescence, NR2F1 induced the expression of NANOG (Nanog homeobox) and SOX2, 2 important pluripotency genes (Fig. 1A). At least NANOG was required for the quiescence phase of DTCs. These findings suggest a specific dormancy function for NR2F1 in cancer cells that may be associated with preservation of quiescence and/or the later metastasis initiating capacity of DTCs. Intriguingly, this coordination of “stemness” pathways with quiescence is similar to that observed in normal adult stem cells (ASCs),2,9 suggesting that dormant tumor cells may use programs similar to those in ASCs to remain in quiescence but retain a latent ability to reactivate. Our findings highlight that the study of DTC dormancy is revealing a new cancer biology that was previously unappreciated and thus not targeted by therapies.
Implications
Conventional therapies do not seem to effectively target metastases for several reasons, one being that they do not eliminate DTCs. Interestingly, our work has brought us back to the concept of the differentiation therapies proposed years ago that were used to treat and prevent primary tumor.10 Our new data showed that the use of retinoids together with epigenetic modulators converts residual tumor cells into long-term dormancy, mimicking spontaneous dormancy programs. From a clinical perspective, this reprogramming treatment would induce dormancy of proliferative residual tumor cells and keep them from reactivating, leading to a chronically asymptomatic disease (Fig. 1B.1). However, targeting survival pathways or promoting terminal differentiation in DTCs may lead to their long-term elimination (Fig. 1B.2). Animal models that reproduce what occurs in the clinic (i.e., tumor surgery and adjuvant therapies) could be used to test dormancy-inducing therapies like the one we proposed.3 Another contribution of our work was defining biomarkers of dormant DTCs that we have now validated in human samples. These data should facilitate determination of the state of DTCs (dormant versus active) as well as monitoring therapy responses.
Finally, our findings suggest that, although common to different tumors, the dormancy programs might have different functions that may depend on organ-specific signals. Along these lines, we found that NR2F1 in the BM was linked to quiescence and survival of DTCs. To further dissect how NR2F1 orchestrates these programs we are currently generating epigenome-wide maps of NR2F1 in dormant DTCs from different microenvironments. Conceivably, the design of therapies that establish strong terminal differentiation could prevent all DTCs from growing into metastasis regardless of the organ site.
Funding
MSS is supported by a DoD-BCRP Grant BC112380.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
MSS would like to acknowledge all authors of the original report.3
References
- 1.Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, et al.. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 2000; 342:525-33; PMID:10684910; http://dx.doi.org/ 10.1056/NEJM200002243420801 [DOI] [PubMed] [Google Scholar]
- 2.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014; 14:611-22; PMID:25118602; http://dx.doi.org/ 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosa MS, Parikh F, Maia AG, Estrada Y, Bosch A, Bragado P, Ekpin E, George A, Zheng Y, Lam HM, et al.. NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat Commun 2015; 6:6170; PMID:25636082; http://dx.doi.org/ 10.1038/ncomms7170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim RS, Avivar-Valderas A, Estrada Y, Bragado P, Sosa MS, Aguirre-Ghiso JA, Segall JE. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PloS One 2012; 7:e35569; PMID:22530051; http://dx.doi.org/ 10.1371/journal.pone.0035569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chery L, Lam HM, Coleman I, Lakely B, Coleman R, Larson S, Aguirre-Ghiso JA, Xia J, Gulati R, Nelson PS, et al.. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 2014; 5:9939-51.; PMID:25301725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson VC, Day TK, Bianco-Miotto T, Selth LA, Han G, Thomas M, Buchanan G, Scher HI, Nelson CC, Greenberg NM, et al.. A gene signature identified using a mouse model of androgen receptor-dependent prostate cancer predicts biochemical relapse in human disease. Int J Cancer 2011; 131(3):662-72; http://dx.doi.org/ 10.1002/ijc.26414 [DOI] [PubMed] [Google Scholar]
- 7.Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, Schewe DM, Aguirre-Ghiso JA. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol 2013; 15:1351-61; PMID:24161934; http://dx.doi.org/ 10.1038/ncb2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al.. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med 2011; 208:2641-55; PMID:22124112; http://dx.doi.org/ 10.1084/jem.20110840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 2013; 14:329-40; PMID:23698583; http://dx.doi.org/ 10.1038/nrm3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geyer C, Papadimitrakopoulou V, Hong WK. Chemoprevention in head and neck cancer: basic science and clinical application. Semin Radiat Oncol 1998; 8:292-301; PMID:9873107; http://dx.doi.org/ 10.1016/S1053-4296(98)80027-1 [DOI] [PubMed] [Google Scholar]