ABSTRACT
The BCL-2/BCL-XL inhibitor navitoclax has shown promise for the treatment of cancer but on-target toxicities have limited its utility. Recently, the generation of selective BCL-2 family inhibitors has enabled a careful dissection of BCL-2 biology, and early work indicates that these molecules have improved therapeutic profiles for the treatment of cancer.
KEYWORDS: A-1331852, apoptosis, chemical parsing, navitoclax, venetoclax
The intrinsic mitochondrial pathway of programmed cell death (apoptosis) is regulated by the B-cell lymphoma-2 (BCL-2) family of proteins, which contain up to 4 BCL-2 homology (BH) motifs. Antiapoptotic proteins like BCL-2, BCL-2 related protein, long isoform (BCL-XL), and myeloid cell leukemia-1 (MCL-1) bind the BH3 motifs of specific proapoptotic counterparts, including BH3-only proteins such as BCL-2 interacting mediator of cell death (BIM) and the multidomain proteins BCL-2 antagonist/killer (BAK) and BCL-2 associated X protein (BAX). Antiapoptotic proteins are often overexpressed in cancer cells, where they sequester their proapoptotic counterparts to maintain survival. This makes the BCL-2 family of anti-apoptotic proteins attractive therapeutic targets, albeit targets that are very challenging to drug. In the last 2 decades, drug discovery scientists have been able to target the BH3-binding grooves of BCL-2, BCL-XL and MCL-1 with small-molecule BH3 mimetics, which effectively compete for binding with the pro-death proteins and free them to trigger apoptosis.
ABT-737 and ABT-263 (navitoclax) inhibit both BCL-2 and BCL-XL, and were the first BH3 mimetics shown to be capable of antitumor activity in vivo.1,2 Navitoclax went on to demonstrate antileukemic activity in early stage clinical trials but its dosing was limited by thrombocytopenia. Concurrent preclinical studies suggested that this toxicity was the result of BCL-XL inhibition,3,4 prompting the hypothesis that selective BCL-2 inhibition could achieve antileukemic activity while sparing platelets. After another campaign of structure-based drug design, the navitoclax team was able to generate the potent and selective BCL-2 inhibitor ABT-199, now known as venetoclax.5 Preclinical studies with venetoclax validated the hypothesis that a BCL-2–selective inhibitor would spare platelets and improve upon the efficacy of navitoclax. Clinical proof of this concept was first achieved in subjects with chronic lymphocytic leukemia (CLL), in whom single doses of venetoclax induced rapid and extensive reductions in circulating tumor burden.
As the venetoclax story demonstrates, selective BCL-2 family inhibitors can be powerful tools for dissecting cell survival dependencies and translating those findings into improved strategies for cancer therapy. Knowing this, our team had also been at work generating BCL-XL–selective inhibitors, and these efforts culminated in the recently described molecules A-1155463 and A-1331852.6,7 Together with venetoclax, these compounds provide a molecular toolkit for parsing the various effects of navitoclax into its BCL-2– and/or BCL-XL–dependent components. By using these compounds in simple cell killing experiments one can easily determine which BCL-2 family proteins a given cell population depends on for survival. For example, the killing of certain cancer cell lines can be attributed specifically to BCL-2 or BCL-XL inhibition, whereas others can only be killed when both targets are inhibited.7 There are also cases in which inhibition of either BCL-2 or BCL-XL suffices to kill a given cell line and, not surprisingly, cells that show resistance to all 3 classes of inhibitor.
Armed with this molecular toolkit, our team set out to answer another set of questions. Preclinical work had demonstrated that navitoclax can synergize with taxanes to kill solid tumor cells in vitro and in vivo; however, dosing of this combination in the clinic was limited by neutropenia.8 Using a combination of approaches, including a number of tumor xenograft studies, it was demonstrated that BCL-XL inhibition suffices to recapitulate the activity of navitoclax when combined with docetaxel.7 Conversely, granulocyte colony forming assays using human bone marrow and in vivo rodent studies pointed strongly to BCL-2 inhibition as the driver of exacerbated neutropenia. Thus, the story in solid tumors turned out to be the reverse of what had been observed in hematologic malignancies (Fig. 1): in this case it was BCL-XL inhibition that drove the efficacy of the navitoclax-docetaxel combination, whereas BCL-2 inhibition was the culprit in exacerbating neutropenia. And so, by employing this collection of selective BCL-2 family inhibitors, the team was able to identify another improved therapeutic strategy, in this case for the treatment of solid tumors.
Figure 1.
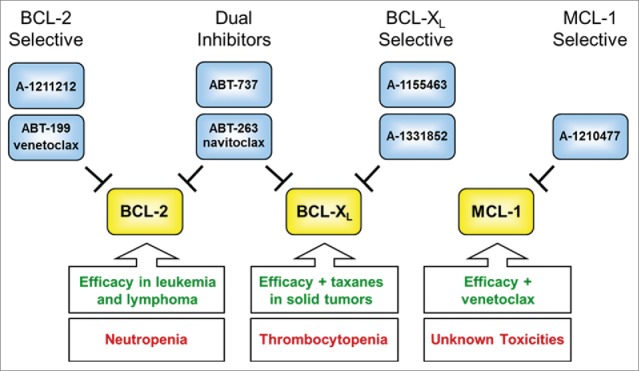
A molecular toolkit of BCL-2 family inhibitors. The antiapoptotic proteins BCL-2 (B-cell lymphoma-2), BCL-XL (BCL-2 related protein, long isoform), and MCL-1 (myeloid cell leukemia-1) bind to the BCL-2 homology 3 (BH3) motifs of proapoptotic proteins, thereby preventing apoptosis. This diagram depicts the selectivity of various small-molecule BH3 mimetics that have been generated as BCL-2 family inhibitors. Using the latest generation of selective molecules, scientists are beginning to define the roles of these proteins in maintaining the survival of certain normal and malignant cell populations. These findings are helping to define improved strategies for the treatment of both hematologic malignancies and solid tumors.
The description of potent, selective, and orally bioavailable BH3 mimetics like the BCL-2–selective inhibitor venetoclax and the BCL-XL–selective inhibitor A-1331852 represents a leap forward in our ability to dissect cell survival dependencies. More importantly, we are finding that these molecules have the potential to exhibit improved therapeutic profiles. With the recent description of MCL-1–selective inhibitors9,10 this toolkit of BH3 mimetics continues to expand. “Chemical parsing” enabled by these tools should help to define the roles of BCL-2, BCL-XL, and MCL-1 in a variety of normal and diseased tissue types. Ultimately the hope is that, like venetoclax, other selective BCL-2 family inhibitors will find uses in the clinic for the treatment of cancer and other diseases.
Disclosure of Potential Conflicts of Interest
The author is an employee of AbbVie. AbbVie and Genentech participated in the review and approval of this publication.
Funding
Financial support for this research was provided by AbbVie & Genentech.
References
- 1.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al.. An inhibitor of Bcl-2 family proteins induces regression of solid tumors. Nature 2005; 435:677-681; PMID:15902208; http://dx.doi.org/ 10.1038/nature03579 [DOI] [PubMed] [Google Scholar]
- 2.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al.. ABT-263: A Potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68:3421-3428; PMID:18451170; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5836 [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, Iciek LA, Morgan SJ, Nasarre MC, Nelson R, et al.. BCL-2 family proteins are essential for platelet survival. Cell Death Differ 2007; 14:943-951; PMID:17205078; http://dx.doi.org/ 10.1038/sj.cdd.4402072 [DOI] [PubMed] [Google Scholar]
- 4.Mason K, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, et al.. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128:1173-1186; PMID:17382885; http://dx.doi.org/ 10.1016/j.cell.2007.01.037 [DOI] [PubMed] [Google Scholar]
- 5.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, et al.. ABT-199, a potent and selective BCL-2 inhibitor, achieves anti-tumor activity while sparing platelets. Nat Med 2013; 19:202-208; PMID:23291630; http://dx.doi.org/ 10.1038/nm.3048 [DOI] [PubMed] [Google Scholar]
- 6.Tao ZF, Hasvold L, Wang L, Wang X, Petros AM, Park CH, Boghaert E, Catron ND, Chen J, Colman PM, et al.. Discovery of a potent and selective BCL-XL inhibitor with in vivo activity. ACS Med Chem Lett 2014; 5:1088-1093; PMID:25313317; http://dx.doi.org/ 10.1021/ml5001867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, Belmont LD, Nimmer P, Xiao Y, Ma XM, et al.. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 2015; 7:279ra40; PMID:25787766; http://dx.doi.org/ 10.1126/scitranslmed.aaa4642 [DOI] [PubMed] [Google Scholar]
- 8.Puglisi M, van Doorn L, Blanco-Codesido M, De Jonge MJ, Moran K, Yang J, Busman T, Franklin C, Mabry M, Krivoshik A, et al.. A phase I safety and pharmacokinetic (PK) study of navitoclax (N) in combination with docetaxel (D) in patients (pts) with solid tumors. J Clin Oncol 2011; 29:supplemental abstract 2518 [Google Scholar]
- 9.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, et al.. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis 2015; 6:e1590; PMID:25590800; http://dx.doi.org/ 10.1038/cddis.2014.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruncko M, Wang L, Sheppard GS, Phillips DC, Tahir SK, Xue J, Erickson S, Fidanze S, Fry E, Hasvold L, et al.. Structure-guided design of a series of MCL-1 inhibitors with high affinity and selectivity. J Med Chem 58:2180-2194; PMID:25679114; http://dx.doi.org/ 10.1021/jm501258m [DOI] [PubMed] [Google Scholar]


