ABSTRACT
E2F transcription factors are key regulators of cellular proliferation, and altered E2F activity is a common feature of tumor cells. Thus, E2F targeting is being explored as a therapeutic strategy in cancer. Importantly, recent mouse knockout studies show that concomitant loss of E2f1/E2f2 activity is associated with increased genomic instability and oncogenic potential in normal differentiating cells, a finding that might have implications for cancer therapy.
Keywords: Apoptosis, DNA damage response, E2, p53, replication stress, senescence
The retinoblastoma protein (RB1, best known as pRB) pathway plays a central role in the control of cell proliferation and cell death.1 Components of this pathway, such as CDKN2A, CCND1, and the RB gene itself, are frequently mutated in cancer cells. Inactivation of the pRB pathway leads to G1 checkpoint alterations and genomic instability that are thought to contribute to tumorigenesis. The oncogenic stress induced by loss of pRB function is known to activate the other major tumor suppressor, p53 (TP53, best known as p53), and the combined suppression of pRB and p53 pathways is critical for disabling the cellular defense against neoplasia.1
E2F transcription factors (E2F1–8) are key downstream effectors of pRB. They regulate the expression of a wide range of transcriptional targets involved in DNA replication and repair, cell cycle progression, apoptosis, or differentiation, which underlies their relevance in cell fate regulatory networks.2 Loss of pRB function is thought to lead to inappropriate activation of E2F factors, followed by increased cellular proliferation rates and tumorigenesis.2 Thus, inhibition of E2F activity has been proposed as a therapeutic strategy for the treatment of cancer and it has been shown that small-molecule E2F inhibitors are effective against cancer cells.3-5 Our recent work raises a cautionary note on the suitability of this approach by providing evidence that loss of E2F activity has harmful effects on differentiating cells that could potentially outweigh the therapeutic benefits of E2F targeting.
To dissect the contribution of E2F factors to cell fate and tumorigenesis, our laboratory and others have generated gain-of-function and loss-of-function mouse models of individual E2fs or combinations of E2fs. These studies have revealed the complexity of the functional roles played by individual E2fs, which are often dependent on the cellular context. Regarding E2F1 and E2F2, there are experimental data supporting both positive and negative regulatory roles in cellular proliferation and tumorigenesis, through mechanisms that remain largely unresolved.6
We have recently described a double knockout (DKO) mouse model (E2f1−/−/E2f2−/−) in which the combined loss of E2f1 and E2f2 activity results in unscheduled DNA replication and cellular division in differentiating cells.7,8 E2f1−/−/E2f2−/− DKO cells show overexpression of E2f target genes involved in DNA replication or cell cycle progression (including Mcm genes, Orc1, Cdc6, Ccnd3, Ccna2, Cdc25a, or Cdc25c), and repression of cell cycle inhibitors (Cdkn2c). Furthermore, the activities of both G1 and mitotic cyclin/Cdks appear to be aberrantly elevated in DKO cells. These results suggest a key role for E2f1/E2f2 transcription factors in restraining DNA synthesis and promoting cell cycle exit during terminal differentiation.
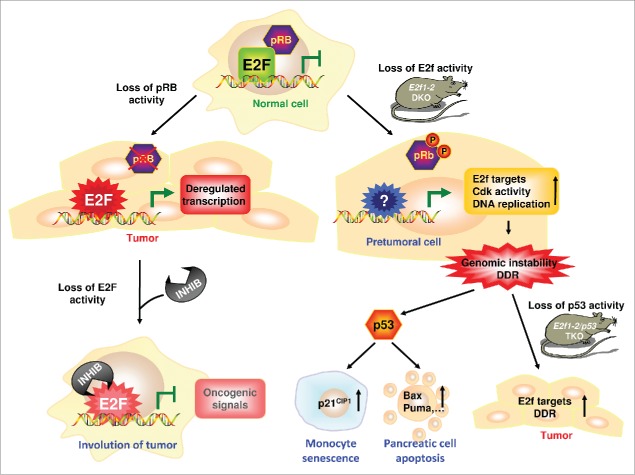
We have examined in detail the consequences of unscheduled DNA replication and cell cycle progression in cells lacking E2f1 and E2f2, finding evidence (increased γ-H2ax levels and activation of the p53 pathway) of a strong DNA damage response (DDR) in cells that are undergoing terminal differentiation, including bone-marrow-derived macrophages and pancreatic cells (Fig. 1). This DDR was associated with senescence in macrophages and with apoptosis in pancreatic cells, leading to generalized tissue atrophy and diabetes.
Figure 1.
Influence of cellular context on the outcome of E2F depletion. Tumor cells commonly exhibit disruption of the pRB pathway, which deregulates E2F function. Loss of E2F activity in tumor cells by treatment with inhibitor molecules (INHIB) prevents tumor growth. In contrast, loss of E2f activity by targeted inactivation (E2f1−/−/E2f2−/− DKO) within a non-tumoral cell elicits a DNA damage response (DDR) and genomic instability. Consequently, this abnormal condition leads to apoptosis or senescence in a p53-functional context, but is oncogenic in a p53-deficient context (E2f1−/−/E2f2−/−/p53−/− TKO).
There are several mechanisms by which E2f1/E2f2 depletion might lead to replication stress. The increased expression of genes involved in formation of the DNA replication complex could lead to illegitimate replication origin firing and fork stalling, as shown after overexpression of CDT1.9 Furthermore, recent work has shown that controlled Cdk activity is key to maintaining genome integrity during S phase.10 Thus, the accumulation of aberrant G1 and mitotic Cdk activities in cells lacking E2f1 and E2f2 may also indirectly affect the efficiency of DNA replication processes.
Importantly, we have shown that inhibition of DNA replication by the DNA polymerase inhibitor aphidicolin prevented accumulation of the DDR mediators γ-H2ax, p53, and Cdkn1a (best known as p21CIP1) in DKO pancreatic and monocyte cells, which places p53 activation downstream of the DNA replication stress in our systems. We have further shown that functional inactivation of the p53 pathway restored most of the phenotypic defects associated with E2f1/E2f2 loss. Interestingly, elimination of p21CIP1 could rescue senescence, but not apoptosis, of DKO cells, suggesting that the program elicited by p53 in E2f1−/−/E2f2−/− DKO mice is primarily aimed to arrest cell cycle in macrophages and to activate apoptosis in pancreatic cells.
Strikingly, depletion of p53 led to the development of T-cell lymphoblastic leukemia/lymphomas in E2f1−/−/E2f2−/− mice, significantly reducing their lifespan. These findings imply that loss of E2f1/E2f2 increases the oncogenic potential in normal cells through the generation of genomic instability. The outcome of these cells will depend on their levels of functional p53.
In summary, our results show that depletion of E2f1/E2f2 can lead to apoptosis or senescence in normally differentiating p53-competent cells, but promotes tumor development upon loss of p53. These findings support the notion that cell context is critical for the outcome of E2F activity, and have important implications in the field of cancer therapy. Ongoing studies aimed to develop E2F inhibitors as anticancer agents should take into account the fact that exacerbating DNA replication rates in normal cells by depleting E2f1/E2f2 could lead to atrophy in a p53-competent context but might be oncogenic in a p53-deficient context.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Jose Antonio Rodriguez for helpful discussions and comments on the manuscript.
Funding
This work was supported by grants from the Spanish Ministry (SAF2012–33551), the Basque Government (IE12–331 and IT634–13), and the University of the Basque Country UPV/EHU (UFI11/20).
References
- 1.Manning AL, Dyson NJ. pRB, a tumor suppressor with a stabilizing presence. Trends Cell Biol 2011; 21:433-41; PMID:22318235; http://dx.doi.org/ 10.1038/nrc3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Heuvel S, Dyson NJ. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 2008; 9:713-24; PMID:18719710; http://dx.doi.org/ 10.1038/nrm2469 [DOI] [PubMed] [Google Scholar]
- 3.Kaelin WG. Jr. E2F1 as a target: promoter-driven suicide ans small molecule modulators. Cancer Biol ther 2003; 2(4 Suppl 1):S48-54; PMID:14508080 [PubMed] [Google Scholar]
- 4.Ma Y, Kurtyka CA, Boyapalle S, Sung SS, Lawrence H, Guida W, Cress WD. A small-molecule E2F inhibitor blocks growth in a melanoma culture model. Cancer Res 2008; 68:6292-9; PMID:18676853; http://dx.doi.org/ 10.1158/0008-5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie X, Bansal N, Shaik T, Kerrigan JE, Minko T, Garbuzenko O, Abali EE, Johnson-Farley N, Banerjee D, Scotto KW, Bertino JR. A novel peptide that inhibits E2F transcription and regresses prostate tumor xenografts. Oncotarget 2014; 5:901-7; PMID:24658650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 2009; 9:785-97; PMID:19851314; http://dx.doi.org/ 10.1038/nrc2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iglesias-Ara A, Zenarruzabeitia O, Fernandez-Rueda J, Sanchez-Tillo E, Field SJ, Celada A, Zubiaga AM. Accelerated DNA replication in E2F1- and E2F2-deficient macrophages leads to induction of the DNA damage response and p21(CIP1)-dependent senescence. Oncogene 2010; 29: 5579-90; PMID:20676136; http://dx.doi.org/ 10.1038/onc.2010.296 [DOI] [PubMed] [Google Scholar]
- 8.Iglesias-Ara A, Zenarruzabeitia O, Buelta L, Merino J, Zubiaga AM. E2F1 and E2F2 prevent replicative stress and subsequent p53-dependent organ involution. Cell Death Differ 2015; Feb 6; PMID:25656653; http://dx.doi.org/ 10.1038/cdd.2015.4 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson IF, Li A, Blow JJ. Deregulated replication licensing causes DNA fragmentation consistent with head-to-tail fork collision. Mol Cell 2006; 24: 433-43; PMID:17081992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck H1, Nähse V, Larsen MS, Groth P, Clancy T, Lees M, Jørgensen M, Helleday T, Syljuåsen RG, Sørensen CS. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J Cell Biol 2010; 188: 629-38; PMID:20194642; http://dx.doi.org/ 10.1083/jcb.200905059 [DOI] [PMC free article] [PubMed] [Google Scholar]